The Practical Implications of Re-Referencing in ERP Studies: The Case of N400 in the Picture–Word Verification Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
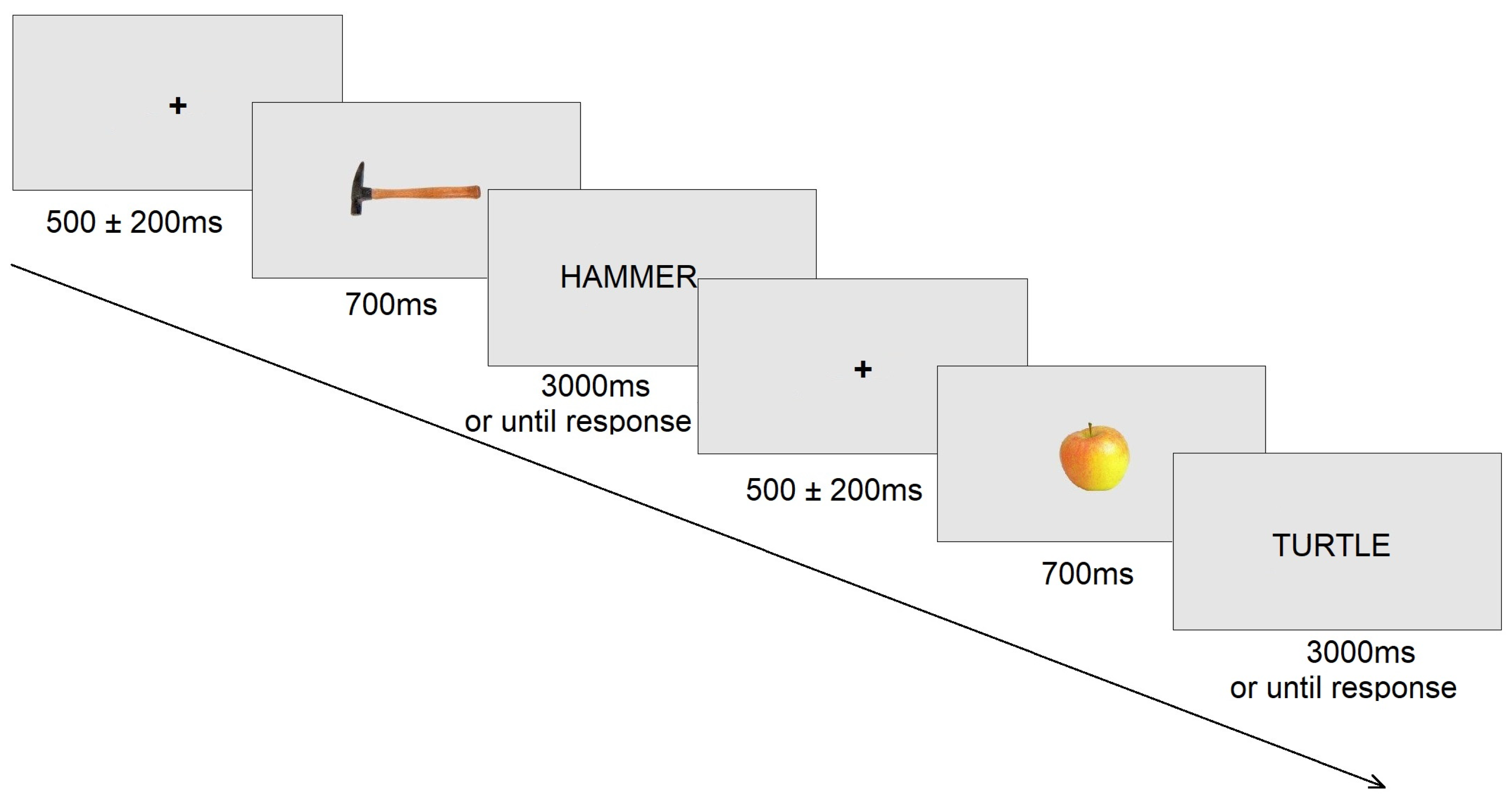
2.3. Procedure
2.4. EEG Recording and ERP Processing
2.5. Data Analysis
3. Results
3.1. Behavioral Results
3.2. ERP Results
3.3. Statistical Parametric Scalp Mapping (SPSM) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niso, G.; Krol, L.R.; Combrisson, E.; Dubarry, A.S.; Elliott, M.A.; François, C.; Héjja-Brichard, Y.; Herbst, S.K.; Jerbi, K.; Kovic, V.; et al. Good Scientific Practice in EEG and MEG Research: Progress and Perspectives. NeuroImage 2022, 257, 119056. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R.; Miller, G.A.; Ritter, W.; Ruchkin, D.S.; Rugg, M.D.; et al. Guidelines for Using Human Event-Related Potentials to Study Cognition: Recording Standards and Publication Criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; St Louis, E.K., Frey, L.C., Eds.; American Epilepsy Society: Chicago, IL, USA, 2016; ISBN 978-0-9979756-0-4. [Google Scholar]
- Brain Products. Choosing Your Reference—And Why It Matters [Press Release]. Available online: https://pressrelease.brainproducts.com/referencing/ (accessed on 22 March 2024).
- Luck, S.J. An Introduction to the Event-Related Potential Technique, 2nd ed.; The MIT Press: Cambridge, MA, USA, 2014; ISBN 978-0-262-52585-5. [Google Scholar]
- Offner, F.F. The EEG as Potential Mapping: The Value of the Average Monopolar Reference. Electroencephalogr. Clin. Neurophysiol. 1950, 2, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Yao, D. A Method to Standardize a Reference of Scalp EEG Recordings to a Point at Infinity. Physiol. Meas. 2001, 22, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.; Perrin, F.; Pernier, J. A Theoretical Justification of the Average Reference in Topographic Evoked Potential Studies. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1985, 62, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. The Clinical Use of the “Average” Reference Electrode in Monopolar Recording. Electroencephalogr. Clin. Neurophysiol. 1950, 2, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.A.; Miriani, R.M.; Langhals, N.B.; Joseph, M.D.; Anderson, D.J.; Kipke, D.R. Using a Common Average Reference to Improve Cortical Neuron Recordings from Microelectrode Arrays. J. Neurophysiol. 2009, 101, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic ERP Analyses: A Step-by-Step Tutorial Review. Brain Topogr. 2008, 20, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. Issues in the Application of the Average Reference: Review, Critiques, and Recommendations. Behav. Res. Methods Instrum. Comput. 1998, 30, 34–43. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; ISBN 978-0-19-505038-7. [Google Scholar]
- Dong, L.; Liu, X.; Zhao, L.; Lai, Y.; Gong, D.; Liu, T.; Yao, D. A Comparative Study of Different EEG Reference Choices for Event-Related Potentials Extracted by Independent Component Analysis. Front. Neurosci. 2019, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Hu, Z.; Li, Y.; Ye, C.; Liu, Q. Electrophysiological Correlates of Change Detection during Delayed Matching Task: A Comparison of Different References. Front. Neurosci. 2017, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, P.; Yao, D. A Comparative Study of Different References for EEG Default Mode Network: The Use of the Infinity Reference. Clin. Neurophysiol. 2010, 121, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yao, D. Why Do We Need to Use a Zero Reference? Reference Influences on the ERPs of Audiovisual Effects: Reference Influence on ERPs. Psychophysiology 2013, 50, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.; Yang, H.; Xu, J.; Mo, S.; Lai, H.; Wu, T.; Zhang, J. Influence of EEG References on N170 Component in Human Facial Recognition. Front. Neurosci. 2019, 13, 705. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Fan, C.; Wang, M.; Li, L. A Comparative Study of Average, Linked Mastoid, and REST References for ERP Components Acquired during FMRI. Front. Neurosci. 2017, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L. REST: A Good Idea but Not the Gold Standard. Clin. Neurophysiol. 2010, 121, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Kutas, M.; Federmeier, K.D. Thirty Years and Counting: Finding Meaning in the N400 Component of the Event-Related Brain Potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; Ostrosky-Solis, F.; Perez, M.; Bobes, M.A.; Rangel, L.E. ERP Assessment of Semantic Memory in Alzheimer’s Disease. Int. J. Psychophysiol. 1997, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.M.; Woodward, S.H.; Sullivan, E.V.; Isaacks, B.G.; Tinklenberg, J.R.; Yesavage, J.A.; Roth, W.T. N400 Evidence of Abnormal Responses to Speech in Alzheimer’s Disease. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 235–246. [Google Scholar] [CrossRef]
- Olichney, J.M.; Yang, J.-C.; Taylor, J.; Kutas, M. Cognitive Event-Related Potentials: Biomarkers of Synaptic Dysfunction Across the Stages of Alzheimer’s Disease. J. Alzheimers Dis. 2011, 26, 215–228. [Google Scholar] [CrossRef]
- Coderre, E.L.; Cohn, N.; Slipher, S.K.; Chernenok, M.; Ledoux, K.; Gordon, B. Visual and Linguistic Narrative Comprehension in Autism Spectrum Disorders: Neural Evidence for Modality-Independent Impairments. Brain Lang. 2018, 186, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Márquez-García, A.V.; Vakorin, V.A.; Kozhemiako, N.; Magnuson, J.R.; Iarocci, G.; Ribary, U.; Moreno, S.; Doesburg, S.M. Children with Autism Spectrum Disorder Show Atypical Electroencephalographic Response to Processing Contextual Incongruencies. Sci. Rep. 2022, 12, 8948. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.C.; Valasek, C.A.; Minati, L.; Boggio, P.S. Altered Semantic Integration in Autism beyond Language: A Cross-Modal Event-Related Potentials Study. Neuroreport 2013, 24, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Baeuchl, C.; Hoppstädter, M. Insights from Simultaneous EEG-fMRI and Patient Data Illuminate the Role of the Anterior Medial Temporal Lobe in N400 Generation. Neuropsychologia 2024, 193, 108762. [Google Scholar] [CrossRef]
- Olichney, J.M.; Taylor, J.R.; Gatherwright, J.; Salmon, D.P.; Bressler, A.J.; Kutas, M.; Iragui-Madoz, V.J. Patients with MCI and N400 or P600 Abnormalities Are at Very High Risk for Conversion to Dementia. Neurology 2008, 70, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Díaz Rivera, M.N.; Amoruso, L.; Bocanegra, Y.; Suárez, J.X.; Moreno, L.; Muñoz, E.; Birba, A.; García, A.M. Electrophysiological Alterations during Action Semantic Processing in Parkinson’s Disease. Neurobiol. Aging 2024, 136, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, H.; Tachibana, H.; Sugita, M.; Okita, T. Recognition Memory in Normal Aging and Parkinson’s Disease: Behavioral and Electrophysiologic Measures. Cogn. Brain Res. 2001, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Tachibana, H.; Sugita, M. Memory Function in Aging and Parkinson’s Disease-An Event-related Potential Study. Jpn. J. Geriat 1998, 35, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Olichney, J.M.; Riggins, B.R.; Hillert, D.G.; Nowacki, R.; Tecoma, E.; Kutas, M.; Iragui, V.J. Reduced Sensitivity of the N400 and Late Positive Component to Semantic Congruity and Word Repetition in Left Temporal Lobe Epilepsy. Clin. Electroencephalogr. 2002, 33, 111–118. [Google Scholar] [CrossRef]
- Tian, Z.; Huang, S.; Wen, S.; Zhang, Q.; Huang, K.; Gui, Y.; Hu, B.; Feng, L.; Wang, Q. Event-Related Potentials Reveal Visual Episodic Memory Deficits in Patients with Temporal Lobe Epilepsy. Epilepsy Behav. 2023, 148, 109460. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.F. Semantic Activation and Verbal Working Memory Maintenance in Schizophrenic Thought Disorder: Insights from Electrophysiology and Lexical Amibiguity. Clin. EEG Neurosci. 2008, 39, 103–107. [Google Scholar] [CrossRef]
- Shin, K.S.; Kang, D.-H.; Choi, J.-S.; Kim, Y.Y.; Kwon, J.S. Neuropsychological Correlates of N400 Anomalies in Patients with Schizophrenia: A Preliminary Report. Neurosci. Lett. 2008, 448, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Basma, B.; Savage, R.; Bertone, A. The N400 in Readers with Dyslexia: A Systematic Review and Meta-Analysis. Int. J. Psychophysiol. 2024, 196, 112283. [Google Scholar] [CrossRef]
- Simmons, J.P.; Nelson, L.D.; Simonsohn, U. False-Positive Psychology: Undisclosed Flexibility in Data Collection and Analysis Allows Presenting Anything as Significant. Psychol. Sci. 2011, 22, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Gaspelin, N. How to Get Statistically Significant Effects in Any ERP Experiment (and Why You Shouldn’t). Psychophysiology 2017, 54, 146–157. [Google Scholar] [CrossRef]
- Ghosh Hajra, S.; Liu, C.C.; Song, X.; Fickling, S.D.; Cheung, T.P.L.; D’Arcy, R.C.N. Multimodal Characterization of the Semantic N400 Response within a Rapid Evaluation Brain Vital Sign Framework. J. Transl. Med. 2018, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.F.; Phillips, C.; Poeppel, D. A Cortical Network for Semantics: (De)Constructing the N400. Nat. Rev. Neurosci. 2008, 9, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Van Petten, C.; Luka, B.J. Neural Localization of Semantic Context Effects in Electromagnetic and Hemodynamic Studies. Brain Lang. 2006, 97, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Tucker, D.M.; Kutas, M.; Posner, M.I. Topography of the N400: Brain Electrical Activity Reflecting Semantic Expectancy. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1993, 88, 188–209. [Google Scholar] [CrossRef] [PubMed]
- Petten, C.V.; Kutas, M. The Use of Event-Related Potentials in the Study of Brain Asymmetries. Int. J. Neurosci. 1988, 39, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, B.; Wang, Y.; Zhou, X. Electrophysiological Responses to Expectancy Violations in Semantic and Gambling Tasks: A Comparison of Different EEG Reference Approaches. Front. Neurosci. 2018, 12, 169. [Google Scholar] [CrossRef]
- Šoškić, A.; Jovanović, V.; Styles, S.J.; Kappenman, E.S.; Ković, V. How to Do Better N400 Studies: Reproducibility, Consistency and Adherence to Research Standards in the Existing Literature. Neuropsychol. Rev. 2021, 32, 577–600. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-Related Potentials in Clinical Research: Guidelines for Eliciting, Recording, and Quantifying Mismatch Negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Martin, A.E.; Nieuwland, M.S. How Robust Are Prediction Effects in Language Comprehension? Failure to Replicate Article-Elicited N400 Effects. Lang. Cogn. Neurosci. 2017, 32, 954–965. [Google Scholar] [CrossRef]
- Kappenman, E.S.; Farrens, J.L.; Zhang, W.; Stewart, A.X.; Luck, S.J. ERP CORE: An Open Resource for Human Event-Related Potential Research. NeuroImage 2021, 225, 117465. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Cui, Y.; Yang, G.; He, L.; Liu, Q.; Yin, G. How Different EEG References Influence Sensor Level Functional Connectivity Graphs. Front. Neurosci. 2017, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Qin, Y.; Hu, S.; Dong, L.; Bringas Vega, M.L.; Valdés Sosa, P.A. Which Reference Should We Use for EEG and ERP Practice? Brain Topogr. 2019, 32, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. Best Practices for Repeated Measures ANOVAs of ERP Data: Reference, Regional Channels, and Robust ANOVAs. Int. J. Psychophysiol. 2017, 111, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Bringas Vega, M.L.; Nunez, P.; Riera, J.; Zhang, R.; Valdes-Sosa, P.A. Editorial: Through a Glass, Darkly: The Influence of the EEG Reference on Inference About Brain Function and Disorders. Front. Neurosci. 2019, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, W.A.; Gibbs, F.A. A Balanced Non-Cephalic Reference Electrode. Electroencephalogr. Clin. Neurophysiol. 1951, 3, 237–240. [Google Scholar] [CrossRef]
- Petrusic, I.; Jovanovic, V.; Kovic, V.; Savic, A. Characteristics of N400 Component Elicited in Patients Who Have Migraine with Aura. J. Headache Pain. 2021, 22, 157. [Google Scholar] [CrossRef] [PubMed]
- Petrusic, I.; Jovanovic, V.; Kovic, V.; Savic, A.M. P3 Latency as a Biomarker for the Complexity of Migraine with Aura: Event-Related Potential Study. Cephalalgia 2022, 42, 1022–1030. [Google Scholar] [CrossRef]
- Jovanović, V.; Petrušić, I.; Savić, A.; Ković, V. Processing of Visual Hapaxes in Picture Naming Task: An Event-Related Potential Study. Int. J. Psychophysiol. 2024, 203, 112394. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kutas, M.; Iragui, V. The N400 in a Semantic Categorization Task across 6 Decades. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1998, 108, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An Open-Source, Graphical Experiment Builder for the Social Sciences. Behav. Res. 2012, 44, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Garrett, D.R.; Luck, S.J. Optimal Filters for ERP Research II: Recommended Settings for Seven Common ERP Components. Psychophysiology 2024, 61, e14530. [Google Scholar] [CrossRef] [PubMed]
- Yao, D. High-Resolution EEG Mappings: A Spherical Harmonic Spectra Theory and Simulation Results. Clin. Neurophysiol. 2000, 111, 81–92. [Google Scholar] [CrossRef]
- Dong, L.; Li, F.; Liu, Q.; Wen, X.; Lai, Y.; Xu, P.; Yao, D. MATLAB Toolboxes for Reference Electrode Standardization Technique (REST) of Scalp EEG. Front. Neurosci. 2017, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, Y.; Peter, V.; Sharma, M. Effect of EEG Referencing Methods on Auditory Mismatch Negativity. Front. Neurosci. 2017, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Stewart, A.X.; Simmons, A.M.; Rhemtulla, M. Standardized Measurement Error: A Universal Metric of Data Quality for Averaged Event-related Potentials. Psychophysiology 2021, 58, e13793. [Google Scholar] [CrossRef] [PubMed]

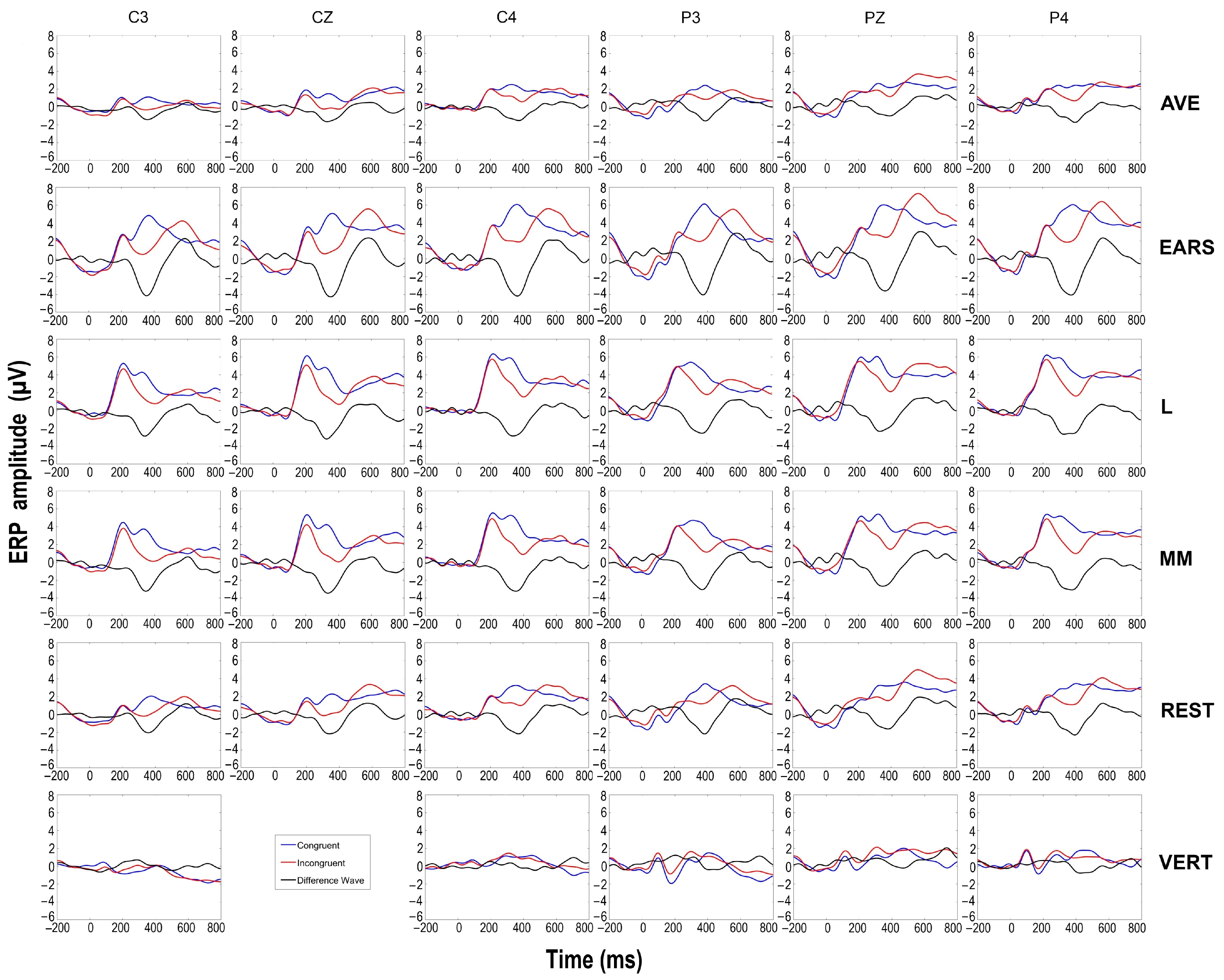
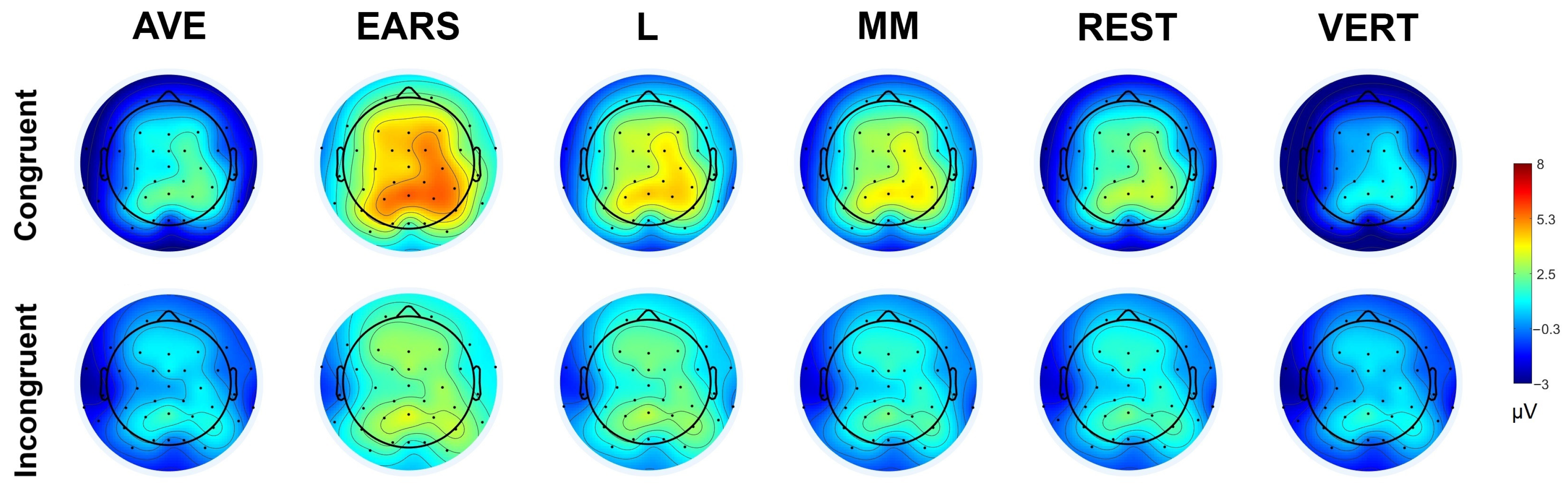
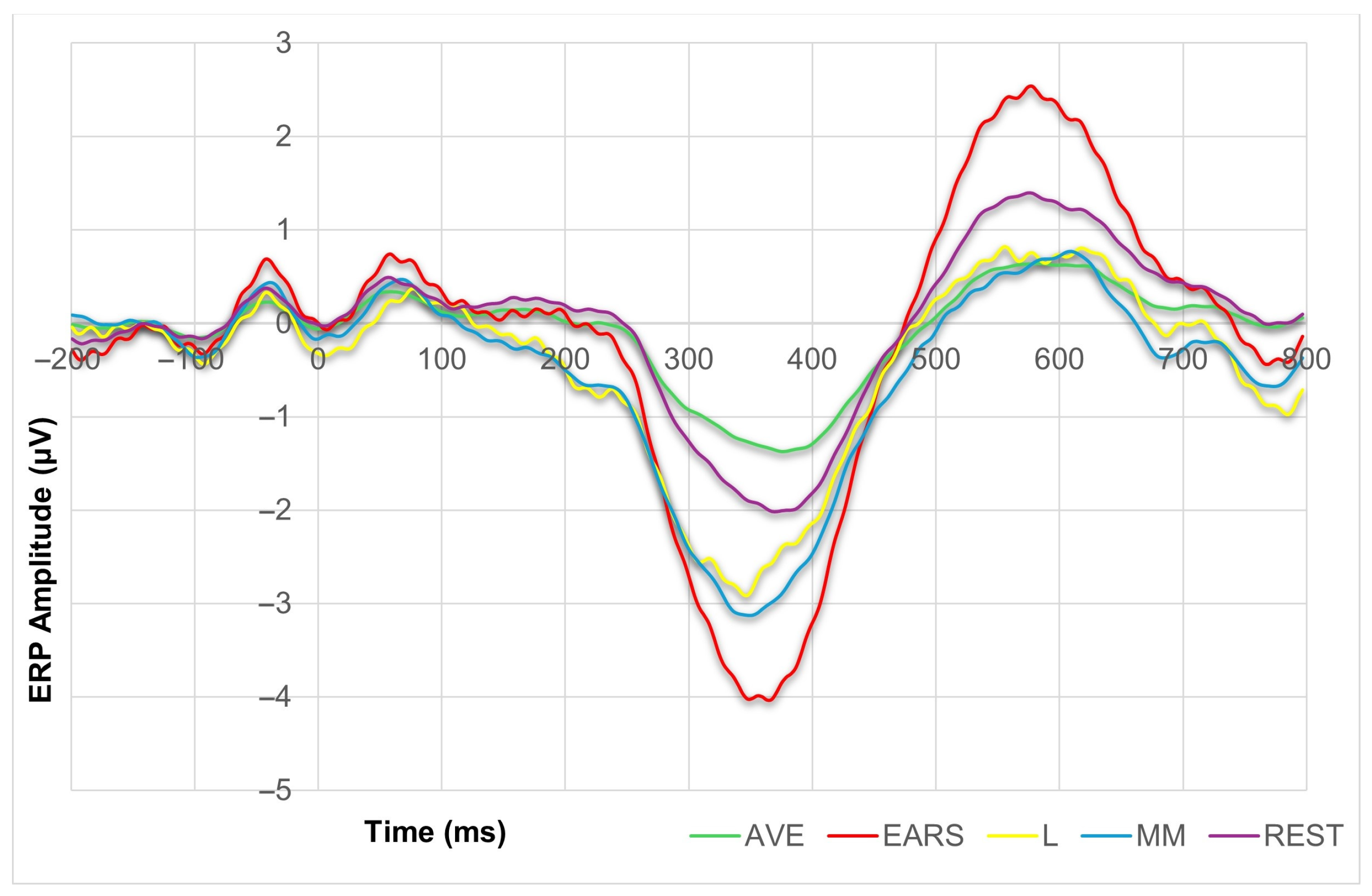

| Congruent | Incongruent | F(1, 16) | ηp2 | |||
|---|---|---|---|---|---|---|
| M | SE | M | SE | |||
| AVE | 1.77 | 0.23 | 0.89 | 0.17 | 19.40 *** | 0.548 |
| EARS | 4.97 | 0.65 | 2.68 | 0.43 | 14.42 ** | 0.474 |
| L | 3.82 | 0.67 | 2.13 | 0.46 | 7.52 ** | 0.320 |
| MM | 3.44 | 0.44 | 1.48 | 0.37 | 19.16 *** | 0.545 |
| REST | 2.62 | 0.36 | 1.42 | 0.21 | 14.38 ** | 0.473 |
| VERT | 0.80 | 0.43 | 0.72 | 0.27 | 0.03 | 0.002 |
| Reference | AVE | EARS | L | MM | REST |
|---|---|---|---|---|---|
| AVE | - | 0.981 | 0.945 | 0.959 | 0.999 |
| EARS | 0.981 | - | 0.986 | 0.995 | 0.988 |
| L | 0.945 | 0.986 | - | 0.994 | 0.956 |
| MM | 0.959 | 0.995 | 0.994 | - | 0.968 |
| REST | 0.999 | 0.988 | 0.956 | 0.968 | - |
| A | MM | EARS | L | AVE | REST | VERT | B | MM | EARS | L | AVE | REST | VERT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FP2 | 0.407 | 0.168 | 0.833 | 0.017 | 0.111 | 0.025 | FP2 | −0.852 | −1.444 | 0.215 | 2.662 | 1.688 | 2.471 |
| FP1 | 0.753 | 0.675 | 0.353 | 0.007 | 0.03 | 0.013 | FP1 | 0.32 | −0.427 | 0.957 | 3.061 | 2.382 | 2.78 |
| FT10 | 0.835 | 0.702 | 0.353 | 0.005 | 0.055 | 0.007 | FT10 | 0.212 | −0.389 | 0.957 | 3.272 | 2.07 | 3.113 |
| F8 | 0.444 | 0.308 | 0.896 | 0.213 | 0.651 | 0.012 | F8 | −0.785 | −1.054 | −0.133 | 1.298 | 0.461 | 2.821 |
| F7 | 0.648 | 0.35 | 0.915 | 0.16 | 0.392 | 0.046 | F7 | −0.465 | −0.962 | 0.108 | 1.475 | 0.879 | 2.163 |
| FT9 | 0.723 | 0.233 | 0.675 | 0.061 | 0.141 | 0.032 | FT9 | −0.361 | −1.238 | 0.428 | 2.014 | 1.549 | 2.349 |
| T8 | 0.038 | 0.038 | 0.142 | 0.796 | 0.373 | 0.269 | T8 | −2.257 | −2.26 | −1.543 | −0.263 | −0.917 | 1.145 |
| FC6 | 0.133 | 0.127 | 0.399 | 0.974 | 0.597 | 0.098 | FC6 | −1.585 | −1.608 | −0.867 | −0.033 | −0.539 | 1.756 |
| F4 | 0.048 | 0.039 | 0.138 | 0.424 | 0.215 | 0.525 | F4 | −2.143 | −2.254 | −1.56 | −0.821 | −1.29 | 0.649 |
| FZ | 0.004 | 0.006 | 0.058 | 0.357 | 0.085 | 0.267 | FZ | −3.34 | −3.179 | −2.045 | −0.948 | −1.838 | 1.149 |
| F3 | 0.033 | 0.02 | 0.184 | 0.782 | 0.289 | 0.262 | F3 | −2.328 | −2.582 | −1.388 | −0.282 | −1.096 | 1.163 |
| FC5 | 0.048 | 0.014 | 0.265 | 0.771 | 0.509 | 0.108 | FC5 | −2.143 | −2.77 | −1.154 | 0.296 | −0.676 | 1.702 |
| T7 | 0.042 | 0.033 | 0.139 | 0.354 | 0.196 | 0.755 | T7 | −2.214 | −2.337 | −1.557 | −0.955 | −1.349 | 0.317 |
| FC2 | 0.001 | 0.001 | 0.005 | 0.001 | 0.001 | 0.288 | FC2 | −4.132 | −4 | −3.255 | −3.834 | −3.908 | −1.1 |
| FCZ | 0.027 | 0.039 | 0.169 | 0.414 | 0.23 | 0.446 | FCZ | −2.428 | −2.249 | −1.44 | −0.84 | −1.248 | 0.781 |
| FC1 | 0.002 | 0.005 | 0.029 | 0.026 | 0.017 | 0.949 | FC1 | −3.622 | −3.244 | −2.406 | −2.455 | −2.652 | 0.065 |
| C4 | 0.002 | 0.002 | 0.006 | 0.03 | 0.01 | 0.856 | C4 | −3.613 | −3.636 | −3.191 | −2.389 | −2.904 | −0.184 |
| CZ | 0.013 | 0.014 | 0.088 | 0.086 | 0.054 | *** | CZ | −2.785 | −2.77 | −1.819 | −1.83 | −2.078 | *** |
| C3 | 0 | 0.001 | 0.011 | 0.001 | 0.001 | 0.981 | C3 | −4.784 | −4.066 | −2.874 | −4.274 | −3.888 | 0.025 |
| CP6 | 0.002 | 0.004 | 0.015 | 0.022 | 0.014 | 0.838 | CP6 | −3.702 | −3.318 | −2.731 | −2.545 | −2.743 | −0.208 |
| CP2 | 0 | 0 | 0.007 | 0 | 0 | 0.274 | CP2 | −4.896 | −4.462 | −3.098 | −4.627 | −4.473 | −1.133 |
| CP1 | 0 | 0.001 | 0.008 | 0 | 0.001 | 0.855 | CP1 | −4.801 | −4.128 | −3.043 | −4.474 | −4.087 | −0.185 |
| CP5 | 0.085 | 0.052 | 0.292 | 0.944 | 0.526 | 0.119 | CP5 | −1.835 | −2.098 | −1.089 | 0.071 | −0.648 | 1.646 |
| P4 | 0 | 0.002 | 0.01 | 0.005 | 0.005 | 0.611 | P4 | −4.366 | −3.642 | −2.913 | −3.242 | −3.255 | −0.519 |
| PZ | 0.009 | 0.009 | 0.078 | 0.112 | 0.049 | 0.541 | PZ | −2.952 | −2.972 | −1.885 | −1.68 | −2.132 | 0.625 |
| P3 | 0 | 0.001 | 0.006 | 0 | 0.001 | 0.938 | P3 | −5.333 | −4.098 | −3.16 | −4.367 | −4.026 | 0.08 |
| TP10 | *** | 0.966 | 0.346 | 0.007 | 0.052 | 0.003 | TP10 | *** | 0.044 | 0.971 | 3.087 | 2.099 | 3.552 |
| P8 | 0.004 | 0.021 | 0.109 | 0.917 | 0.392 | 0.201 | P8 | −3.316 | −2.548 | −1.696 | −0.106 | −0.879 | 1.334 |
| O2 | 0.009 | 0.011 | 0.154 | 0.726 | 0.293 | 0.247 | O2 | −2.99 | −2.894 | −1.498 | −0.356 | −1.087 | 1.202 |
| O1 | 0.025 | 0.059 | 0.231 | 0.908 | 0.499 | 0.197 | O1 | −2.468 | −2.032 | −1.244 | −0.117 | −0.692 | 1.345 |
| P7 | 0.026 | 0.02 | 0.085 | 0.749 | 0.289 | 0.274 | P7 | −2.449 | −2.581 | −1.836 | −0.325 | −1.096 | 1.132 |
| TP9 | *** | 0.106 | *** | 0.133 | 0.324 | 0.091 | TP9 | *** | −1.714 | *** | 1.585 | 1.017 | 1.798 |
| PO10 | 0.734 | 0.344 | 0.938 | 0.064 | 0.256 | 0.017 | PO10 | −0.346 | −0.976 | −0.079 | 1.986 | 1.179 | 2.661 |
| OZ | 0.382 | 0.226 | 0.824 | 0.064 | 0.19 | 0.018 | OZ | −0.899 | −1.258 | 0.227 | 1.994 | 1.368 | 2.629 |
| PO9 | 0.135 | 0.069 | 0.413 | 0.293 | 0.786 | 0.092 | PO9 | −1.572 | −1.951 | −0.841 | 1.088 | 0.276 | 1.795 |
| LM | 0.476 | *** | 0.127 | 0.02 | 0.012 | 0.016 | LM | 0.73 | *** | 1.609 | 2.595 | 2.848 | 2.684 |
| RM | 0.473 | *** | 0.125 | 0.007 | 0.003 | 0.014 | RM | 0.735 | *** | 1.618 | 3.065 | 3.423 | 2.752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, V.; Petrušić, I.; Ković, V.; Savić, A.M. The Practical Implications of Re-Referencing in ERP Studies: The Case of N400 in the Picture–Word Verification Task. Diagnostics 2025, 15, 156. https://doi.org/10.3390/diagnostics15020156
Jovanović V, Petrušić I, Ković V, Savić AM. The Practical Implications of Re-Referencing in ERP Studies: The Case of N400 in the Picture–Word Verification Task. Diagnostics. 2025; 15(2):156. https://doi.org/10.3390/diagnostics15020156
Chicago/Turabian StyleJovanović, Vojislav, Igor Petrušić, Vanja Ković, and Andrej M. Savić. 2025. "The Practical Implications of Re-Referencing in ERP Studies: The Case of N400 in the Picture–Word Verification Task" Diagnostics 15, no. 2: 156. https://doi.org/10.3390/diagnostics15020156
APA StyleJovanović, V., Petrušić, I., Ković, V., & Savić, A. M. (2025). The Practical Implications of Re-Referencing in ERP Studies: The Case of N400 in the Picture–Word Verification Task. Diagnostics, 15(2), 156. https://doi.org/10.3390/diagnostics15020156







