Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Sample Collection and Analysis
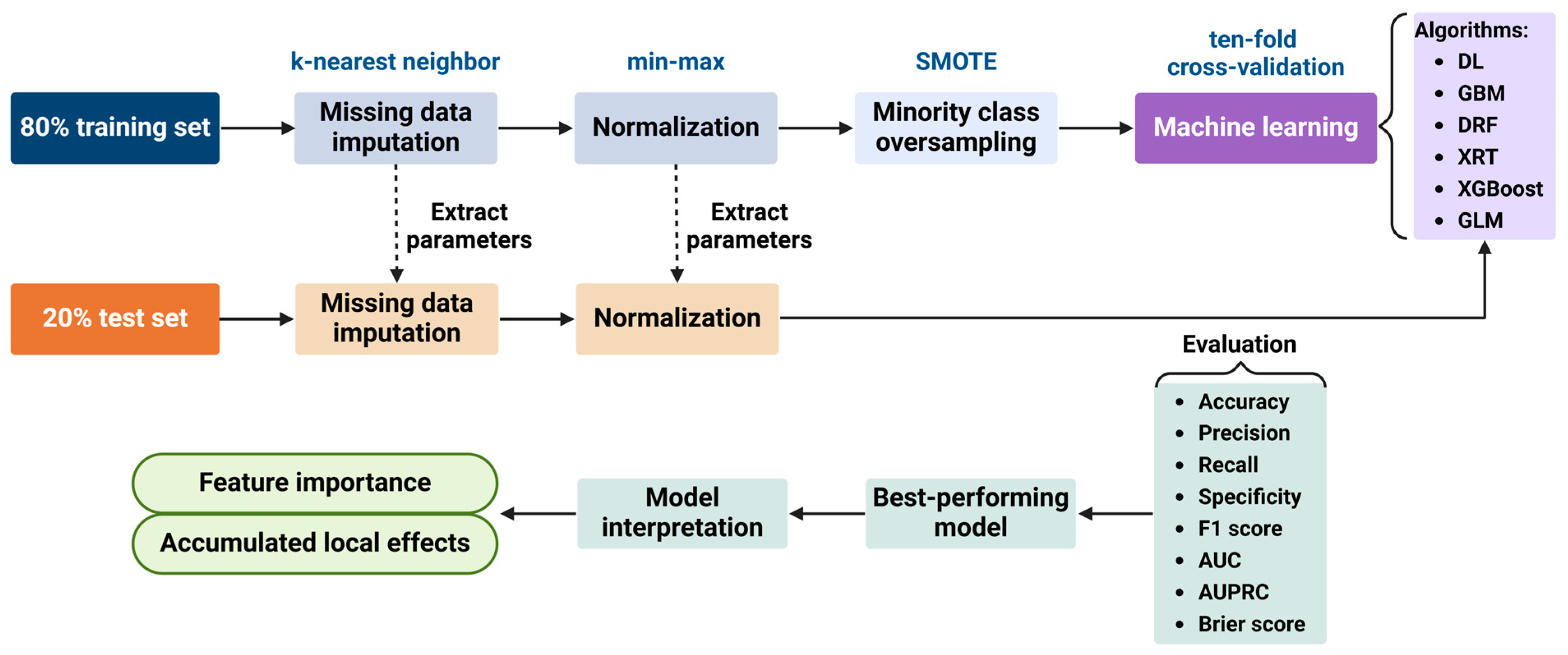
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
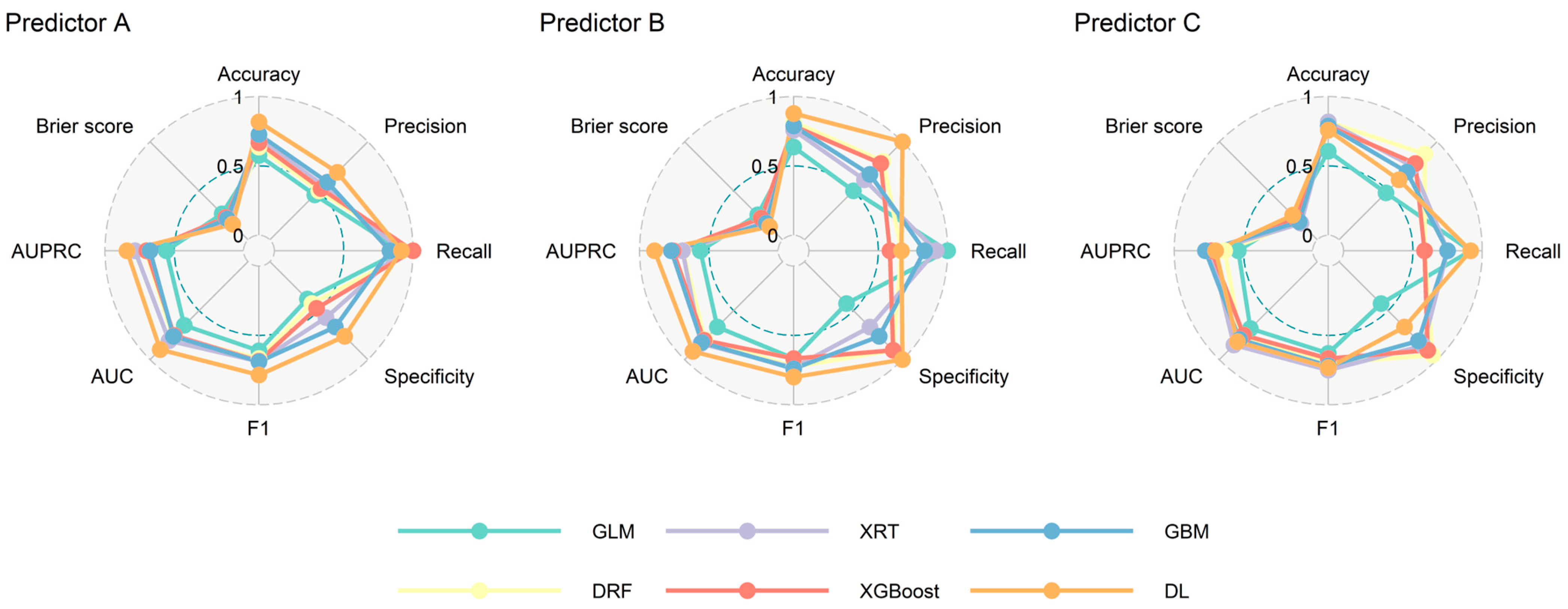
3.2. Model Performance
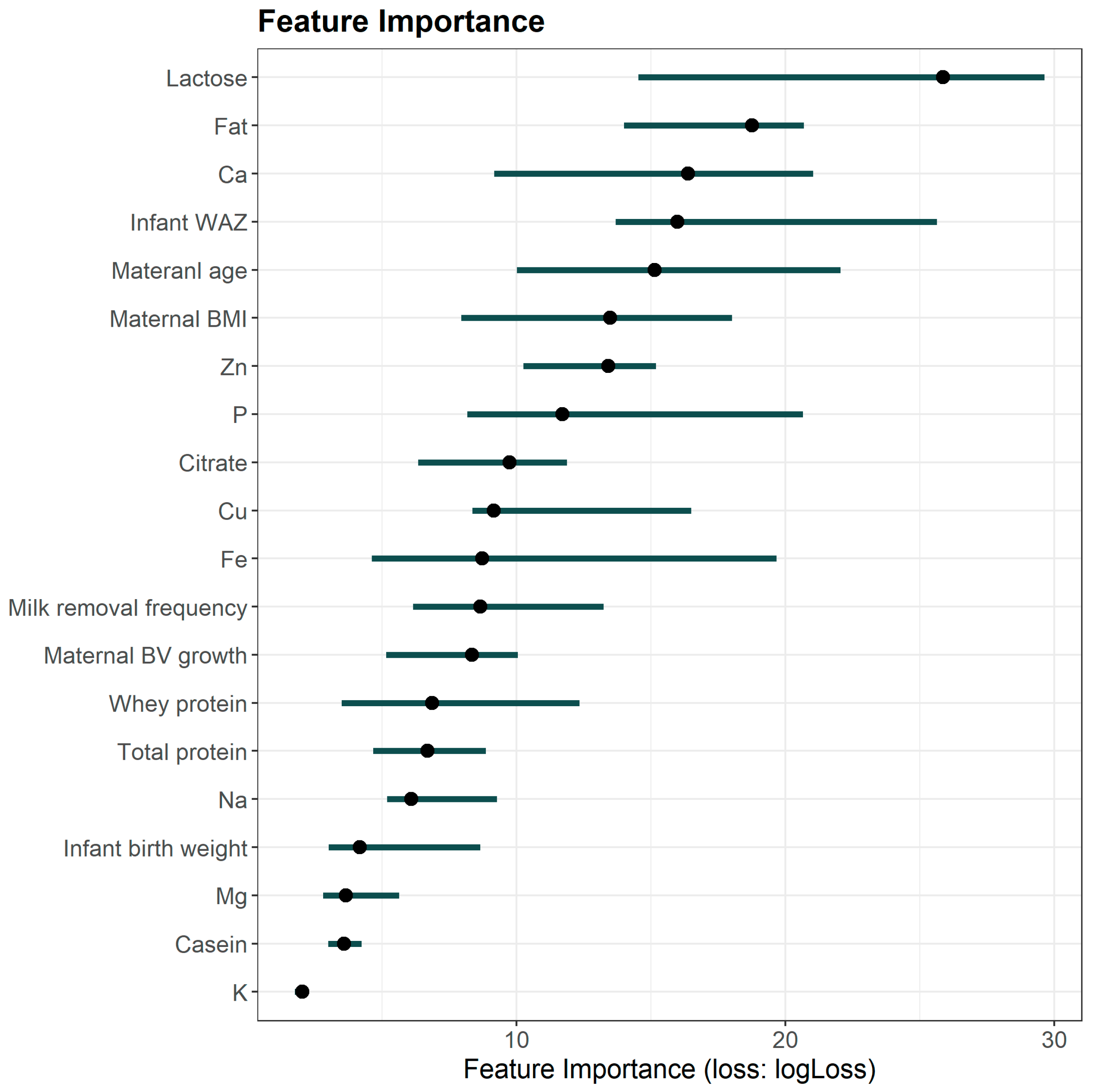
3.3. Prediction Variables Interpretation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Breastfeeding Recommendations. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_2 (accessed on 2 March 2023).
- Li, R.; Fein, S.B.; Chen, J.; Grummer-Strawn, L.M. Why mothers stop breastfeeding: Mothers’ self-reported reasons for stopping during the first year. Pediatrics 2008, 122 (Suppl. 2), S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.L.; Dodds, L.; Legge, A.; Bryanton, J.; Semenic, S. Factors influencing the reasons why mothers stop breastfeeding. Can. J. Public Health 2014, 105, e179–e185. [Google Scholar] [CrossRef]
- Morrison, A.H.; Gentry, R.; Anderson, J. Mothers’ Reasons for Early Breastfeeding Cessation. MCN Am. J. Matern. Child. Nurs. 2019, 44, 325–330. [Google Scholar] [CrossRef]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef]
- Marasco, L.A. Unsolved Mysteries of the Human Mammary Gland: Defining and Redefining the Critical Questions from the Lactation Consultant’s Perspective. J. Mammary Gland. Biol. Neoplasia 2014, 19, 271–288. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. 2010 Australian National Infant Feeding Survey: Indicator Results; Australian Institute of Health and Welfare: Darlinghurst, Australia, 2012. [Google Scholar]
- Netting, M.J.; Moumin, N.A.; Knight, E.J.; Golley, R.K.; Makrides, M.; Green, T.J. The Australian Feeding Infants and Toddler Study (OzFITS 2021): Breastfeeding and Early Feeding Practices. Nutrients 2022, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.-J.; Hartmann, P.E. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.; Algert, C.S.; Ampt, A.; Sywak, M.S.; Roberts, C.L. The impact of cosmetic breast implants on breastfeeding: A systematic review and meta-analysis. Int. Breastfeed. J. 2014, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Garbin, C.P.; Deacon, J.P.; Rowan, M.K.; Hartmann, P.E.; Geddes, D.T. Association of nipple piercing with abnormal milk production and breastfeeding. JAMA 2009, 301, 2550–2551. [Google Scholar] [CrossRef]
- Rivera, O.C.; Geddes, D.T.; Barber-Zucker, S.; Zarivach, R.; Gagnon, A.; Soybel, D.I.; Kelleher, S.L. A common genetic variant in zinc transporter ZnT2 (Thr288Ser) is present in women with low milk volume and alters lysosome function and cell energetics. Am. J. Physiol. Cell Physiol. 2020, 318, C1166–C1177. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; Gagnon, A.; Rivera, O.C.; Hicks, S.D.; Carney, M.C.; Alam, S. Milk-derived miRNA profiles elucidate molecular pathways that underlie breast dysfunction in women with common genetic variants in SLC30A2. Sci. Rep. 2019, 9, 12686. [Google Scholar] [CrossRef]
- Ito, O.; Kawazoe, T.; Suzuki, S.; Muneuchi, G.; Saso, Y.; Hamamoto, Y.; Imai, T.; Hata, Y. Mammary hypoplasia resulting from hormone receptor deficiency. Plast. Reconstr. Surg. 2004, 113, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Kam, R.L.; Amir, L.H.; Cullinane, M. Is There an Association Between Breast Hypoplasia and Breastfeeding Outcomes? A Systematic Review. Breastfeed. Med. 2021, 16, 594–602. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.; Robertson, S.; Friedman, A.; Klaus, M. Effect of frequent breast-feeding on early milk production and infant weight gain. Pediatrics 1983, 72, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Perrella, S.L.; Lai, C.T.; Taylor, N.L.; Geddes, D.T. Causes of Low Milk Supply: The Roles of Estrogens, Progesterone, and Related External Factors. Adv. Nutr. 2024, 15, 100129. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. The Impact of Maternal Obesity on Human Milk Macronutrient Composition: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Britten, F.L.; Lai, C.T.; Geddes, D.T.; Callaway, L.K.; Duncan, E.L. Is Secretory Activation Delayed in Women with Type Two Diabetes? A Pilot Study. Nutrients 2022, 14, 1323. [Google Scholar] [CrossRef]
- Azulay Chertok, I.R.; Haile, Z.T.; Shuisong, N.; Kennedy, M. Differences in Human Milk Lactose and Citrate Concentrations Based on Gestational Diabetes Status. Breastfeed. Med. 2020, 15, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hennigar, S.R.; Alam, S.; Nishida, K.; Kelleher, S.L. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015, 290, 13064–13078. [Google Scholar] [CrossRef] [PubMed]
- Medina Poeliniz, C.; Engstrom, J.L.; Hoban, R.; Patel, A.L.; Meier, P. Measures of Secretory Activation for Research and Practice: An Integrative Review. Breastfeed. Med. 2020, 15, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.; Gardner, H.; Hartmann, P. Normal Human Lactation: Closing the gap. F1000Research 2018, 7, 801. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Hartmann, P.E. Initiation of human lactation: Secretory differentiation and secretory activation. J. Mammary Gland. Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Esquerra-Zwiers, A.L.; Mulder, C.; Czmer, L.; Perecki, A.; Goris, E.D.; Lai, C.T.; Geddes, D. Associations of Secretory Activation Breast Milk Biomarkers with Breastfeeding Outcome Measures. J. Pediatr. 2023, 253, 259–265.e2. [Google Scholar] [CrossRef]
- Murase, M.; Wagner, E.A.; Chantry, C.J.; Dewey, K.G.; Nommsen-Rivers, L.A. The Relation between Breast Milk Sodium to Potassium Ratio and Maternal Report of a Milk Supply Concern. J. Pediatr. 2017, 181, 294–297.e3. [Google Scholar] [CrossRef]
- Hoban, R.; Medina Poeliniz, C.; Somerset, E.; Tat Lai, C.; Janes, J.; Patel, A.L.; Geddes, D.; Meier, P.P. Mother’s Own Milk Biomarkers Predict Coming to Volume in Pump-Dependent Mothers of Preterm Infants. J. Pediatr. 2021, 228, 44–52.e3. [Google Scholar] [CrossRef] [PubMed]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef] [PubMed]
- Molnar, C. Interpretable Machine Learning: A Guide for Making Black Box Models Explainable; Lulu Press: Morrisville, NC, USA, 2020. [Google Scholar]
- Oliver-Roig, A.; Rico-Juan, J.R.; Richart-Martínez, M.; Cabrero-García, J. Predicting exclusive breastfeeding in maternity wards using machine learning techniques. Comput. Methods Programs Biomed. 2022, 221, 106837. [Google Scholar] [CrossRef]
- Oyebode, O.; Lomotey, R.; Orji, R. “I Tried to Breastfeed but…”: Exploring Factors Influencing Breastfeeding Behaviours Based on Tweets Using Machine Learning and Thematic Analysis. IEEE Access 2021, 9, 61074–61089. [Google Scholar] [CrossRef]
- Açikgöz, A.; Çakirli, M.; Şahin, B.M.; Çelik, Ö. Predicting mothers’ exclusive breastfeeding for the first 6 months: Interface creation study using machine learning technique. J. Eval. Clin. Pract. 2024, 30, 1000–1007. [Google Scholar] [CrossRef]
- Walle, A.D.; Abebe Gebreegziabher, Z.; Ngusie, H.S.; Kassie, S.Y.; Lambebo, A.; Zekarias, F.; Dejene, T.M.; Kebede, S.D. Prediction of delayed breastfeeding initiation among mothers having children less than 2 months of age in East Africa: Application of machine learning algorithms. Front. Public Health 2024, 12, 1413090. [Google Scholar] [CrossRef]
- Kent, J.C.; Hepworth, A.R.; Sherriff, J.L.; Cox, D.B.; Mitoulas, L.R.; Hartmann, P.E. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Lutes, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef]
- Jin, X.; Lai, C.T.; Perrella, S.L.; McEachran, J.L.; Gridneva, Z.; Geddes, D.T. Maternal Breast Growth and Body Mass Index Are Associated with Low Milk Production in Women. Nutrients 2024, 16, 2854. [Google Scholar] [CrossRef]
- World Health Organization. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 1 June 2024).
- Meier, P.P.; Engstrom, J.L.; Zuleger, J.L.; Motykowski, J.E.; Vasan, U.; Meier, W.A.; Hartmann, P.E.; Williams, T.M. Accuracy of a user-friendly centrifuge for measuring creamatocrits on mothers’ milk in the clinical setting. Breastfeed. Med. 2006, 1, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Atwood, C.S.; Hartmann, P.E. Collection of fore and hind milk from the sow and the changes in milk composition during suckling. J. Dairy Res. 1992, 59, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Lönnerdal, B. Human milk proteins: Separation of whey proteins and their analysis by polyacrylamide gel electrophoresis, fast protein liquid chromatography (FPLC) gel filtration, and anion-exchange chromatography. Am. J. Clin. Nutr. 1989, 49, 464–470. [Google Scholar] [CrossRef]
- Khan, S.; Casadio, Y.S.; Lai, C.T.; Prime, D.K.; Hepworth, A.R.; Trengove, N.J.; Hartmann, P.E. Investigation of short-term variations in casein and whey proteins in breast milk of term mothers. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 136–141. [Google Scholar] [CrossRef]
- Moellering, H.; Gruber, W. Determination of citrate with citrate lyase. Anal. Biochem. 1966, 17, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.T.; Gardner, H.; Geddes, D. Comparison of Inductively Coupled Plasma Optical Emission Spectrometry with an Ion Selective Electrode to Determine Sodium and Potassium Levels in Human Milk. Nutrients 2018, 10, 1218. [Google Scholar] [CrossRef]
- Esquerra-Zwiers, A.; Vroom, A.; Geddes, D.; Lai, C.T. Use of a portable point-of-care instrumentation to measure human milk sodium and potassium concentrations. Breastfeed. Med. 2022, 17, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.W.; Kim, J.H. Human milk macronutrient analysis using point-of-care near-infrared spectrophotometry. J. Perinatol. 2011, 31, 339–343. [Google Scholar] [CrossRef]
- Costa, A.; Lopez-Villalobos, N.; Sneddon, N.W.; Shalloo, L.; Franzoi, M.; De Marchi, M.; Penasa, M. Invited review: Milk lactose—Current status and future challenges in dairy cattle. J. Dairy Sci. 2019, 102, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lonnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Hoban, R.; Patel, A.L.; Medina Poeliniz, C.; Lai, C.T.; Janes, J.; Geddes, D.; Meier, P.P. Human Milk Biomarkers of Secretory Activation in Breast Pump-Dependent Mothers of Premature Infants. Breastfeed. Med. 2018, 13, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F. Lactose: Chemistry and Properties. In Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents; Springer: New York, NY, USA, 2009; pp. 1–15. [Google Scholar] [CrossRef]
- Sadovnikova, A.; Garcia, S.C.; Hovey, R.C. A Comparative Review of the Extrinsic and Intrinsic Factors Regulating Lactose Synthesis. J. Mammary Gland Biol. Neoplasia 2021, 26, 197–215. [Google Scholar] [CrossRef]
- Zhao, F.-Q. Biology of Glucose Transport in the Mammary Gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Collier, R.J.; McNamara, J.P.; Head, H.H.; Croom, W.J.; Wilcox, C.J. Effects of thyroxine and growth hormone treatment of dairy cows on mammary uptake of glucose, oxygen and other milk fat precursors. J. Anim. Sci. 1988, 66, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, A. Changes in plasma and milk concentrations of glucose and IGF-1 in response to exogenous growth hormone in lactating goats. J. Dairy Res. 1999, 66, 207–214. [Google Scholar] [CrossRef]
- Hannan, F.M.; Elajnaf, T.; Vandenberg, L.N.; Kennedy, S.H.; Thakker, R.V. Hormonal regulation of mammary gland development and lactation. Nat. Rev. Endocrinol. 2023, 19, 46–61. [Google Scholar] [CrossRef]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2017, 69 (Suppl. 2), 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; LaPensee, C.R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.E.; Harvatine, K.J.; Ross, A.C.; Wagner, E.A.; Riddle, S.W.; Gernand, A.D.; Nommsen-Rivers, L.A. Fatty Acid Transfer from Blood to Milk Is Disrupted in Mothers with Low Milk Production, Obesity, and Inflammation. J. Nutr. 2023, 152, 2716–2726. [Google Scholar] [CrossRef]
- Hang, J.; Rillema, J.A. Prolactin’s effects on lipoprotein lipase (LPL) activity and on LPL mRNA levels in cultured mouse mammary gland explants. Proc. Soc. Exp. Biol. Med. 1997, 214, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Field, B.; Coore, H.G. Control of rat mammary-gland pyruvate dehydrogenase by insulin and prolactin. Biochem. J. 1976, 156, 333. [Google Scholar] [CrossRef]
- Flint, D.J.; Clegg, R.A.; Vernon, R.G. Prolactin and the regulation of adipose-tissue metabolism during lactation in rats. Mol. Cell. Endocrinol. 1981, 22, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004, 113, e465–e471. [Google Scholar] [CrossRef]
- Weitzman, J.K.-P.D.N. Krebs citric acid cycle: Half a century and still turning. In Proceedings of the Biochemical Society Symposium, Oxford, UK, 13–15 April 1987; pp. 1–198. [Google Scholar]
- Faulkner, A.; Peaker, M. Reviews of the progress of Dairy Science: Secretion of citrate into milk. J. Dairy Res. 1982, 49, 159–169. [Google Scholar] [CrossRef]
- Kent, J.C.; Arthur, P.G.; Retallack, R.W.; Hartmann, P.E. Calcium, phosphate and citrate in human milk at initiation of lactation. J. Dairy Res. 1992, 59, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Garnsworthy, P.C.; Masson, L.L.; Lock, A.L.; Mottram, T.T. Variation of Milk Citrate with Stage of Lactation and De Novo Fatty Acid Synthesis in Dairy Cows. J. Dairy Sci. 2006, 89, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Morriss, F.H., Jr.; Brewer, E.D.; Spedale, S.B.; Riddle, L.; Temple, D.M.; Caprioli, R.M.; West, M.S. Relationship of human milk pH during course of lactation to concentrations of citrate and fatty acids. Pediatrics 1986, 78, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Uniacke-Lowe, T.; Ryan, A.C.; Kelly, A.L. The composition and physico-chemical properties of human milk: A review. Trends Food Sci. Technol. 2021, 112, 608–621. [Google Scholar] [CrossRef]
- McCormick, N.H.; Hennigar, S.R.; Kiselyov, K.; Kelleher, S.L. The biology of zinc transport in mammary epithelial cells: Implications for mammary gland development, lactation, and involution. J. Mammary Gland Biol. Neoplasia 2014, 19, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.O. Trace element transport in the mammary gland. Annu. Rev. Nutr. 2007, 27, 165–177. [Google Scholar] [CrossRef]
- Turcksin, R.; Bel, S.; Galjaard, S.; Devlieger, R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: A systematic review. Matern. Child Nutr. 2014, 10, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Glaria, A.A.; Davie, P.; Beake, S.; Bick, D. Breastfeeding experiences and support for women who are overweight or obese: A mixed-methods systematic review. Matern. Child Nutr. 2020, 16, e12865. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A. Does Insulin Explain the Relation between Maternal Obesity and Poor Lactation Outcomes? An Overview of the Literature. Adv. Nutr. 2016, 7, 407–414. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Dena-Beltrán, J.L.; Ruiz-Herrera, X.; Ocampo-Ruiz, A.L.; Martínez de la Escalera, G.; Clapp, C.; Macotela, Y. Obesity-derived alterations in the lactating mammary gland: Focus on prolactin. Mol. Cell. Endocrinol. 2023, 559, 111810. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.P.; Lefevre, C.; Wong, C.S.; Nicholas, K.R.; Sharp, J.A. Insulin regulates human mammosphere development and function. Cell Tissue Res. 2021, 384, 333–352. [Google Scholar] [CrossRef]
- Neville, M.C.; Webb, P.; Ramanathan, P.; Mannino, M.P.; Pecorini, C.; Monks, J.; Anderson, S.M.; MacLean, P. The insulin receptor plays an important role in secretory differentiation in the mammary gland. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1103–E1114. [Google Scholar] [CrossRef]
- Buonfiglio, D.C.; Ramos-Lobo, A.M.; Freitas, V.M.; Zampieri, T.T.; Nagaishi, V.S.; Magalhães, M.; Cipolla-Neto, J.; Cella, N.; Donato, J., Jr. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 2016, 6, 22421. [Google Scholar] [CrossRef]
- de Los Ríos, E.A.; Ruiz-Herrera, X.; Tinoco-Pantoja, V.; López-Barrera, F.; Martínez de la Escalera, G.; Clapp, C.; Macotela, Y. Impaired prolactin actions mediate altered offspring metabolism induced by maternal high-fat feeding during lactation. FASEB J. 2018, 32, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Kitano, N.; Nomura, K.; Kido, M.; Murakami, K.; Ohkubo, T.; Ueno, M.; Sugimoto, M. Combined effects of maternal age and parity on successful initiation of exclusive breastfeeding. Prev. Med. Rep. 2016, 3, 121–126. [Google Scholar] [CrossRef]
- Colombo, L.; Crippa, B.L.; Consonni, D.; Bettinelli, M.E.; Agosti, V.; Mangino, G.; Bezze, E.N.; Mauri, P.A.; Zanotta, L.; Roggero, P.; et al. Breastfeeding Determinants in Healthy Term Newborns. Nutrients 2018, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Wang, W.; Wang, F.; Jiang, H.; Wang, L. Factors associated with exclusive breastfeeding during postpartum in Lanzhou city, China: A cross-sectional study. Front. Public Health 2023, 11, 1089764. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Gallagher, D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 2010, 26, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Breda, E.; Oberg, A.L.; Powell, C.C.; Dalla Man, C.; Basu, A.; Vittone, J.L.; Klee, G.G.; Arora, P.; Jensen, M.D.; et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003, 52, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired lactation: Review of delayed lactogenesis and insufficient lactation. J. Midwifery Women’s Health 2021, 66, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Henly, S.J.; Anderson, C.M.; Avery, M.D.; Hills-Bonuyk, S.G.; Potter, S.; Duckett, L.J. Anemia and insufficient milk in first-time mothers. Birth 1995, 22, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.T.; Jin, X.; Zhou, X.; Stinson, L.F.; Wlodek, M.E.; Perrella, S.L.; Gridneva, Z.; Geddes, D.T. Biomarkers of Low Milk Supply. Proceedings 2023, 93, 5. [Google Scholar] [CrossRef]


| Characteristics | NMS | LMS | p-Value |
|---|---|---|---|
| Maternal and infant factors | |||
| MP (mL/24 h) | 846 ± 208 (602–1682, 106) | 416 ± 140 (81–597, 58) | <0.001 |
| Infant age at MP measurement (months) | 2.9 ± 1.1 (1.0–5.3, 106) | 2.8 ± 1.2 (1.1–5.1, 58) | 0.429 |
| Infant age at sample collection (months) | 3.1 ± 1.2 (1.0–5.4, 106) | 2.9 ± 1.3 (1.0–5.3, 58) | 0.466 |
| Maternal age (years) | 33.4 ± 4.5 (22.7–46.2, 106) | 35.2 ± 4.4 (25.0–49.0, 58) | 0.014 |
| Maternal BMI (kg/m2) | 28.2 ± 6.0 (19.5–64.5, 101) | 28.9 ± 5.2 (19.4–42.9, 54) | 0.223 |
| Maternal BV growth during pregnancy (cm3) | 191 ± 146 (0–730, 78) | 170 ± 169 (0–700, 47) | 0.161 |
| Infant birth weight (g) | 3446 ± 445 (2310–5040, 106) | 3354 ± 475 (2270–5045, 58) | 0.084 |
| Infant WAZ | −0.3 ± 0.9 (−2.9–1.8, 100) | −0.7 ± 0.8 (−2.6–1.0, 54) | 0.004 |
| Infant sex: male | 52 (49.1%) | 28 (49.1%) | 1.000 |
| Parity: primiparous | 65 (61.3%) | 36 (62.1%) | 1.000 |
| Delivery mode: vaginal | 58 (55.2%) | 31 (54.4%) | 1.000 |
| Milk removal frequency (times/24 h) | 13.4 ± 4.3 (4–28, 106) | 12.4 ± 4.5 (5–24, 58) | 0.115 |
| Exclusive breastfeeding | 96 (90.6%) | 24 (41.4%) | <0.001 |
| Milk composition | |||
| Fat (g/L) | 51.2 ± 17.6 (22.6–110.2, 106) | 44.3 ± 15.8 (20.2–83.6, 58) | 0.020 |
| Lactose (g/L) | 71.6 ± 24.3 (33.8–206.4, 106) | 81.5 ± 15.8 (50.0–140.0, 58) | <0.001 |
| Total protein (g/L) | 13.1 ± 4.3 (3.1–26.6, 106) | 11.7 ± 4.1 (4.4–23.6, 58) | 0.055 |
| Casein (g/L) | 1.1 ± 0.5 (0.2–2.6, 106) | 1.0 ± 0.6 (0.1–2.6, 58) | 0.234 |
| Whey protein (g/L) | 8.6 ± 2.9 (2.6–22.9, 106) | 8.0 ± 3.0 (1.1–20.1, 58) | 0.160 |
| Citrate (mg/L) | 524.9 ± 271.7 (128.7–1227.5, 106) | 363.6 ± 170.8 (121.0–781.8, 58) | <0.001 |
| Ca (mg/L) | 254.6 ± 60.7 (127.6–418.5, 106) | 252.4 ± 50.3 (123.1–338.8, 58) | 0.802 |
| Cu (mg/L) | 0.3 ± 0.3 (0.0–2.1, 106) | 0.4 ± 0.3 (0.0–1.9, 58) | 0.173 |
| Fe (mg/L) | 0.5 ± 0.4 (0.1–1.7, 106) | 0.7 ± 0.9 (0.0–6.6, 58) | 0.625 |
| K (mg/L) | 574.9 ± 131.0 (237.0–882.3, 106) | 575.6 ± 127.5 (281.1–853.7, 58) | 0.654 |
| Mg (mg/L) | 30.9 ± 6.0 (17.3–46.4, 106) | 30.7 ± 6.4 (11.8–40.6, 58) | 0.893 |
| Na (mg/L) | 126.4 ± 66.6 (23.1–413.6, 106) | 143.9 ± 88.1 (29.7–663.2, 58) | 0.063 |
| P (mg/L) | 139.1 ± 31.1 (68.2–225.7, 106) | 132.8 ± 28.0 (55.5–207.3, 58) | 0.192 |
| Zn (mg/L) | 1.9 ± 1.5 (0.3–6.3, 106) | 2.1 ± 1.3 (0.3–5.1, 58) | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Lai, C.T.; Perrella, S.L.; Zhou, X.; Hassan, G.M.; McEachran, J.L.; Gridneva, Z.; Taylor, N.L.; Wlodek, M.E.; Geddes, D.T. Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches. Diagnostics 2025, 15, 191. https://doi.org/10.3390/diagnostics15020191
Jin X, Lai CT, Perrella SL, Zhou X, Hassan GM, McEachran JL, Gridneva Z, Taylor NL, Wlodek ME, Geddes DT. Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches. Diagnostics. 2025; 15(2):191. https://doi.org/10.3390/diagnostics15020191
Chicago/Turabian StyleJin, Xuehua, Ching Tat Lai, Sharon L. Perrella, Xiaojie Zhou, Ghulam Mubashar Hassan, Jacki L. McEachran, Zoya Gridneva, Nicolas L. Taylor, Mary E. Wlodek, and Donna T. Geddes. 2025. "Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches" Diagnostics 15, no. 2: 191. https://doi.org/10.3390/diagnostics15020191
APA StyleJin, X., Lai, C. T., Perrella, S. L., Zhou, X., Hassan, G. M., McEachran, J. L., Gridneva, Z., Taylor, N. L., Wlodek, M. E., & Geddes, D. T. (2025). Milk Composition Is Predictive of Low Milk Supply Using Machine Learning Approaches. Diagnostics, 15(2), 191. https://doi.org/10.3390/diagnostics15020191





