Diagnostic Approach to Biliary Strictures
Abstract
1. Introduction
2. Prevalence and Etiology
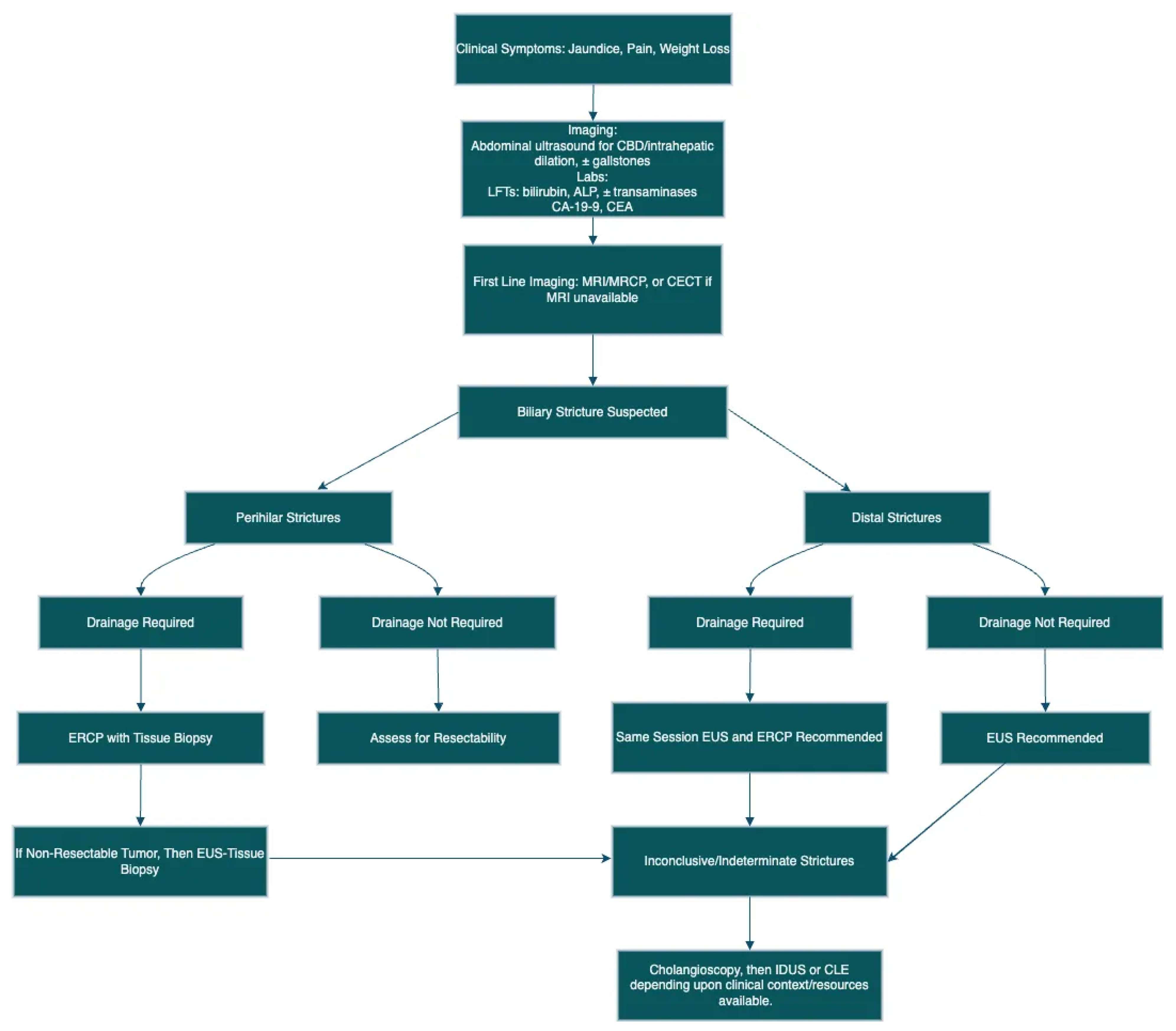
3. Laboratory Tests
4. Cross-Sectional Imaging
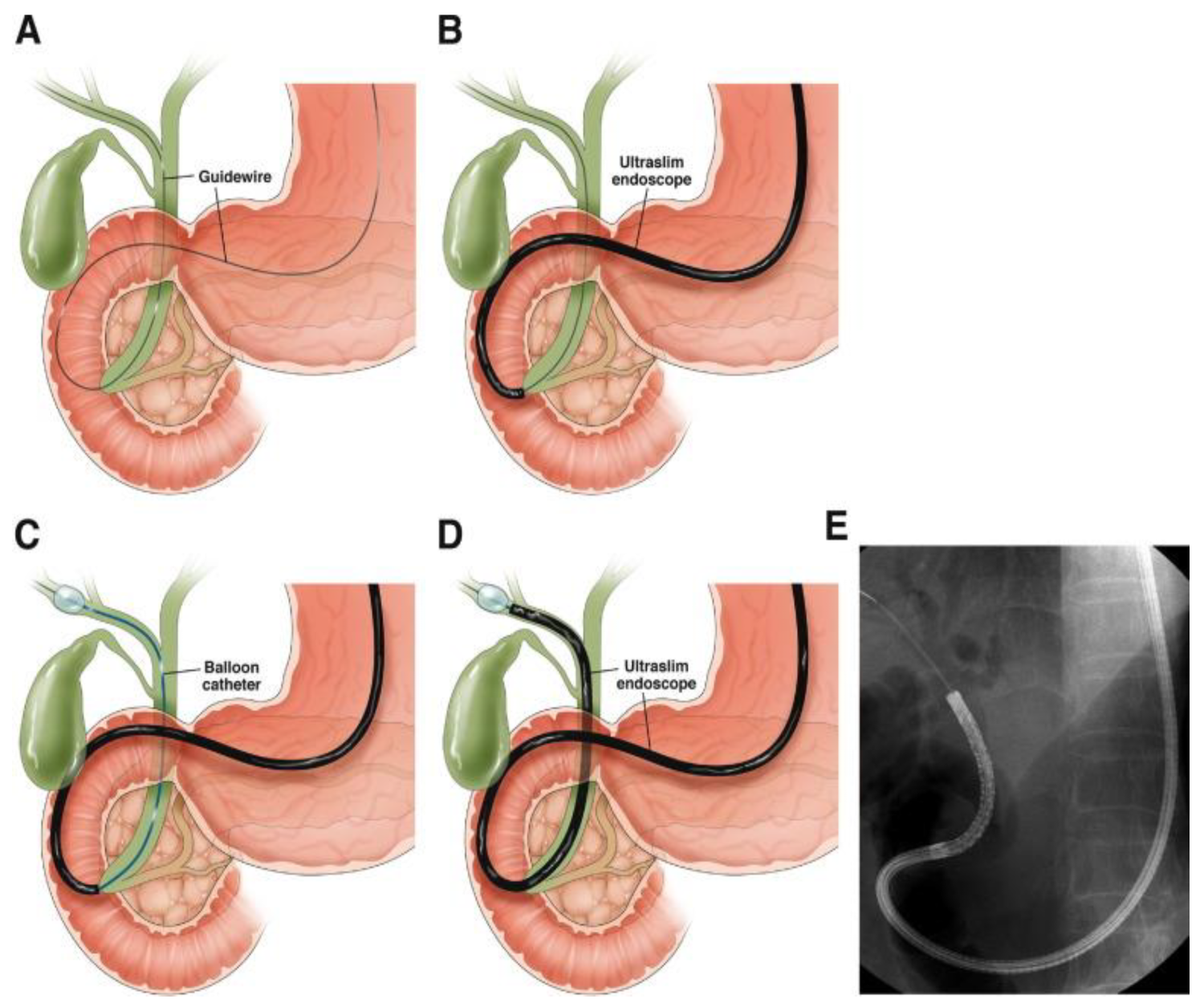
5. Endoscopic Evaluation
5.1. Extrahepatic Strictures
5.2. Perihilar Structures
5.3. Indeterminate Biliary Strictures
| Cholangioscopy Method | Description | Advantages | Limitations |
|---|---|---|---|
| Single-Operator Cholangioscopy (SOC) | Operated through duodenoscope by a single endoscopist | High success rate, stable positioning | High cost, steep learning curve |
| Dual-Operator Cholangioscopy (DOC) | Requires two operators using an ultra-thin reusable endoscope | Better control and maneuverability | High equipment cost, fragile equipment |
| Direct Cholangioscopy (DC) | Direct access using ultra-slim endoscope | High-definition imaging, potential for virtual chromoendoscopy | Technically challenging, potential severe adverse events |
6. Artificial Intelligence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, T.; Boike, J.R. Biliary Strictures: Etiologies and Medical Management. Semin. Interv. Radiol. 2021, 38, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.S.; Calvert, T.J.; Chokshi, R.J. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J. Surg. Res. 2013, 184, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, A.K.P.; Tirumani, S.H.; Prasad, S.R.; Fasih, N.; McInnes, M. Benign biliary strictures: A current comprehensive clinical and imaging review. AJR Am. J. Roentgenol. 2011, 197, W295–W306. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.X.; Jayasekeran, V.; Chong, A.K. Benign biliary strictures: Prevalence, impact, and management strategies. Clin. Exp. Gastroenterol. 2019, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Fujii-Lau, L.L.; Thosani, N.C.; Al-Haddad, M.; Acoba, J.; Wray, C.J.; Zvavanjanja, R.; Amateau, S.K.; Buxbaum, J.L.; Calderwood, A.H.; Chalhoub, J.M.; et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the diagnosis of malignancy in biliary strictures of undetermined etiology: Summary and recommendations. Gastrointest. Endosc. 2023, 98, 685–693. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Liu, X.M.; Yan, X.P.; Zshang, H.K.; Ma, F.; Guo, Y.G.; Fan, C.; Wang, S.; Shi, A.; Wang, B.; Wang, H.; et al. Magnetic Anastomosis for Biliojejunostomy: First Prospective Clinical Trial. World J. Surg. 2018, 42, 4039–4045. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef]
- Chapoy, P.R.; Kendall, R.S.; Fonkalsrud, E.; Ament, M.E. Congenital stricture of the common hepatic duct: An unusual case without jaundice. Gastroenterology 1981, 80, 380–383. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Olson, K.A.; Gershwin, M.E. Evaluation of indeterminate biliary strictures. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 28–37. [Google Scholar] [CrossRef]
- Carpelan-Holmström, M.; Louhimo, J.; Stenman, U.H.; Alfthan, H.; Haglund, C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002, 22, 2311–2316. [Google Scholar] [PubMed]
- Ni, X.G.; Bai, X.F.; Mao, Y.L.; Shao, Y.F.; Wu, J.X.; Shan, Y.; Wang, C.F.; Wang, J.; Tian, Y.T.; Liu, Q.; et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur. J. Surg. Oncol. 2005, 31, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9—Tumor marker: Past, present, and future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.H.; Myung, S.J.; Lim, B.C.; Park, E.T.; Yoo, K.S.; Seo, D.W.; Lee, S.K.; Min, Y.I. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am. J. Gastroenterol. 1999, 94, 1941–1946. [Google Scholar] [CrossRef]
- Ong, S.L.; Sachdeva, A.; Garcea, G.; Gravante, G.; Metcalfe, M.S.; Lloyd, D.M.; Berry, D.P.; Dennison, A.R. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig. Dis. Sci. 2008, 53, 3213–3217. [Google Scholar] [CrossRef]
- Juntermanns, B.; Radunz, S.; Heuer, M.; Hertel, S.; Reis, H.; Neuhaus, J.P.; Vernadakis, S.; Trarbach, T.; Paul, A.; Kaiser, G.M. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur. J. Med. Res. 2010, 15, 357–361. [Google Scholar] [CrossRef]
- Marrelli, D.; Caruso, S.; Pedrazzani, C.; Neri, A.; Fernandes, E.; Marini, M.; Pinto, E.; Roviello, F. CA19-9 serum levels in obstructive jaundice: Clinical value in benign and malignant conditions. Am. J. Surg. 2009, 198, 333–339. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; Teli, M.; Jardine, N.; Puntis, M.C. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2009, 8, 620–626. [Google Scholar]
- Elmunzer, B.J.; Maranki, J.L.; Gómez, V.; Tavakkoli, A.; Sauer, B.G.; Limketkai, B.N.; Brennan, E.A.; Attridge, E.M.; Brigham, T.J.; Wang, A.Y. ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures. Am. J. Gastroenterol. 2023, 118, 405–426. [Google Scholar] [CrossRef]
- Qumseya, B.J.; Jamil, L.H.; Elmunzer, B.J.; Riaz, A.; Ceppa, E.P.; Thosani, N.C.; Buxbaum, J.L.; Storm, A.C.; Sawhney, M.S.; Pawa, S.; et al. ASGE guideline on the role of endoscopy in the management of malignant hilar obstruction. Gastrointest. Endosc. 2021, 94, 222–234.e22. [Google Scholar] [CrossRef] [PubMed]
- Lalani, T.; Couto, C.A.; Rosen, M.P.; Baker, M.E.; Blake, M.A.; Cash, B.D.; Fidler, J.L.; Greene, F.L.; Hindman, N.M.; Katz, D.S.; et al. ACR appropriateness criteria jaundice. J. Am. Coll. Radiol. JACR 2013, 10, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, T.H.; Bender, J.S.; Duncan, M.D.; Ahrendt, S.A.; Harmon, J.W.; Regan, F. Utility of magnetic resonance cholangiography in the evaluation of biliary obstruction. J. Am. Coll. Surg. 1999, 189, 63–71, discussion 71–72. [Google Scholar] [CrossRef]
- Singh, A.; Mann, H.S.; Thukral, C.L.; Singh, N.R. Diagnostic Accuracy of MRCP as Compared to Ultrasound/CT in Patients with Obstructive Jaundice. J. Clin. Diagn. Res. JCDR 2014, 8, 103–107. [Google Scholar] [CrossRef]
- Rösch, T.; Meining, A.; Frühmorgen, S.; Zillinger, C.; Schusdziarra, V.; Hellerhoff, K.; Classen, M.; Helmberger, H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest. Endosc. 2002, 55, 870–876. [Google Scholar] [CrossRef]
- Kaltenthaler, E.C.; Walters, S.J.; Chilcott, J.; Blakeborough, A.; Vergel, Y.B.; Thomas, S. MRCP compared to diagnostic ERCP for diagnosis when biliary obstruction is suspected: A systematic review. BMC Med. Imaging 2006, 6, 9. [Google Scholar] [CrossRef]
- Expert Panel on Gastrointestinal Imaging; Hindman, N.M.; Arif-Tiwari, H.; Kamel, I.R.; Al-Refaie, W.B.; Bartel, T.B.; Cash, B.D.; Chernyak, V.; Goldstein, A.; Grajo, J.R.; et al. ACR Appropriateness Criteria® Jaundice. J. Am. Coll. Radiol. JACR 2019, 16, S126–S140. [Google Scholar] [CrossRef]
- Gorris, M.; van Huijgevoort, N.C.M.; Fockens, P.; Meijer, S.L.; Verheij, J.; Voermans, R.P.; van Wanrooij, R.L.J.; Lekkerkerker, S.J.; van Hooft, J.E. Comparison of two intraductal brush cytology devices for suspected malignant biliary strictures: Randomized controlled trial. Surg. Endosc. 2023, 37, 4566–4573. [Google Scholar] [CrossRef]
- Karsenti, D.; Privat, J.; Charissoux, A.; Perrot, B.; Leblanc, S.; Chaput, U.; Boytchev, I.; Levy, J.; Schaefer, M.; Bourgaux, J.-F.; et al. Multicenter randomized trial comparing diagnostic sensitivity and cellular abundance with aggressive versus standard biliary brushing for bile duct stenosis without mass syndrome. Endoscopy 2023, 55, 796–803. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Zoutendijk, R.; Van der Schaar, P.J.; Didden, P.; Kelder, H.; Brosens, L.A.; Van Santvoort, H.C.; Raicu, M.G.; Vleggaar, F. Optimization of ERCP Technique to Improve the Sensitivity of Biliary Brushing: A Systematic Review and Meta-analysis. J. Gastrointest. Liver Dis. JGLD 2024, 33, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Jin, Y.; Ding, Z.; Lin, R. Effect of modified biopsy forceps on the diagnosis of malignant biliary strictures: A randomized controlled trial. Chin. Med. J. 2024, 137, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Mohamadnejad, M.; Islami, F.; Keshtkar, A.; Biglari, M.; Malekzadeh, R.; Eloubeidi, M.A. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 290–298.e1. [Google Scholar] [CrossRef] [PubMed]
- Korc, P.; Sherman, S. ERCP tissue sampling. Gastrointest. Endosc. 2016, 84, 557–571. [Google Scholar] [CrossRef]
- Kipp, B.R.; Stadheim, L.M.; Halling, S.A.; Pochron, N.L.; Harmsen, S.; Nagorney, D.M.; Sebo, T.J.; Therneau, T.M.; Gores, G.J.; de Groen, P.C.; et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1675–1681. [Google Scholar] [CrossRef]
- Smoczynski, M.; Jablonska, A.; Matyskiel, A.; Lakomy, J.; Dubowik, M.; Marek, I.; Biernat, W.; Limon, J. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest. Endosc. 2012, 75, 65–73. [Google Scholar] [CrossRef]
- Weilert, F.; Bhat, Y.M.; Binmoeller, K.F.; Kane, S.; Jaffee, I.M.; Shaw, R.E.; Cameron, R.; Hashimoto, Y.; Shah, J.N. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: Results of a prospective, single-blind, comparative study. Gastrointest. Endosc. 2014, 80, 97–104. [Google Scholar] [CrossRef]
- Chhoda, A.; Dawod, S.; Grimshaw, A.; Gunderson, C.; Mahadev, S. Evaluation of diagnostic yield of EUS among patients with asymptomatic common bile duct dilation: Systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 890–901.e8. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Broering, D.C.; Knoefel, W.T.; Rogiers, X.; Swain, P.; Thonke, F.; Bobrowski, C.; Topalidis, T.; Soehendra, N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am. J. Gastroenterol. 2004, 99, 45–51. [Google Scholar] [CrossRef]
- Levy, M.J.; Heimbach, J.K.; Gores, G.J. Endoscopic ultrasound staging of cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012, 28, 244–252. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, M.; Ashida, R.; Kita, E.; Katanuma, A.; Itoi, T.; Mikata, R.; Nishikawa, K.; Matsubayashi, H.; Takayama, Y.; et al. Needle tract seeding after endoscopic ultrasound-guided tissue acquisition of pancreatic tumors: A nationwide survey in Japan. Dig.Endosc. 2022, 34, 1442–1455. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Micames, C.; Jowell, P.S.; White, R.; Paulson, E.; Nelson, R.; Morse, M.; Hurwitz, H.; Pappas, T.; Tyler, D.; McGrath, K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest. Endosc. 2003, 58, 690–695. [Google Scholar] [CrossRef]
- Chiang, A.; Theriault, M.; Salim, M.; James, P.D. The incremental benefit of EUS for the identification of malignancy in indeterminate extrahepatic biliary strictures: A systematic review and meta-analysis. Endosc. Ultrasound. 2019, 8, 310–317. [Google Scholar]
- Moura, D.T.H.; de Moura, E.G.H.; Matuguma, S.E.; Dos Santos, M.E.; Moura, E.T.H.; Baracat, F.I.; Artifon, E.; Cheng, S.; Bernardo, W.M.; Chacon, D.; et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: A prospective comparative study. Endosc. Int. Open 2018, 6, E769–E777. [Google Scholar] [CrossRef]
- Sobhrakhshankhah, E.; Sohrabi, M.; Norouzi, H.R.; Zamani, F.; Ajdarkosh, H.; Nikkhah, M.; Khoonsari, M.R.; Faraji, A.H. Tissue Sampling through Endoscopic Ultrasound-Guided Fine Needle Aspiration versus Endoscopic Retrograde Cholangiopancreatographic Brushing Cytology Technique in Suspicious Malignant Biliary Stricture. Middle East J. Dig. Dis. 2021, 13, 294–301. [Google Scholar] [CrossRef]
- Jo, J.H.; Cho, C.M.; Jun, J.H.; Chung, M.J.; Kim, T.H.; Seo, D.W.; Kim, J.; Park, D.H.; Research Group for Endoscopic Ultrasonography in KSGE. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: A multicenter experience. J. Gastroenterol. Hepatol. 2019, 34, 799–805. [Google Scholar] [CrossRef]
- Chu, Y.L.; Wang, X.F.; Gao, X.Z.; Qiao, X.L.; Liu, F.; Yu, S.Y.; Zhang, J. Endoscopic ultrasonography in tandem with endoscopic retrograde cholangiopancreatography in the management of suspected distal obstructive jaundice. Eur. J. Gastroenterol. Hepatol. 2013, 25, 455–459. [Google Scholar] [CrossRef]
- Bang, J.Y.; Kirtane, S.; Krall, K.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 197–202. [Google Scholar] [CrossRef]
- De Moura, D.T.H.; Moura, E.G.H.D.; Bernardo, W.M.; De Moura, E.T.H.; Baraca, F.I.; Kondo, A.; Matuguma, S.E.; Artifon, E.L.A. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound. 2018, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Schoeman, M.N.; Ruszkiewicz, A. Clinical utility of EUS before cholangioscopy in the evaluation of difficult biliary strictures. Gastrointest. Endosc. 2013, 78, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Estrada, J.D.; Rossi, F.; Dziura, J.; Jamidar, P.A.; Siddiqui, U.D. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: Combined or separate procedures? J. Clin. Gastroenterol. 2011, 45, 711–713. [Google Scholar] [CrossRef]
- Guillén Graf, A.M.; Reyna Aréchiga, A.I.; Escareño Pérez, C.E.; Bosques-Padilla, F.; Jáquez Quintana, J.O. Digital single-operator cholangioscopy in the evaluation of biliary strictures after liver transplantation. Rev. Esp. Enferm. Dig. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Gabbiadini, R.; Tringali, A.; De Angelis, C.G.; Mosca, P.; Maurano, A.; Di Mitri, R.; Manno, M.; Mariani, A.; Cereatti, F.; et al. Digital single-operator cholangioscopy in diagnostic and therapeutic bilio-pancreatic diseases: A prospective, multicenter study. Dig. Liver Dis. 2022, 54, 1243–1249. [Google Scholar]
- Weber, A.; von Weyhern, C.; Fend, F.; Schneider, J.; Neu, B.; Meining, A.; Weidenbach, H.; Schmid, R.M.; Prinz, C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 1097–1101. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Wu, J.C.; Bie, L.; Xia, L.; Gong, B. Diagnostic Utility of Endoscopic Retrograde Cholangiography/Intraductal Ultrasound (ERC/IDUS) in Distinguishing Malignant from Benign Bile Duct Obstruction. Dig. Dis. Sci. 2016, 61, 610–617. [Google Scholar] [CrossRef]
- Slivka, A.; Gan, I.; Jamidar, P.; Costamagna, G.; Cesaro, P.; Giovannini, M.; Caillol, F.; Kahaleh, M. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: Results of a prospective multicenter international study. Gastrointest. Endosc. 2015, 81, 282–290. [Google Scholar] [CrossRef]
- Illimoottil, M.; Ginat, D. Recent Advances in Deep Learning and Medical Imaging for Head and Neck Cancer Treatment: MRI, CT, and PET Scans. Cancers 2023, 15, 3267. [Google Scholar] [CrossRef]
- Crinò, S.F.; Napoleon, B.; Facciorusso, A.; Lakhtakia, S.; Borbath, I.; Caillol, F.; Pham, K.D.-C.; Rizzatti, G.; Forti, E.; Palazzo, L.; et al. Endoscopic Ultrasound-guided Radiofrequency Ablation Versus Surgical Resection for Treatment of Pancreatic Insulinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 2834–2843.e2. [Google Scholar] [CrossRef]
- Facciorusso, A.; Kovacevic, B.; Yang, D.; Vilas-Boas, F.; Martínez-Moreno, B.; Stigliano, S.; Rizzatti, G.; Sacco, M.; Arevalo-Mora, M.; Villarreal-Sanchez, L.; et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: A recursive partitioning analysis. Endoscopy 2022, 54, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Ramai, D.; Gkolfakis, P.; Khan, S.R.; Papanikolaou, I.S.; Triantafyllou, K.; Tringali, A.; Chandan, S.; Mohan, B.P.; Adler, D.G. Comparative efficacy of different methods for difficult biliary cannulation in ERCP: Systematic review and network meta-analysis. Gastrointest. Endosc. 2022, 95, 60–71.e12. [Google Scholar] [CrossRef] [PubMed]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Mangiavillano, B.; Moon, J.H.; Crinò, S.F.; Larghi, A.; Pham, K.D.-C.; Teoh, A.Y.B.; Paduano, D.; Lee, Y.N.; Yoo, H.W.; Shin, I.S.; et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest. Endosc. 2022, 95, 115–122. [Google Scholar] [CrossRef]
- Jain, A.J.; Chun, Y.S. ASO Author Reflections: Hilar Biliary Strictures-A Persistent Diagnostic and Management Dilemma. Ann. Surg. Oncol. 2024, 31, 3112–3113. [Google Scholar] [CrossRef]
- Kulaksiz, H.; Strnad, P.; Römpp, A.; von Figura, G.; Barth, T.; Esposito, I.; Schirmacher, P.; Henne-Bruns, D.; Adler, G.; Stiehl, A. A novel method of forceps biopsy improves the diagnosis of proximal biliary malignancies. Dig. Dis. Sci. 2011, 56, 596–601. [Google Scholar] [CrossRef]
- Gerges, C.; Beyna, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; van Geenen, E.; Neuhaus, H.; Reddy, D.N.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Walter, D.; Peveling-Oberhag, J.; Schulze, F.; Bon, D.; Zeuzem, S.; Friedrich-Rust, M.; Albert, J.G. Intraductal biopsies in indeterminate biliary stricture: Evaluation of histopathological criteria in fluoroscopy- vs. cholangioscopy guided technique. Dig. Liver Dis. 2016, 48, 765–770. [Google Scholar] [CrossRef]
- Kumar, D.; Dayal, V.M.; Jha, S.K.; Jha, A.K.; Kumar, R.K. A Randomized Comparative Study of the Use of Individual Modality and Combination of Endoscopic Retrograde Cholangiopancreatography (ERCP) and Digital Single-Operator Cholangioscopy (DSOC) for Diagnosis of Indeterminate Biliary Strictures. Adv. Biomed. Res. 2024, 13, 4. [Google Scholar] [CrossRef]
- Kahaleh, M.; Gaidhane, M.; Shahid, H.M.; Tyberg, A.; Sarkar, A.; Ardengh, J.C.; Kedia, P.; Andalib, I.; Gress, F.; Sethi, A.; et al. Digital single-operator cholangioscopy interobserver study using a new classification: The Mendoza Classification (with video). Gastrointest. Endosc. 2022, 95, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Tyberg, A.; Slivka, A.; Adler, D.G.; Desai, A.P.; Sejpal, D.V.; Pleskow, D.K.; Bertani, H.; Gan, S.-I.; Shah, R.; et al. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J. Clin. Gastroenterol. 2022, 56, e94–e97. [Google Scholar] [CrossRef] [PubMed]
- Robles-Medranda, C.; Baquerizo-Burgos, J.; Alcivar-Vasquez, J.; Kahaleh, M.; Raijman, I.; Kunda, R.; Puga-Tejada, M.; Egas-Izquierdo, M.; Arevalo-Mora, M.; Mendez, J.C.; et al. Artificial intelligence for diagnosing neoplasia on digital cholangioscopy: Development and multicenter validation of a convolutional neural network model. Endoscopy 2023, 55, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, D.; Zhou, J.D.; Ni, M.; Yan, P.; Zhang, Z.; Yu, T.; Zhan, Q.; Shen, Y.; Zhou, L.; et al. A real-time interpretable artificial intelligence model for the cholangioscopic diagnosis of malignant biliary stricture (with videos). Gastrointest. Endosc. 2023, 98, 199–210.e10. [Google Scholar] [CrossRef]
- Rey Rubiano, A.M.; González-Teshima, L.Y.; Arango, L.; Blanco-Avellaneda, C.; Carvajal Gutiérrez, J.J.; Castaño-Llano, R.; Zuleta, M.A.G.; González, C.; Peñaloza-Ramírez, A.; Morales, R.P.; et al. Clinical practice guideline on the use of single-operator cholangioscopy in the diagnosis of indeterminate biliary stricture and the treatment of difficult biliary stones. Surg. Endosc. 2024, 38, 499–510. [Google Scholar] [CrossRef]
- Moon, J.H.; Terheggen, G.; Choi, H.J.; Neuhaus, H. Peroral cholangioscopy: Diagnostic and therapeutic applications. Gastroenterology 2013, 144, 276–282. [Google Scholar] [CrossRef]
- Milluzzo, S.M.; Landi, R.; Perri, V.; Familiari, P.; Boškoski, I.; Pafundi, P.C.; Farina, A.; Ricci, R.; Spada, C.; Costamagna, G.; et al. Diagnostic accuracy and interobserver agreement of cholangioscopy for indeterminate biliary strictures: A single-center experience. Dig. Liver Dis. 2024, 56, 847–852. [Google Scholar] [CrossRef]
- Deprez, P.H.; Garces Duran, R.; Moreels, T.; Furneri, G.; Demma, F.; Verbeke, L.; Van der Merwe, S.W.; Laleman, W. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy 2018, 50, 109–118. [Google Scholar] [CrossRef]
- Pallio, S.; Sinagra, E.; Santagati, A.; D’Amore, F.; Pompei, G.; Conoscenti, G.; Romeo, F.; Borina, E.; Melita, G.; Rossi, F.; et al. Use of catheter-based cholangioscopy in the diagnosis of indeterminate stenosis: A multicenter experience. Minerva Gastroenterol. 2024, 70, 29–35. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, N.; Wu, Q.; Linghu, E. The new criteria for differential diagnosis of indeterminate biliary stricture under super minimally invasive peroral cholangioscopy. Chin. Med. J. 2024, 137, 255–256. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Song, M.; Sun, Z.; Han, X.; Ren, J.; Jiao, D. False-negative factors of percutaneous transluminal clamp biopsy for suspected malignant biliary stricture: 194 cases analyzed from a single center. Insights Imaging 2024, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.K. Intraductal ultrasonography for biliary strictures. Clin. Endosc. 2023, 56, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.Q.; Zheng, C.; Xie, W.W.; Xu, L.; Wu, J.L.; Zhang, D.Q.; Chen, Y.-G.; Niu, S.-S.; Zhan, X.; Zhou, Y.-B. Diagnostic value of new biliary biopsy cannulae for malignant bile duct strictures via endoscopic retrograde cholangiopancreatography pathway. Asian J. Surg. 2024, 47, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Nie, L.; Xu, J.; Li, H.; Zhu, X.; Wei, M.; Yao, J. A machine learning-based predictive model for biliary stricture attributable to malignant tumors: A dual-center retrospective study. Front. Oncol. 2024, 14, 1406512. [Google Scholar] [CrossRef]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Esteva, A.; Chou, K.; Yeung, S.; Naik, N.; Madani, A.; Mottaghi, A.; Liu, Y.; Topol, E.; Dean, J.; Socher, R. Deep learning-enabled medical computer vision. NPJ Digit. Med. 2021, 4, 5. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Ribeiro, T.; Ferreira, J.P.S.; Boas, F.V.; Afonso, J.; Santos, A.L.; Parente, M.P.; Jorge, R.N.; Pereira, P.; Macedo, G. Artificial intelligence for automatic diagnosis of biliary stricture malignancy status in single-operator cholangioscopy: A pilot study. Gastrointest. Endosc. 2022, 95, 339–348. [Google Scholar] [CrossRef]
- Ribeiro, T.; Saraiva, M.M.; Afonso, J.; Ferreira, J.P.S.; Boas, F.V.; Parente, M.P.L.; Jorge, R.N.; Pereira, P.; Macedo, G. Automatic Identification of Papillary Projections in Indeterminate Biliary Strictures Using Digital Single-Operator Cholangioscopy. Clin. Transl. Gastroenterol. 2021, 12, e00418. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Liu, J.; Zhu, L.; Ding, X.; Chen, D.; Wu, H.; Lu, Z.; Zhou, W.; Zhang, L.; et al. A deep learning-based system for bile duct annotation and station recognition in linear endoscopic ultrasound. EBioMedicine 2021, 65, 103238. [Google Scholar] [CrossRef]
- Marya, N.B.; Hartley, C.; Powers, P.D.; Bois, M.C.; Kerr, S.E.; Graham, R.P.; Levy, M.J.; Norton, D.; Gleeson, F.; Abu Dayyeh, B.K.; et al. Development of a Computer-aided Prediction Tool for Evaluating Brushing Samples of Biliary Strictures. Clin. Gastroenterol. Hepatol. 2024, 22, 185–187.e3. [Google Scholar] [CrossRef] [PubMed]
- Njei, B.; McCarty, T.R.; Mohan, B.P.; Fozo, L.; Navaneethan, U. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann. Gastroenterol. 2023, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Robles-Medranda, C.; Baquerizo-Burgos, J.; Puga-Tejada, M.; Cunto, D.; Egas-Izquierdo, M.; Mendez, J.C.; Arevalo-Mora, M.; Vasquez, J.A.; Lukashok, H.; Tabacelia, D. Cholangioscopy-based convoluted neuronal network vs. confocal laser endomicroscopy in identification of neoplastic biliary strictures. Endosc. Int. Open 2024, 12, E1118–E1126. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte-Ciro, J.; Baquerizo-Burgos, J.; Carvajal-Gutierrez, J.; Mendez, J.C.; Robles-Medranda, C. Usefulness of artificial intelligence-assisted digital single-operator cholangioscopy as a second-opinion consultation tool during interhospital assessment of an indeterminate biliary stricture: A case report. VideoGIE 2023, 8, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Lourdusamy, V.; Konjeti, R.; Vargo, J.J.; Parsi, M.A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 81, 168–176. [Google Scholar] [CrossRef]
- Goyal, H.; Mann, R.; Gandhi, Z.; Perisetti, A.; Zhang, Z.; Sharma, N.; Saligram, S.; Inamdar, S.; Tharian, B. Application of artificial intelligence in pancreaticobiliary diseases. Ther. Adv. Gastrointest. Endosc. 2021, 14, 2631774521993059. [Google Scholar] [CrossRef]

Malignant
|
Key Benign Biliary Conditions
|
| Tumor Marker | Sensitivity (%) | Specificity (%) | Diagnostic Utility | Limitations |
|---|---|---|---|---|
| CA 19-9 | 74 | 41.5 | Prognosis, recurrence, staging | Elevated in benign conditions like cholangitis |
| CEA | Variable | Low | Prognosis, malignancy detection | Not specific for pancreato-biliary malignancies |
| Imaging Technique | Sensitivity (%) | Specificity (%) | Advantages | Limitations |
|---|---|---|---|---|
| Ultrasound | 31–100 | 71–97 | Cost-effective, accessible, non-invasive | Limited in differentiating stricture origin |
| MRI/MRCP | 81–100 | 84–100 | High-resolution imaging, no radiation | Expensive, requires expertise |
| CECT | 89 | 96 | Fast, good spatial resolution | Radiation exposure, contrast required |
| Method | Sensitivity (%) | Procedure Type | Strengths | Limitations |
|---|---|---|---|---|
| ERCP Brush Cytology | 40 | Endoscopic | Widely available | Low sensitivity |
| ERCP with Biopsy | 60 | Endoscopic | Higher sensitivity than brushing | Invasive, risk of complications |
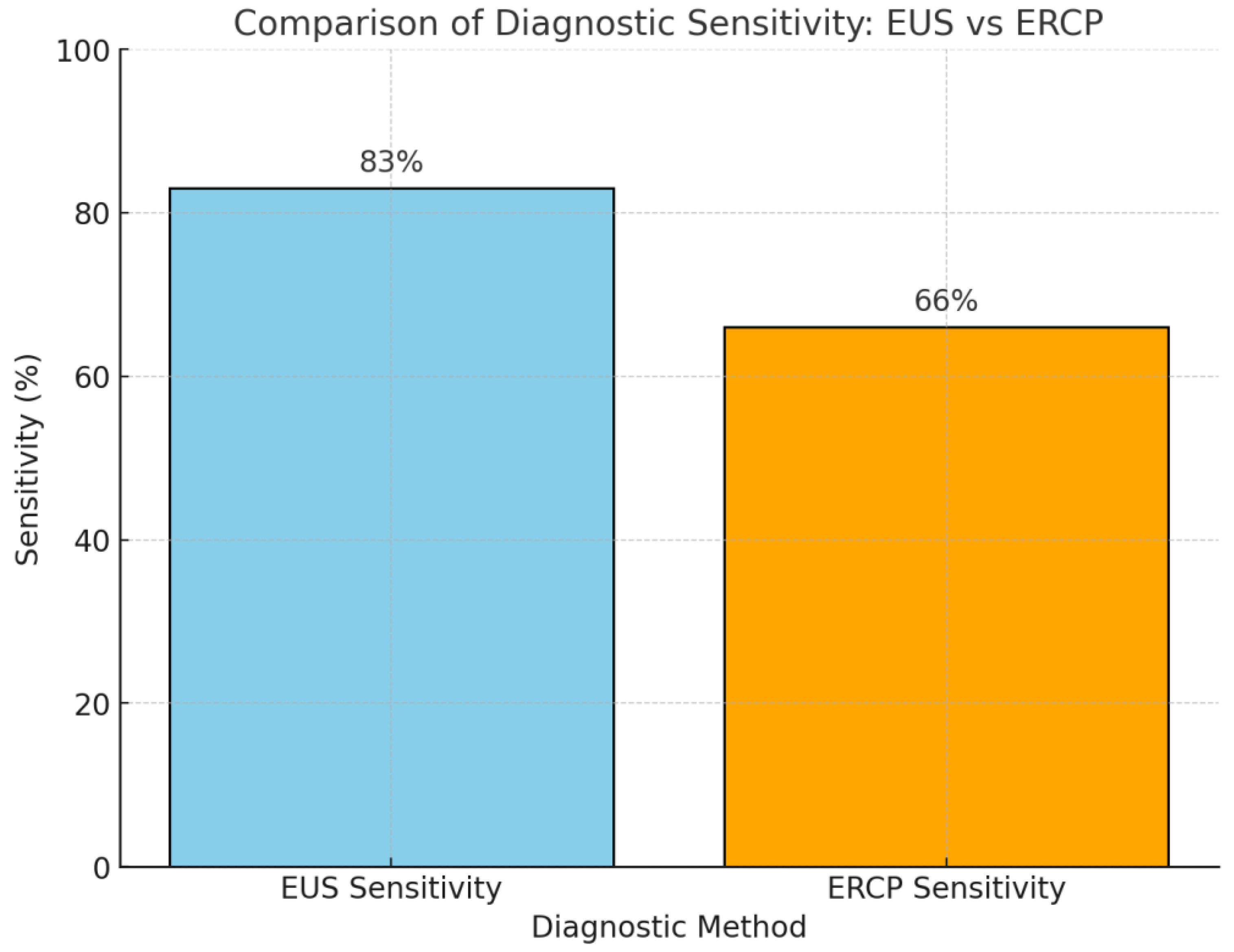
| EUS-FNA | 83 | Endoscopic Ultrasound | High sensitivity for distal strictures | Risk of bile leakage, requires experience |
| Cholangioscopy Biopsy | 80 | Direct Visualization | High precision, direct visualization | Expensive, requires specialized training |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, D.; Singh, S.; Crinò, S.F.; Boskoski, I.; Spada, C.; Fuccio, L.; Samanta, J.; Dhar, J.; Spadaccini, M.; Gkolfakis, P.; et al. Diagnostic Approach to Biliary Strictures. Diagnostics 2025, 15, 325. https://doi.org/10.3390/diagnostics15030325
Raza D, Singh S, Crinò SF, Boskoski I, Spada C, Fuccio L, Samanta J, Dhar J, Spadaccini M, Gkolfakis P, et al. Diagnostic Approach to Biliary Strictures. Diagnostics. 2025; 15(3):325. https://doi.org/10.3390/diagnostics15030325
Chicago/Turabian StyleRaza, Daniyal, Sahib Singh, Stefano Francesco Crinò, Ivo Boskoski, Cristiano Spada, Lorenzo Fuccio, Jayanta Samanta, Jahnvi Dhar, Marco Spadaccini, Paraskevas Gkolfakis, and et al. 2025. "Diagnostic Approach to Biliary Strictures" Diagnostics 15, no. 3: 325. https://doi.org/10.3390/diagnostics15030325
APA StyleRaza, D., Singh, S., Crinò, S. F., Boskoski, I., Spada, C., Fuccio, L., Samanta, J., Dhar, J., Spadaccini, M., Gkolfakis, P., Maida, M. F., Machicado, J., Spampinato, M., & Facciorusso, A. (2025). Diagnostic Approach to Biliary Strictures. Diagnostics, 15(3), 325. https://doi.org/10.3390/diagnostics15030325









