Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
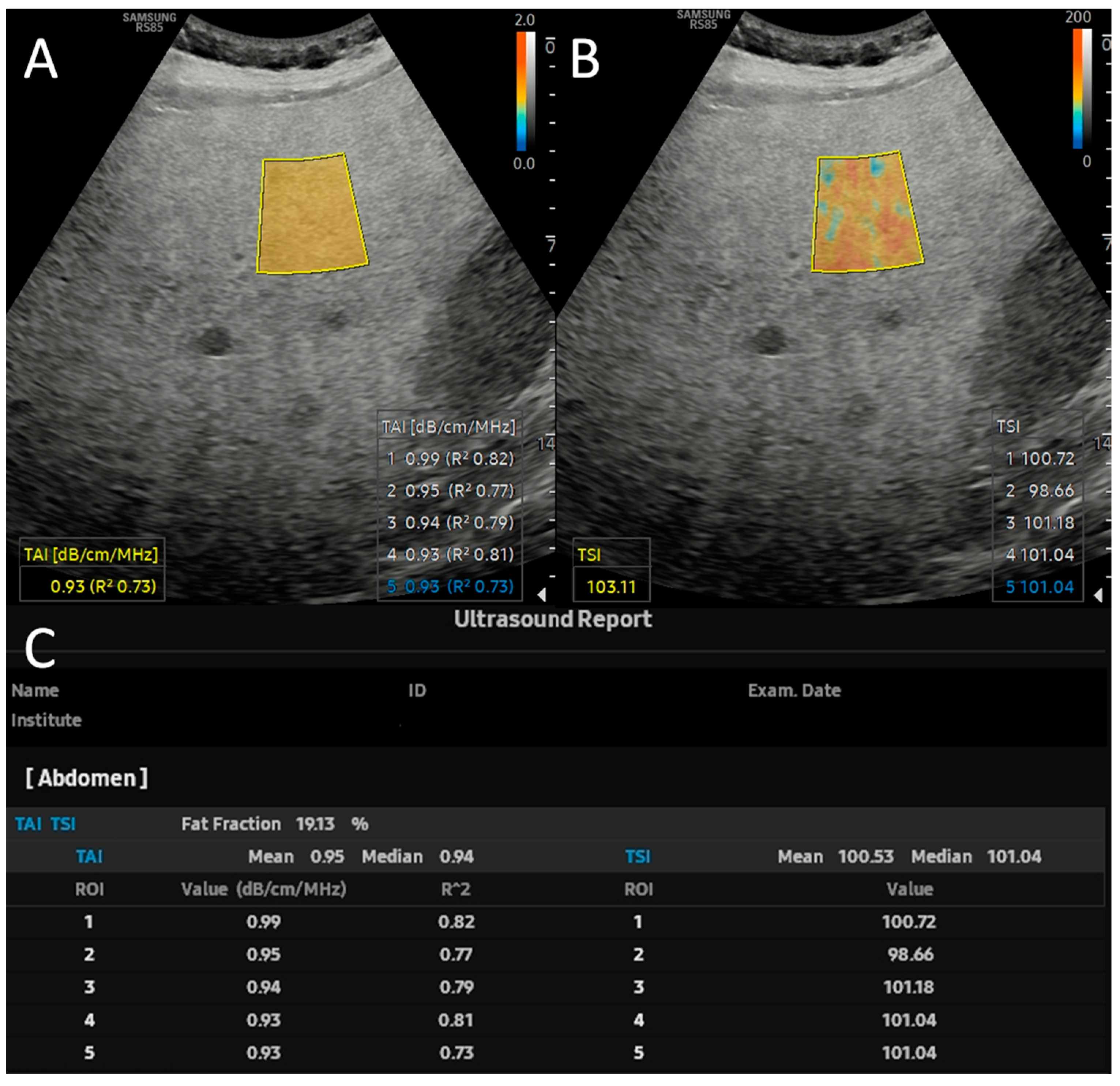
2.2. Quantitative Ultrasound and USFF Measurements
2.3. Chemical-Shift-Encoded Quantitative MRI
2.4. Training and Validation of QUS-Based Prediction Models
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of the Study Cohort
3.2. Variable Selection and Training of Models
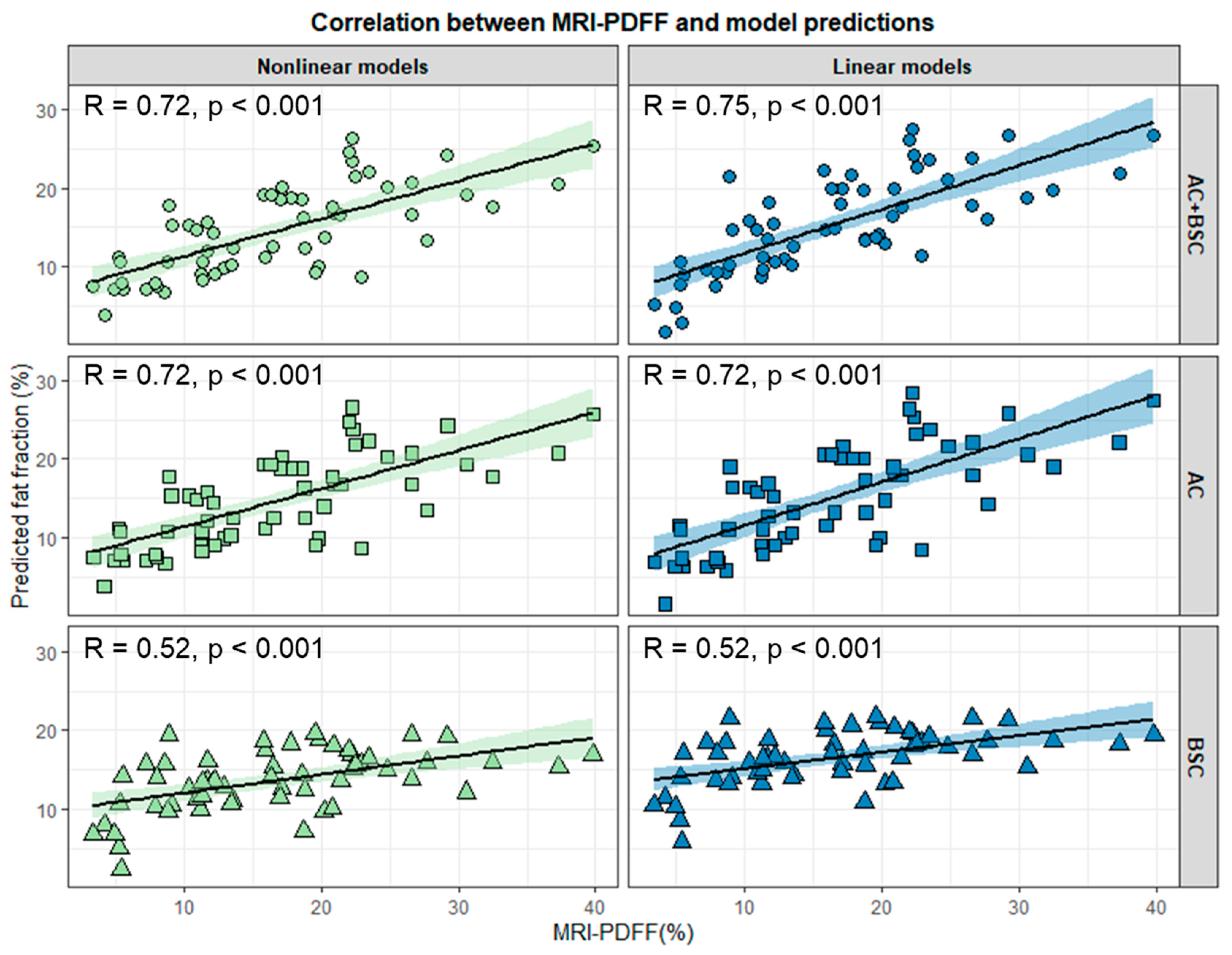
3.3. Evaluation of Models’ Performance in the Test Set
3.4. Grid Search of Ultrasound Parameters
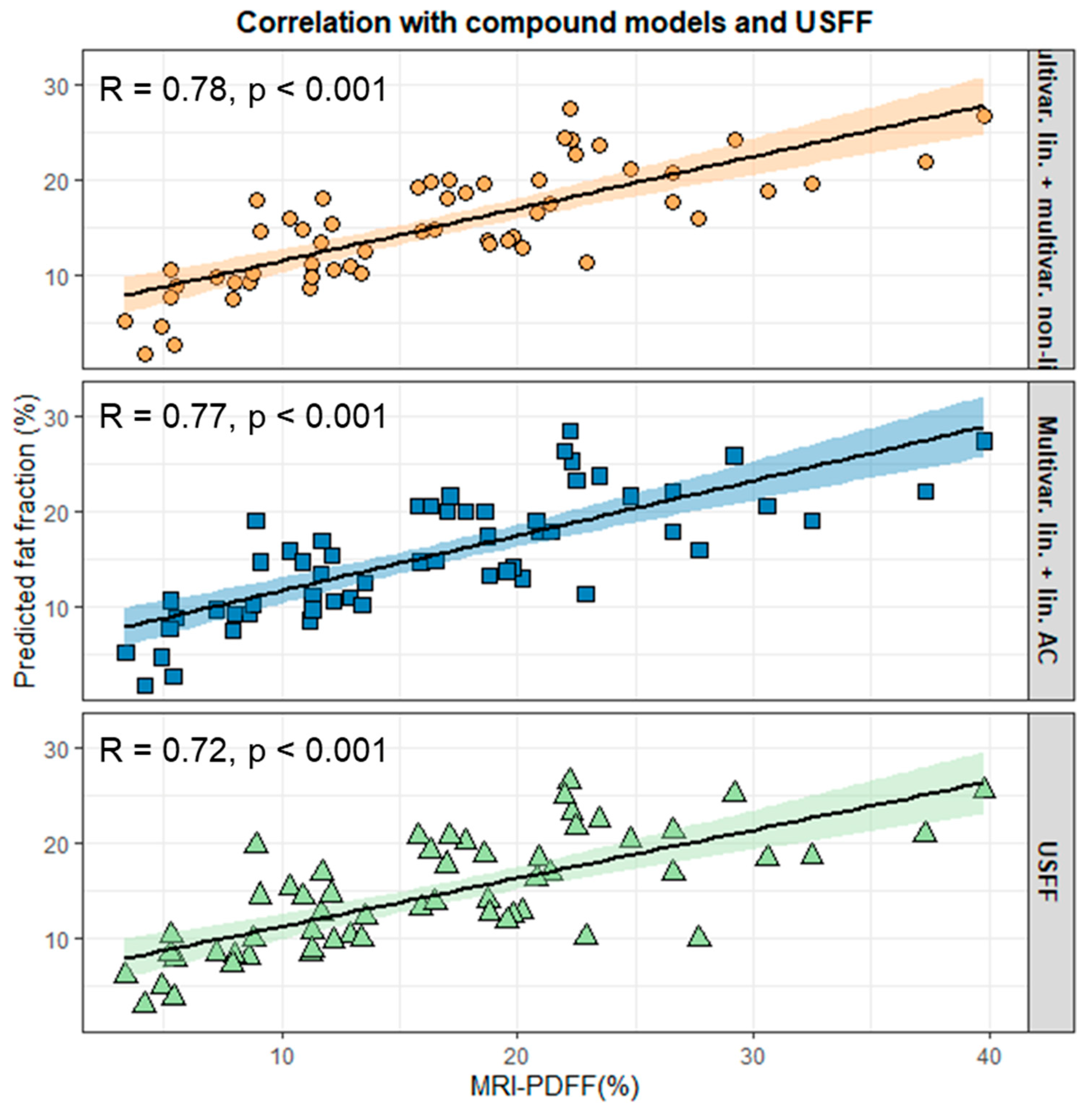
3.5. Construction and Validation of Compound Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-SWE | Two-dimensional shear wave elastography |
| AC | Attenuation coefficient |
| ATI | Attenuation imaging |
| BMI | Body mass index |
| BSC | Backscatter coefficient |
| BSC-D | Backscatter-distribution coefficient |
| CAC | Compound nonlinear AC model |
| CAP | Controlled attenuation parameter |
| CMT | Compound nonlinear multivariable model |
| CSE-MRI | Chemical-shift-encoded magnetic resonance imaging |
| ICC | Intraclass correlation coefficient |
| IQR/M | Interquartile range median ratio |
| LL | Log-likelihood |
| LOAs | Limits of agreement |
| LR | Likelihood ratio |
| LS | Liver stiffness |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated liver disease |
| MRI-PDFF | Magnetic resonance imaging proton-density fat fraction |
| MRS | Magnetic resonance spectroscopy |
| NLLS | Nonlinear least squares |
| OR | Odds ratio |
| QUS | Quantitative ultrasound |
| ROI | Region of interest |
| SLD | Skin-to-liver distance |
| SD | Standard deviation |
| T2DM | Type 2 diabetes |
| TAI | Tissue attenuation imaging |
| TSI | Tissue scatter-distribution imaging |
| UDFF | Ultrasound-derived fat fraction |
| UEFF | Ultrasound-estimated fat fraction |
| USFF | Ultrasound fat fraction |
References
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing Epidemiology, Global Trends and Implications for Outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2024, 18, 827–833. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Spagnuolo, R.; Scarlata, G.G.M.; Scarpellini, E.; Boccuto, L.; Luzza, F. Ultrasound Prevalence and Clinical Features of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Diseases: A Real-Life Cross-Sectional Study. Medicina 2023, 59, 1935. [Google Scholar] [CrossRef]
- Horvath, B.; Allende, D.; Xie, H.; Guirguis, J.; Jeung, J.; Lapinski, J.; Patil, D.; McCullough, A.J.; Dasarathy, S.; Liu, X. Interobserver Variability in Scoring Liver Biopsies with a Diagnosis of Alcoholic Hepatitis. Alcohol. Clin. Exp. Res. 2017, 41, 1568–1573. [Google Scholar] [CrossRef]
- Nalbantoglu, I.L.K.; Brunt, E.M. Role of Liver Biopsy in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 9026–9037. [Google Scholar] [CrossRef]
- Segura-Azuara, N.d.l.Á.; Varela-Chinchilla, C.D.; Trinidad-Calderón, P.A. MAFLD/NAFLD Biopsy-Free Scoring Systems for Hepatic Steatosis, NASH, and Fibrosis Diagnosis. Front. Med. 2022, 8, 774079. [Google Scholar] [CrossRef]
- Wan, F.; Pan, F.; Ayonrinde, O.T.; Adams, L.A.; Mori, T.A.; Beilin, L.J.; O’Sullivan, T.A.; Olynyk, J.K.; Oddy, W.H. Validation of Fatty Liver Disease Scoring Systems for Ultrasound Diagnosed Non-Alcoholic Fatty Liver Disease in Adolescents. Dig. Liver Dis. 2021, 53, 746–752. [Google Scholar] [CrossRef]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef]
- Zsombor, Z.; Zsély, B.; Rónaszéki, A.D.; Stollmayer, R.; Budai, B.K.; Palotás, L.; Bérczi, V.; Kalina, I.; Maurovich Horvat, P.; Kaposi, P.N. Comparison of Vendor-Independent Software Tools for Liver Proton Density Fat Fraction Estimation at 1.5 T. Diagnostics 2024, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Rónaszéki, A.D.; Budai, B.K.; Csongrády, B.; Stollmayer, R.; Hagymási, K.; Werling, K.; Fodor, T.; Folhoffer, A.; Kalina, I.; Győri, G.; et al. Tissue Attenuation Imaging and Tissue Scatter Imaging for Quantitative Ultrasound Evaluation of Hepatic Steatosis. Medicine 2022, 101, e29708. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal Threshold of Controlled Attenuation Parameter with MRI-PDFF as the Gold Standard for the Detection of Hepatic Steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef]
- Labyed, Y.; Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 2020, 39, 2427–2438. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Cho, S.J.; Byun, Y.-H.; Jee, J.H.; Kang, M. Development and Validation of Multivariable Quantitative Ultrasound for Diagnosing Hepatic Steatosis. Sci. Rep. 2023, 13, 15235. [Google Scholar] [CrossRef]
- Han, A.; Byra, M.; Heba, E.; Andre, M.P.; Erdman, J.W.; Loomba, R.; Sirlin, C.B.; O’Brien, W.D. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat with Radiofrequency Ultrasound Data Using One-Dimensional Convolutional Neural Networks. Radiology 2020, 295, 342–350. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoon, J.H.; Lee, G. Two-Dimensional Convolutional Neural Network Using Quantitative US for Noninvasive Assessment of Hepatic Steatosis in NAFLD. Radiology 2023, 307, e221510. [Google Scholar] [CrossRef]
- Kaposi, P.N.; Zsombor, Z.; Rónaszéki, A.D.; Budai, B.K.; Csongrády, B.; Stollmayer, R.; Kalina, I.; Győri, G.; Bérczi, V.; Werling, K.; et al. The Calculation and Evaluation of an Ultrasound-Estimated Fat Fraction in Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease. Diagnostics 2023, 13, 3353. [Google Scholar] [CrossRef]
- Barr, R.G.; Wilson, S.R.; Rubens, D.; Garcia-Tsao, G.; Ferraioli, G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Skorpil, M. Multi-scale Graph-cut Algorithm for Efficient Water-fat Separation. Magn. Reson. Med. 2017, 78, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Barr, R.G.; Berzigotti, A.; Sporea, I.; Wong, V.W.-S.; Reiberger, T.; Karlas, T.; Thiele, M.; Cardoso, A.C.; Ayonrinde, O.T.; et al. WFUMB Guidelines/Guidance on Liver Multiparametric Ultrasound. Part 2: Guidance on Liver Fat Quantification. Ultrasound Med. Biol. 2024, 50, 1088–1098. [Google Scholar] [CrossRef]
- Nelson, S.M.; Hoskins, J.D.; Lisanti, C.; Chaudhuri, J. Ultrasound Fatty Liver Indicator: A Simple Tool for Differentiating Steatosis From Nonalcoholic Steatohepatitis: Validity in the Average Obese Population. J. Ultrasound Med. 2020, 39, 749–759. [Google Scholar] [CrossRef]
- Biciusca, T.; Stan, S.I.; Balteanu, M.A.; Cioboata, R.; Ghenea, A.E.; Danoiu, S.; Bumbea, A.-M.; Biciusca, V. The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics 2023, 13, 3316. [Google Scholar] [CrossRef]
- Yin, H.; Fan, Y.; Yu, J.; Xiong, B.; Zhou, B.; Sun, Y.; Wang, L.; Zhu, Y.; Xu, H. Quantitative US Fat Fraction for Noninvasive Assessment of Hepatic Steatosis in Suspected Metabolic-Associated Fatty Liver Disease. Insights Into Imaging 2024, 15, 159. [Google Scholar] [CrossRef]
- Wang, P.; Song, D.; Han, J.; Zhang, J.; Chen, H.; Gao, R.; Shen, H.; Li, J. Comparing Three Ultrasound-Based Techniques for Diagnosing and Grading Hepatic Steatosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Acad. Radiol. 2024, in press. [Google Scholar] [CrossRef]
- Dillman, J.R.; Thapaliya, S.; Tkach, J.A.; Trout, A.T. Quantification of Hepatic Steatosis by Ultrasound: Prospective Comparison With MRI Proton Density Fat Fraction as Reference Standard. Am. J. Roentgenol. 2022, 219, 784–791. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M. Inter-Platform Reproducibility of Ultrasound-Based Fat Fraction for Evaluating Hepatic Steatosis in Nonalcoholic Fatty Liver Disease. Insights Imaging 2024, 15, 46. [Google Scholar] [CrossRef]
- An, Z.; Liu, Q.; Zeng, W.; Wang, Y.; Zhang, Q.; Pei, H.; Xin, X.; Yang, S.; Lu, F.; Zhao, Y.; et al. Relationship between Controlled Attenuated Parameter and Magnetic Resonance Imaging-Proton Density Fat Fraction for Evaluating Hepatic Steatosis in Patients with NAFLD. Hepatol. Commun. 2022, 6, 1975–1986. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.-J.; Shin, H.J.; Yoon, H.; Kim, S.; Koh, H.; Lee, M.-J. Quick Assessment with Controlled Attenuation Parameter for Hepatic Steatosis in Children Based on MRI-PDFF as the Gold Standard. BMC Pediatr. 2019, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, H.; Zhou, D.; Zhao, Q.; Wang, K.; Fan, Y.; Chen, K.; Han, H.; Xu, H. A Prospective Comparison of Three Ultrasound-Based Techniques in Quantitative Diagnosis of Hepatic Steatosis in NAFLD. Abdom. Radiol. 2024, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Park, S.J. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Using Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Korean J. Radiol. 2021, 22, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Şendur, H.N.; Özdemir Kalkan, D.; Cerit, M.N.; Kalkan, G.; Şendur, A.B.; Özhan Oktar, S. Hepatic Fat Quantification With Novel Ultrasound Based Techniques: A Diagnostic Performance Study Using Magnetic Resonance Imaging Proton Density Fat Fraction as Reference Standard. Can. Assoc. Radiol. J. 2023, 74, 362–369. [Google Scholar] [CrossRef] [PubMed]


| Training Set (n = 60) | Test Set (n = 57) | |
|---|---|---|
| AGE (years) | 57.29 ± 13.64 | 50.33 ± 13.16 |
| BMI (kg/cm2) | 29.78 ± 4.18 | 30.57 ± 3.68 |
| Female/male | 26/34 (43.3%/56.6%) | 26/31 (45.6%/54.4%) |
| T2DM | 15 (25%) | 7 (12.3%) |
| Ultrasound parameters | ||
| SLD (cm) | 2.16 ± 0.51 | 2.41 ± 0.47 |
| PDFF (%) | 14.12 ± 10.26 | 16.5 ± 8.47 |
| USFF (%) | NA | 14.58 ± 5.93 |
| AC (dB/cm/MHz) | 0.84 ± 0.14 | 0.85 ± 0.12 |
| BSC-D | 100.69 ± 9.22 | 99.61 ± 6.01 |
| LS (kPa) | 7.59 ± 3.4 | 5.38 ± 3.03 |
| Blood tests | ||
| ALT (U/L) | 53 ± 41.66 | 64.75 ± 48.87 |
| AST (U/L) | 42 ± 35.26 | 44.33 ± 24.86 |
| GGT (U/L) | 105.07 ± 192.1 | 89.47 ± 99.87 |
| TBIL (µmol/L) | 15.37 ± 9.44 | 14.18 ± 8.31 |
| ALB (g/L) | 43.16 ± 3.94 | 46.06 ± 3.39 |
| PLT (G/L) | 232.86 ± 69.23 | 272.49 ± 55.21 |
| INR | 1.01 ± 0.08 | 1.07 ± 0.07 |
| APRI | 0.37 ± 0.27 | 0.35 ± 0.22 |
| FIB4 | 1.56 ± 1 | 1.16 ± 0.72 |
| NFS | −22.2 ± 11.86 | −29.92 ± 2.74 |
| HSI | 41.5 ± 5.46 | 43.52 ± 6.71 |
| Female/male | 26/34 (43.3%/56.6%) | 26/31 (45.6%/54.4%) |
| T2DM | 15 (25%) | 7 (12.3%) |
| Steatosis grade 1 | ||
| S0 | 16 (26.7%) | 3 (5.3%) |
| S1 | 16 (26.7%) | 23 (40.4%) |
| S2 | 12 (20%) | 12 (21.1%) |
| S3 | 16 (26.7%) | 19 (33.3%) |
| Fibrosis grade 2 | ||
| F0/1 | 7 (11.6%) | 35 (61.4%) |
| F2 | 40 (66.6%) | 17 (29.8%) |
| F3 | 9 (15%) | 3 (5.3%) |
| F4 | 4 (6.6%) | 2 (3.5%) |
| Regression Model | F-Statistics | b | R2 | p Value # |
|---|---|---|---|---|
| Multivariable | 8.39 | 47.7 * | 0.494 ** | <0.001 |
| AC | 66.28 | 53.04 | 0.533 | <0.001 |
| BSC-D | 20.96 | 0.573 | 0.265 | <0.001 |
| Age | 0.27 | −0.051 | 0.005 | 0.605 |
| BMI | 2.21 | 0.509 | 0.041 | 0.144 |
| LS | 0.003 | −0.022 | <0.001 | 0.955 |
| SLD | 5.86 | 5.99 | 0.092 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boglárka, Z.; Zsombor, Z.; Rónaszéki, A.D.; Egresi, A.; Stollmayer, R.; Himsel, M.; Bérczi, V.; Kalina, I.; Werling, K.; Győri, G.; et al. Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound. Diagnostics 2025, 15, 203. https://doi.org/10.3390/diagnostics15020203
Boglárka Z, Zsombor Z, Rónaszéki AD, Egresi A, Stollmayer R, Himsel M, Bérczi V, Kalina I, Werling K, Győri G, et al. Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound. Diagnostics. 2025; 15(2):203. https://doi.org/10.3390/diagnostics15020203
Chicago/Turabian StyleBoglárka, Zsély, Zita Zsombor, Aladár D. Rónaszéki, Anna Egresi, Róbert Stollmayer, Marco Himsel, Viktor Bérczi, Ildikó Kalina, Klára Werling, Gabriella Győri, and et al. 2025. "Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound" Diagnostics 15, no. 2: 203. https://doi.org/10.3390/diagnostics15020203
APA StyleBoglárka, Z., Zsombor, Z., Rónaszéki, A. D., Egresi, A., Stollmayer, R., Himsel, M., Bérczi, V., Kalina, I., Werling, K., Győri, G., Maurovich-Horvat, P., Folhoffer, A., Hagymási, K., & Kaposi, P. N. (2025). Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound. Diagnostics, 15(2), 203. https://doi.org/10.3390/diagnostics15020203






