Wearables in Chronomedicine and Interpretation of Circadian Health
Abstract
1. Introduction
2. Actigraphy Features: Beyond Sleep
3. Overview of Actigraphic Health Markers
4. Interpretation of Deviant Parameters
4.1. Faded Circadian Oscillation (Amplitude Decrease)
4.2. Phase Deviations
4.3. Fragmentation and Ultradian/Infradian Modifications
4.4. Misalignment (Intrinsic Desynchrony)
4.5. Social Jet Lag: Objective Characterization by Wearables
4.6. Composite Markers
5. Circadian Health Markers from Actigraphy
6. Molecular Insights on the Interaction Between Timed Physical Activity and Brain Health
7. Wearables to Track Circadian Markers in Neurodegenerative Diseases
8. Boosting Brain, Vascular and Metabolic Health by Clock-Enhancing Strategies
8.1. Scheduled Physical Activity
8.2. Light Hygiene and Chronobiotics
8.3. Optimizing Weekly Schedules
9. Perspectives of Actigraphy-Compatible Wearable Technologies/Next-Generation Comprehensive Monitoring Systems
9.1. Functional Near-Infrared Spectroscopy (fNIRS) and Photoplethysmography (PPG)
9.2. Biochemical Analyses
9.3. Improving Light Exposure Monitoring and Analytics
9.4. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- López-Otín, C.; Kroemer, G. Hallmarks of health. Cell 2021, 184, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Agadzhanian, N.A.; Gubin, D.G. Desinkhronoz: Mekhanizmy razvitiia ot molekuliarno-geneticheskogo do organizmennogo urovnia [Desynchronization: Mechanisms of development from molecular to systemic levels]. Usp. Fiziol. Nauk. 2004, 35, 57–72. (In Russian) [Google Scholar] [PubMed]
- Cornelissen, G.; Otsuka, K. Chronobiology of Aging: A Mini-Review. Gerontology 2017, 63, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Foster, R.G.; Klerman, E.B. The circadian system, sleep, and the health/disease balance: A conceptual review. J. Sleep Res. 2022, 31, e13621. [Google Scholar] [CrossRef]
- Gubin, D. Chronotherapeutic Approaches. In Chronobiology and Chronomedicine: From Molecular and Cellular Mechanisms to Whole Body Interdigitating Networks; Cornelissen, G., Hirota, T., Eds.; Royal Society of Chemistry: London, UK, 2024; Volume 23, Chapter 21; pp. 536–577. [Google Scholar]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef]
- Kramer, A.; Lange, T.; Spies, C.; Finger, A.-M.; Berg, D.; Oster, H. Foundations of circadian medicine. PLoS Biol. 2022, 20, e3001567. [Google Scholar] [CrossRef]
- Gubin, D.G. Molecular basis of circadian rhythms and principles of circadian disruption. Usp. Fiziol. Nauk. 2013, 44, 65–87. (In Russian) [Google Scholar] [PubMed]
- Gubin, D.; Cornelissen, G.; Weinert, D.; Vetoshkin, A.S.; Gapon, L.I.; Shurkevich, N.P.; Poshinov, F.A.; Belozerova, N.V.; Danilova, L.A. Circadian disruption and Vascular Variability Disorders (VVD)—Mechanisms linking aging, disease state and Arctic shift-work: Applications for chronotherapy. World Heart J. 2013, 5, 285–306. [Google Scholar]
- Gubin, D.G.; Weinert, D.; Bolotnova, T.V. Age-Dependent Changes of the temporal Order—Causes and Treatment. Curr. Aging Sci. 2015, 9, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef]
- Weinert, D.; Gubin, D. Chronobiological Study Designs. In Chronobiology and Chronomedicine from Molecular and Cellular Mechanisms to Whole Body Interdigitating Networks; Cornelissen, G., Hirota, T., Eds.; Royal Society of Chemistry: London, UK, 2024; Volume 23, Chapter 22; pp. 579–609. [Google Scholar]
- Usmani, I.M.; Dijk, D.-J.; Skeldon, A.C. Mathematical Analysis of Light-sensitivity Related Challenges in Assessment of the Intrinsic Period of the Human Circadian Pacemaker. J. Biol. Rhythm. 2024, 39, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Functional link between distal vasodilation and sleep-onset latency? Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R741–R748. [Google Scholar] [CrossRef]
- Kräuchi, K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med. Rev. 2007, 11, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; de Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P.A. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2020, 43, zsz254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kane, M.; Zhang, Y.; Sun, W.; Song, Y.; Dong, S.; Lin, Q.; Zhu, Q.; Jiang, F.; Zhao, H. Circadian Rhythm Analysis Using Wearable Device Data: Novel Penalized Machine Learning Approach. J. Med. Internet Res. 2021, 23, e18403. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.R.; Perez-Pozuelo, I.; Grandner, M.A. Past, Present, and Future of Multisensory Wearable Technology to Monitor Sleep and Circadian Rhythms. Front. Digit. Health 2021, 3, 721919. [Google Scholar] [CrossRef]
- Shandhi, M.H.; Wang, W.K.; Dunn, J. Taking the time for our bodies: How wearables can be used to assess circadian physiology. Cell Rep. Methods 2021, 1, 100067. [Google Scholar] [CrossRef]
- Tobin, S.Y.; Williams, P.G.; Baron, K.G.; Halliday, T.M.; Depner, C.M. Challenges and Opportunities for Applying Wearable Technology to Sleep. Sleep Med. Clin. 2021, 16, 607–618. [Google Scholar] [CrossRef]
- Lin, C.; Chen, I.-M.; Chuang, H.-H.; Wang, Z.-W.; Lin, H.-H.; Lin, Y.-H. Examining Human-Smartphone Interaction as a Proxy for Circadian Rhythm in Patients with Insomnia: Cross-Sectional Study. J. Med. Internet Res. 2023, 25, e48044. [Google Scholar] [CrossRef]
- de Zambotti, M.; Goldstein, C.; Cook, J.; Menghini, L.; Altini, M.; Cheng, P.; Robillard, R. State of the science and recommendations for using wearable technology in sleep and circadian research. Sleep 2023, 47, zsad325. [Google Scholar] [CrossRef]
- Moorthy, P.; Weinert, L.; Schüttler, C.; Svensson, L.; Sedlmayr, B.; Müller, J.; Nagel, T. Attributes, Methods, and Frameworks Used to Evaluate Wearables and Their Companion mHealth Apps: Scoping Review. JMIR mHealth uHealth 2024, 12, e52179. [Google Scholar] [CrossRef] [PubMed]
- della Monica, C.; Ravindran, K.K.G.; Atzori, G.; Lambert, D.J.; Rodriguez, T.; Mahvash-Mohammadi, S.; Bartsch, U.; Skeldon, A.C.; Wells, K.; Hampshire, A.; et al. A Protocol for Evaluating Digital Technology for Monitoring Sleep and Circadian Rhythms in Older People and People Living with Dementia in the Community. Clocks Sleep 2024, 6, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ganglberger, W.; Panneerselvam, E.; Leone, M.J.; Quadri, S.A.; Goparaju, B.; Tesh, R.A.; Akeju, O.; Thomas, R.J.; Westover, M.B. Sleep staging from electrocardiography and respiration with deep learning. Sleep 2020, 43, zsz306. [Google Scholar] [CrossRef] [PubMed]
- Kranzinger, S.; Baron, S.; Kranzinger, C.; Heib, D.; Borgelt, C. Generalisability of sleep stage classification based on interbeat intervals: Validating three machine learning approaches on self-recorded test data. Behaviormetrika 2023, 51, 341–358. [Google Scholar] [CrossRef]
- Kripke, D.F.; Mullaney, D.; Messin, S.; Wyborney, V.G. Wrist actigraphic measures of sleep and rhythms. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Wilde-Frenz, J.; Schulz, H. Rate and Distribution of Body Movements during Sleep in Humans. Percept. Mot. Ski. 1983, 56, 275–283. [Google Scholar] [CrossRef]
- Borbély, A.A. New techniques for the analysis of the human sleep-wake cycle. Brain Dev. 1986, 8, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef]
- Patterson, M.R.; Nunes, A.A.S.; Gerstel, D.; Pilkar, R.; Guthrie, T.; Neishabouri, A.; Guo, C.C. 40 years of actigraphy in sleep medicine and current state of the art algorithms. npj Digit. Med. 2023, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Zidani, R.; Misset, J.-L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997, 350, 681–686. [Google Scholar] [CrossRef]
- Kramer, G.; Dominguez-Vega, Z.T.; Laarhoven, H.S.; Brandsma, R.; Smit, M.; van der Stouwe, A.M.; Elting, J.W.J.; Maurits, N.M.; Rosmalen, J.G.; Tijssen, M.A. Similar association between objective and subjective symptoms in functional and organic tremor. Park. Relat. Disord. 2019, 64, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Blackwell, T.; Cawthon, P.M.; Ancoli-Israel, S.; Stone, K.L.; Yaffe, K. Association of Circadian Abnormalities in Older Adults with an Increased Risk of Developing Parkinson Disease. JAMA Neurol. 2020, 77, 1270–1278. [Google Scholar] [CrossRef]
- Feigl, B.; Lewis, S.J.; Burr, L.D.; Schweitzer, D.; Gnyawali, S.; Vagenas, D.; Carter, D.D.; Zele, A.J. Efficacy of biologically-directed daylight therapy on sleep and circadian rhythm in Parkinson’s disease: A randomised, double-blind, parallel-group, active-controlled, phase 2 clinical trial. eClinicalMedicine 2024, 69, 102474. [Google Scholar] [CrossRef]
- Winer, J.R.; Lok, R.; Weed, L.; He, Z.; Poston, K.L.; Mormino, E.C.; Zeitzer, J.M. Impaired 24-h activity patterns are associated with an increased risk of Alzheimer’s disease, Parkinson’s disease, and cognitive decline. Alzheimer’s Res. Ther. 2024, 16, 35. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Stefani, O.; Voronin, K.A.; Mezhakova, M.S.; Petrov, I.M.; Borisenkov, M.F.; Markov, A.A.; Gubin, D.G. Wearable Light-and-Motion Dataloggers for Sleep/Wake Research: A Review. Appl. Sci. 2022, 12, 11794. [Google Scholar] [CrossRef]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of Sleep Parameters from Actigraphy and Polysomnography in Older Women: The SOF Study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Kaplan, E.R.; Cailler, M.; Rueschman, M.; Wang, R.; Weng, J.; Taveras, E.M.; Redline, S. Actigraphy-based sleep estimation in adolescents and adults: A comparison with polysomnography using two scoring algorithms. Nat. Sci. Sleep 2018, 10, 13–20. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2018, 14, 1209–1230. [Google Scholar] [CrossRef] [PubMed]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, H.M.; Yao, Z.; Krafty, R.T.; Evans, M.A.; Buysse, D.J.; Kravitz, H.M.; Matthews, K.A.; Gold, E.B.; Harlow, S.D.; Samuelsson, L.B.; et al. Comparing polysomnography, actigraphy, and sleep diary in the home environment: The Study of Women’s Health Across the Nation (SWAN) Sleep Study. SLEEP Adv. 2022, 3, zpac001. [Google Scholar] [CrossRef] [PubMed]
- Winnebeck, E.C.; Fischer, D.; Leise, T.; Roenneberg, T. Dynamics and Ultradian Structure of Human Sleep in Real Life. Curr. Biol. 2017, 28, 49–59.e5. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Reichert, C.F.; Münch, M.; Gabel, V.; Stefani, O.; Chellappa, S.L.; Schmidt, C. Ultradian sleep cycles: Frequency, duration, and associations with individual and environmental factors—A retrospective study. Sleep Health 2023, 10, S52–S62. [Google Scholar] [CrossRef] [PubMed]
- Witting, W.; Kwa, I.H.; Eikelenboom, P.; Mirmiran, M.; Swaab, D. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol. Psychiatry 1990, 27, 563–572. [Google Scholar] [CrossRef]
- Van Someren, E.J.W.; Swaab, D.F.; Colenda, C.C.; Cohen, W.; McCall, W.V.; Rosenquist, P.B. Bright Light Therapy: Improved Sensitivity to Its Effects on Rest-Activity Rhythms in Alzheimer Patients by Application of Nonparametric Methods. Chronobiol. Int. 1999, 16, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á.; Madrid, J.A. A New Integrated Variable Based on Thermometry, Actimetry and Body Position (TAP) to Evaluate Circadian System Status in Humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.; Tserne, T.; Bakutova, L.; Gubin, D. Actimetry-Derived 24 h Rest–Activity Rhythm Indices Applied to Predict MCTQ and PSQI. Appl. Sci. 2022, 12, 6888. [Google Scholar] [CrossRef]
- Danilevicz, I.M.; van Hees, V.T.; van der Heide, F.C.T.; Jacob, L.; Landré, B.; Benadjaoud, M.A.; Sabia, S. Measures of fragmentation of rest activity patterns: Mathematical properties and interpretability based on accelerometer real life data. BMC Med. Res. Methodol. 2024, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Danilenko, K.; Stefani, O.; Kolomeichuk, S.; Markov, A.; Petrov, I.; Voronin, K.; Mezhakova, M.; Borisenkov, M.; Shigabaeva, A.; et al. Blue Light and Temperature Actigraphy Measures Predicting Metabolic Health Are Linked to Melatonin Receptor Polymorphism. Biology 2023, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Liu, F.; Zipunnikov, V.; Bilgel, M.; Rabinowitz, J.A.; An, Y.; Di, J.; Bai, J.; Wanigatunga, S.K.; Wu, M.N.; et al. Evaluating a novel 24-hour rest/activity rhythm marker of preclinical β-amyloid deposition. Sleep 2024, 47, zsae037. [Google Scholar] [CrossRef]
- Blackwell, T.L.; Figueiro, M.G.; Tranah, G.J.; Zeitzer, J.M.; Yaffe, K.; Ancoli-Israel, S.; Kado, D.M.; Ensrud, K.E.; Lane, N.E.; Leng, Y.; et al. Associations of 24-Hour Light Exposure and Activity Patterns and Risk of Cognitive Impairment and Decline in Older Men: The MrOS Sleep Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 78, 1834–1843. [Google Scholar] [CrossRef]
- Xiao, Q.; Durbin, J.; Bauer, C.; Yeung, C.H.C.; Figueiro, M.G. Alignment Between 24-h Light-Dark and Activity-Rest Rhythms Is Associated with Diabetes and Glucose Metabolism in a Nationally Representative Sample of American Adults. Diabetes Care 2023, 46, 2171–2179. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.; Stern, N.; El-Osta, A.; Chai, Z.; Bilu, C.; Einat, H.; Zimmet, P. The Circadian Syndrome Is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016. Nutrients 2022, 14, 5317. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.M.; Lutsey, P.L.; Ogilvie, R.P.; Pankow, J.S.; Bertoni, A.; Michos, E.D.; Punjabi, N.; Redline, S. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy172. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Redline, S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity with Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2019, 42, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Qian, J.; Evans, D.S.; Redline, S.; Lane, N.E.; Ancoli-Israel, S.; Scheer, F.A.; Stone, K.; Osteoporotic Fractures in Men (MrOS) Study Group. Cross-sectional and Prospective Associations of Rest-Activity Rhythms with Metabolic Markers and Type 2 Diabetes in Older Men. Diabetes Care 2020, 43, 2702–2712. [Google Scholar] [CrossRef]
- Xiao, Q.; Matthews, C.E.; Playdon, M.; Bauer, C. The association between rest-activity rhythms and glycemic markers: The US National Health and Nutrition Examination Survey, 2011–2014. Sleep 2021, 45, zsab291. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Vu, T.-H.; Maas, M.B.; Braun, R.I.; Wolf, M.S.; Roenneberg, T.; Daviglus, M.L.; Reid, K.J.; Zee, P.C. Light at night in older age is associated with obesity, diabetes, and hypertension. Sleep 2022, 46, zsac130. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.A.; Qiu, X.; Schwartz, J.; Huang, T.; Scheer, F.A.; Redline, S.; Sofer, T. Light exposure during sleep is bidirectionally associated with irregular sleep timing: The multi-ethnic study of atherosclerosis (MESA). Environ. Pollut. 2023, 344, 123258. [Google Scholar] [CrossRef] [PubMed]
- Gubin, G.D.; Weinert, D. Bioritmy i vozrast [Biorhythms and age]. Usp Fiziol Nauk. 1991, 22, 77–96. (In Russian) [Google Scholar] [PubMed]
- Gubin, D.; Cornélissen, G.; Halberg, F.; Gubin, G.; Uezono, K.; Kawasaki, T. The human blood pressure chronome: A biological gauge of aging. In Vivo 1998, 11, 485–494. [Google Scholar]
- Abbott, S.M.; Malkani, R.G.; Zee, P.C. Circadian disruption and human health: A bidirectional relationship. Eur. J. Neurosci. 2019, 51, 567–583. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Belsky, D.W.; McCall, W.V.; Liu, Y.; Su, S. Blunted Rest–Activity Circadian Rhythm is Associated with Increased Rate of Biological Aging: An Analysis of NHANES 2011–2014. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 78, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Malishevskaya, T.N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol. Int. 2019, 36, 564–577. [Google Scholar] [CrossRef]
- Lyall, L.M.; Wyse, C.A.; Graham, N.; Ferguson, A.; Lyall, D.M.; Cullen, B.; Morales, C.A.C.; Biello, S.M.; Mackay, D.; Ward, J.; et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 2018, 5, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yang, L.; Ai, S.; Liu, Y.; Zhang, W.; Lei, B.; Chen, J.; Liu, Y.; Chan, J.W.Y.; Chan, N.Y.; et al. Association between accelerometer-measured amplitude of rest–activity rhythm and future health risk: A prospective cohort study of the UK Biobank. Lancet Healthy Longev. 2023, 4, e200–e210. [Google Scholar] [CrossRef]
- Cai, R.; Gao, L.; Gao, C.; Yu, L.; Zheng, X.; Bennett, D.A.; Buchman, A.S.; Hu, K.; Li, P. Circadian disturbances and frailty risk in older adults. Nat. Commun. 2023, 14, 7219. [Google Scholar] [CrossRef]
- Brooks, T.G.; Lahens, N.F.; Grant, G.R.; Sheline, Y.I.; FitzGerald, G.A.; Skarke, C. Diurnal rhythms of wrist temperature are associated with future disease risk in the UK Biobank. Nat. Commun. 2023, 14, 5172. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Su, S.; McCall, W.V.; Isales, C.; Snieder, H.; Wang, X. Rest-activity circadian rhythm and impaired glucose tolerance in adults: An analysis of NHANES 2011–2014. BMJ Open Diabetes Res. Care 2022, 10, e002632. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Su, S.; McCall, W.V.; Wang, X. Blunted rest-activity rhythm is associated with increased white blood-cell-based inflammatory markers in adults: An analysis from NHANES 2011–2014. Chronobiol. Int. 2022, 39, 895–902. [Google Scholar] [CrossRef]
- Shim, J.; Fleisch, E.; Barata, F. Wearable-based accelerometer activity profile as digital biomarker of inflammation, biological age, and mortality using hierarchical clustering analysis in NHANES 2011–2014. Sci. Rep. 2023, 13, 9326. [Google Scholar] [CrossRef]
- Tai, Y.; Obayashi, K.; Yamagami, Y.; Saeki, K. Association between circadian skin temperature rhythms and actigraphic sleep measures in real-life settings. J. Clin. Sleep Med. 2023, 19, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Nelaeva, A.A.; Uzhakova, A.E.; Hasanova, Y.V.; Cornelissen, G.; Weinert, D. Disrupted circadian rhythms of body temperature, heart rate and fasting blood glucose in prediabetes and type 2 diabetes mellitus. Chronobiol. Int. 2017, 34, 1136–1148. [Google Scholar] [CrossRef]
- Windred, D.P.; Burns, A.C.; Rutter, M.K.; Yeung, C.H.C.; Lane, J.M.; Xiao, Q.; Saxena, R.; Cain, S.W.; Phillips, A.J. Personal light exposure patterns and incidence of type 2 diabetes: Analysis of 13 million hours of light sensor data and 670,000 person-years of prospective observation. Lancet Reg. Health Eur. 2024, 42, 100943. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Koga, N.; Hidese, S.; Nagashima, A.; Kim, Y.; Higuchi, T.; Kunugi, H. 24-h activity rhythm and sleep in depressed outpatients. J. Psychiatr. Res. 2016, 77, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Partonen, T.; Paunio, T.; Castaneda, A.E.; Marttunen, M.; Urrila, A.S. Advanced phases and reduced amplitudes are suggested to characterize the daily rest-activity cycles in depressed adolescent boys. Chronobiol. Int. 2017, 34, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Minaeva, O.; Booij, S.H.; Lamers, F.; Antypa, N.; Schoevers, R.A.; Wichers, M.; Riese, H. Level and timing of physical activity during normal daily life in depressed and non-depressed individuals. Transl. Psychiatry 2020, 10, 259. [Google Scholar] [CrossRef]
- Roveda, E.; Montaruli, A.; Galasso, L.; Pesenti, C.; Bruno, E.; Pasanisi, P.; Cortellini, M.; Rampichini, S.; Erzegovesi, S.; Caumo, A.; et al. Rest-activity circadian rhythm and sleep quality in patients with binge eating disorder. Chronobiol. Int. 2017, 35, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Cornélissen, G.; Halberg, F.; Oehlerts, G. Excessive circadian amplitude of blood pressure increases risk of ischaemic stroke and nephropathy. J. Med. Eng. Technol. 1997, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cornélissen, G.; Delcourt, A.; Toussaint, G.; Otsuka, K.; Watanabe, Y.; Siegelova, J.; Fiser, B.; Dusek, J.; Homolka, P.; Kumar, A.; et al. Opportunity of detecting pre-hypertension: Worldwide data on blood pressure overswinging. Biomed. Pharmacother. 2005, 59 (Suppl. S1), S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Borer, K.T.; Cornelissen, G.; Halberg, F.; Brook, R.; Rajagopalan, S.; Fay, W. Circadian blood pressure overswinging in a physically fit, normotensive African American woman. Am. J. Hypertens. 2002, 15, 827–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velasquez, M.T.; Beddhu, S.; Nobakht, E.; Rahman, M.; Raj, D.S. Ambulatory Blood Pressure in Chronic Kidney Disease: Ready for Prime Time? Kidney Int. Rep. 2016, 1, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Magee, M.; Sletten, T.L.; Gordon, C.; Lovato, N.; Ambani, K.; Bartlett, D.J.; Kennaway, D.J.; Lack, L.C.; Grunstein, R.R.; et al. Light-based methods for predicting circadian phase in delayed sleep–wake phase disorder. Sci. Rep. 2021, 11, 10878. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Blackwell, T.; Ancoli-Israel, S.; Paudel, M.L.; Ensrud, K.E.; Cauley, J.A.; Redline, S.; Hillier, T.A.; Cummings, S.R.; Stone, K.L.; et al. Circadian Activity Rhythms and Mortality: The Study of Osteoporotic Fractures. J. Am. Geriatr. Soc. 2010, 58, 282–291. [Google Scholar] [CrossRef]
- Tranah, G.J.; Ma, T.B.; Stone, K.L.; Ancoli-Israel, S.; Paudel, M.L.; Ensrud, K.; Cauley, J.A.; Redline, S.; Hillier, T.A.; Cummings, S.R.; et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011, 70, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Saint-Maurice, P.F.; Freeman, J.R.; Russ, D.; Almeida, J.S.; Shams-White, M.M.; Patel, S.; Wolff-Hughes, D.L.; Watts, E.L.; Loftfield, E.; Hong, H.G.; et al. Associations between actigraphy-measured sleep duration, continuity, and timing with mortality in the UK Biobank. Sleep 2023, 47, zsad312. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Neroev, V.; Malishevskaya, T.; Cornelissen, G.; Astakhov, S.Y.; Kolomeichuk, S.; Yuzhakova, N.; Kabitskaya, Y.; Weinert, D. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J. Pineal Res. 2021, 70, e12730. [Google Scholar] [CrossRef]
- Nikbakhtian, S.; Reed, A.B.; Obika, B.D.; Morelli, D.; Cunningham, A.C.; Aral, M.; Plans, D. Accelerometer-derived sleep onset timing and cardiovascular disease incidence: A UK Biobank cohort study. Eur. Heart J. Digit. Health 2021, 2, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.K.; Clerx, W.M.; O’brien, C.S.; Sano, A.; Barger, L.K.; Picard, R.W.; Lockley, S.W.; Klerman, E.B.; Czeisler, C.A. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017, 7, 3216. [Google Scholar] [CrossRef]
- Schlagintweit, J.; Laharnar, N.; Glos, M.; Zemann, M.; Demin, A.V.; Lederer, K.; Penzel, T.; Fietze, I. Effects of sleep fragmentation and partial sleep restriction on heart rate variability during night. Sci. Rep. 2023, 13, 6202. [Google Scholar] [CrossRef]
- Laharnar, N.; Fatek, J.; Zemann, M.; Glos, M.; Lederer, K.; Suvorov, A.V.; Demin, A.V.; Penzel, T.; Fietze, I. A sleep intervention study comparing effects of sleep restriction and fragmentation on sleep and vigilance and the need for recovery. Physiol. Behav. 2019, 215, 112794. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily Melatonin Administration Attenuates Age-Dependent Disturbances of Cardiovascular Rhythms. Curr. Aging Sci. 2015, 9, 5–13. [Google Scholar] [CrossRef]
- Qian, X.; Droste, S.K.; Lightman, S.L.; Reul, J.M.H.M.; Linthorst, A.C.E. Circadian and Ultradian Rhythms of Free Glucocorticoid Hormone Are Highly Synchronized between the Blood, the Subcutaneous Tissue, and the Brain. Endocrinology 2012, 153, 4346–4353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Q.; Pan, Y.; Mace, E.M.; York, B.; Antoulas, A.C.; Dacso, C.C.; O’malley, B.W. A Cell-Autonomous Mammalian 12 hr Clock Coordinates Metabolic and Stress Rhythms. Cell Metab. 2017, 25, 1305–1319.e9. [Google Scholar] [CrossRef]
- Kembro, J.M.; Flesia, A.G.; Acosta-Rodríguez, V.A.; Takahashi, J.S.; Nieto, P.S. Dietary restriction modulates ultradian rhythms and autocorrelation properties in mice behavior. Commun. Biol. 2024, 7, 303. [Google Scholar] [CrossRef]
- Grant, A.D.; Newman, M.; Kriegsfeld, L.J. Ultradian rhythms in heart rate variability and distal body temperature anticipate onset of the luteinizing hormone surge. Sci. Rep. 2020, 10, 20378. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Murakami, S.; Okajima, K.; Shibata, K.; Kubo, Y.; Gubin, D.G.; Beaty, L.A.; Cornelissen, G. Appropriate Circadian-Circasemidian Coupling Protects Blood Pressure from Morning Surge and Promotes Human Resilience and Wellbeing. Clin. Interv. Aging 2023, 18, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Neroev, V.; Malishevskaya, T.; Weinert, D.; Astakhov, S.; Kolomeichuk, S.; Cornelissen, G.; Kabitskaya, Y.; Boiko, E.; Nemtsova, I.; Gubin, D. Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. Int. J. Mol. Sci. 2020, 22, 359. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.J.; Santostasi, G.; Baron, K.G.; Wilson, J.; Kang, J.; Zee, P.C. Timing and Intensity of Light Correlate with Body Weight in Adults. PLoS ONE 2014, 9, e92251. [Google Scholar] [CrossRef]
- Iimuro, S.; Imai, E.; Watanabe, T.; Nitta, K.; Akizawa, T.; Matsuo, S.; Makino, H.; Ohashi, Y.; Hishida, A. Hyperbaric area index calculated from ABPM elucidates the condition of CKD patients: The CKD-JAC study. Clin. Exp. Nephrol. 2014, 19, 114–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edgley, K.; Chun, H.-Y.Y.; Whiteley, W.N.; Tsanas, A. New Insights into Stroke from Continuous Passively Collected Temperature and Sleep Data Using Wrist-Worn Wearables. Sensors 2023, 23, 1069. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social Jetlag and Obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef] [PubMed]
- Caliandro, R.; Streng, A.A.; van Kerkhof, L.W.M.; van der Horst, G.T.J.; Chaves, I. Social Jetlag and Related Risks for Human Health: A Timely Review. Nutrients 2021, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T. How can social jetlag affect health? Nat. Rev. Endocrinol. 2023, 19, 383–384. [Google Scholar] [CrossRef]
- Henderson, S.E.M.; Brady, E.M.; Robertson, N. Associations between social jetlag and mental health in young people: A systematic review. Chronobiol. Int. 2019, 36, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Hu, H.; Akter, S.; Kuwahara, K.; Kochi, T.; Eguchi, M.; Kurotani, K.; Nanri, A.; Kabe, I.; Mizoue, T. Social jetlag is associated with an increased likelihood of having depressive symptoms among the Japanese working population: The Furukawa Nutrition and Health Study. Sleep 2019, 43, zsz204. [Google Scholar] [CrossRef]
- Qu, Y.; Li, T.; Xie, Y.; Tao, S.; Yang, Y.; Zou, L.; Zhang, D.; Zhai, S.; Tao, F.; Wu, X. Association of chronotype, social jetlag, sleep duration and depressive symptoms in Chinese college students. J. Affect. Disord. 2022, 320, 735–741. [Google Scholar] [CrossRef]
- Korman, M.; Tkachev, V.; Reis, C.; Komada, Y.; Kitamura, S.; Gubin, D.; Kumar, V.; Roenneberg, T. COVID-19-mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Sci. Rep. 2020, 10, 22225. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klerman, E.B.; Phillips, A.J.K. Measuring sleep regularity: Theoretical properties and practical usage of existing metrics. Sleep 2021, 44, zsab103. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.E.; Postnova, S.; Sletten, T.L.; Rajaratnam, S.M.; Phillips, A.J. Computational approaches for individual circadian phase prediction in field settings. Curr. Opin. Syst. Biol. 2020, 22, 39–51. [Google Scholar] [CrossRef]
- Kim, D.W.; Zavala, E.; Kim, J.K. Wearable technology and systems modeling for personalized chronotherapy. Curr. Opin. Syst. Biol. 2020, 21, 9–15. [Google Scholar] [CrossRef]
- Brown, L.S.; Hilaire, M.A.S.; McHill, A.W.; Phillips, A.J.K.; Barger, L.K.; Sano, A.; Czeisler, C.A.; Doyle, F.J.; Klerman, E.B. A classification approach to estimating human circadian phase under circadian alignment from actigraphy and photometry data. J. Pineal Res. 2021, 71, e12745. [Google Scholar] [CrossRef]
- Mayer, C.; Kim, D.W.; Zhang, M.; Lee, M.P.; Forger, D.B.; Burgess, H.J.; Moon, C. Predicting circadian phase in community-dwelling later-life adults using actigraphy data. J. Sleep Res. 2024, e14425. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mayer, C.; Cheng, P.; Siddula, A.; Burgess, H.J.; Drake, C.; Goldstein, C.; Walch, O.; Forger, D.B. Predicting circadian phase across populations: A comparison of mathematical models and wearable devices. Sleep 2021, 44, zsab126. [Google Scholar] [CrossRef]
- Gubin, D.G.; Borisenkov, M.F.; Kolomeichuk, S.N.; Markov, A.A.; Weinert, D.; Cornelissen, G.; Stefani, O. Evaluating circadian light hygiene: Methodology and health implications. Russ. Open Med. J. 2024, 13, e0415. [Google Scholar] [CrossRef]
- Gubin, D.; Danilenko, K.; Stefani, O.; Kolomeichuk, S.; Markov, A.; Petrov, I.; Voronin, K.; Mezhakova, M.; Borisenkov, M.; Shigabaeva, A.; et al. Light Environment of Arctic Solstices is Coupled with Melatonin Phase-Amplitude Changes and Decline of Metabolic Health. J. Pineal Res. 2024, 77, e70023. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef]
- Gubin, D.; Boldyreva, J.; Stefani, O.; Kolomeichuk, S.; Danilova, L.; Shigabaeva, A.; Cornelissen, G.; Weinert, D. Higher vulnerability to poor circadian light hygiene in individuals with a history of COVID-19. Chronobiol. Int. 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shurkevich, N.P.; Vetoshkin, A.S.; Kareva, M.A.; Gubin, D.G. Arterial hypertension and COVID-19 in Arctic rotating shift work: The impact of chronostructure disruptions on circadian blood pressure rhythm in relation to echocardiographic parameters. Russ. Open Med. J. 2024, 13, e0408. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Quante, M.; Godbole, S.; James, P.; Hipp, J.A.; Marinac, C.R.; Mariani, S.; Feliciano, E.M.C.; Glanz, K.; Laden, F.; et al. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol. Int. 2017, 34, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Danieletto, M.; Tomalin, L.; Choi, K.H.; Zweig, M.; Golden, E.; Kaur, S.; Helmus, D.; Biello, A.; Pyzik, R.; et al. Use of Physiological Data from a Wearable Device to Identify SARS-CoV-2 Infection and Symptoms and Predict COVID-19 Diagnosis: Observational Study. J. Med. Internet Res. 2021, 23, e26107. [Google Scholar] [CrossRef]
- Doyle, M.M.; Murphy, T.E.; Miner, B.; Pisani, M.A.; Lusczek, E.R.; Knauert, M.P. Enhancing cosinor analysis of circadian phase markers using the gamma distribution. Sleep Med. 2022, 92, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gombert-Labedens, M.; Alzueta, E.; Perez-Amparan, E.; Yuksel, D.; Kiss, O.; de Zambotti, M.; Simon, K.; Zhang, J.; Shuster, A.; Morehouse, A.; et al. Using Wearable Skin Temperature Data to Advance Tracking and Characterization of the Menstrual Cycle in a Real-World Setting. J. Biol. Rhythm. 2024, 39, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Fleisch, E.; Barata, F. Circadian rhythm analysis using wearable-based accelerometry as a digital biomarker of aging and healthspan. npj Digit. Med. 2024, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic Sleep/Wake Identification From Wrist Activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef]
- Sazonov, E.; Sazonova, N.; Schuckers, S.; Neuman, M.; CHIME Study Group. Activity-based sleep–wake identification in infants. Physiol. Meas. 2004, 25, 1291–1304. [Google Scholar] [CrossRef]
- Kolodyazhniy, V.; Späti, J.; Frey, S.; Götz, T.; Wirz-Justice, A.; Kräuchi, K.; Cajochen, C.; Wilhelm, F.H. An Improved Method for Estimating Human Circadian Phase Derived From Multichannel Ambulatory Monitoring and Artificial Neural Networks. Chronobiol. Int. 2012, 29, 1078–1097. [Google Scholar] [CrossRef]
- Stone, J.E.; Aubert, X.L.; Maass, H.; Phillips, A.J.K.; Magee, M.; Howard, M.E.; Lockley, S.W.; Rajaratnam, S.M.W.; Sletten, T.L. Application of a Limit-Cycle Oscillator Model for Prediction of Circadian Phase in Rotating Night Shift Workers. Sci. Rep. 2019, 9, 11032. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Duffy, J.F. Novel Approaches for Assessing Circadian Rhythmicity in Humans: A Review. J. Biol. Rhythm. 2020, 35, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Walch, O.; Huang, Y.; Mayer, C.; Sagong, C.; Castelan, A.C.; Burgess, H.J.; Roth, T.; Forger, D.B.; Drake, C.L. Predicting circadian misalignment with wearable technology: Validation of wrist-worn actigraphy and photometry in night shift workers. Sleep 2020, 44, zsaa180. [Google Scholar] [CrossRef]
- Kim, D.W.; Mayer, C.; Lee, M.P.; Choi, S.W.; Tewari, M.; Forger, D.B. Efficient assessment of real-world dynamics of circadian rhythms in heart rate and body temperature from wearable data. J. R. Soc. Interface 2023, 20, 20230030. [Google Scholar] [CrossRef]
- Neikrug, A.B.; Chen, I.Y.; Palmer, J.R.; McCurry, S.M.; Von Korff, M.; Perlis, M.; Vitiello, M.V. Characterizing Behavioral Activity Rhythms in Older Adults Using Actigraphy. Sensors 2020, 20, 549. [Google Scholar] [CrossRef] [PubMed]
- Werkmann, V.; Glynn, N.W.; Harezlak, J. Extracting actigraphy-based walking features with structured functional principal components. Physiol. Meas. 2024, 45, 085001. [Google Scholar] [CrossRef] [PubMed]
- George, S.V.; Kunkels, Y.K.; Booij, S.; Wichers, M. Uncovering complexity details in actigraphy patterns to differentiate the depressed from the non-depressed. Sci. Rep. 2021, 11, 13447. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.H.; Doan, T.; Takasu, A. Deep Wavelet Convolutional Neural Networks for Multimodal Human Activity Recognition Using Wearable Inertial Sensors. Sensors 2023, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Al Numan, O.; Kabir, R.; Islam, R.; Watanobe, Y. A Robust Deep Feature Extraction Method for Human Activity Recognition Using a Wavelet Based Spectral Visualisation Technique. Sensors 2024, 24, 4343. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Hu, H.; Zhang, Y. Wearable Sensor-Based Human Activity Recognition Method with Multi-Features Extracted from Hilbert-Huang Transform. Sensors 2016, 16, 2048. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Zhu, J.-D. Hilbert–Huang transformation-based time-frequency analysis methods in biomedical signal applications. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zschocke, J.; Kluge, M.; Pelikan, L.; Graf, A.; Glos, M.; Müller, A.; Mikolajczyk, R.; Bartsch, R.P.; Penzel, T.; Kantelhardt, J.W. Detection and analysis of pulse waves during sleep via wrist-worn actigraphy. PLoS ONE 2019, 14, e0226843. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Lin, C.; Chang, H.-C.; Chang, J.-H.; Chuang, H.-H.; Lin, Y.-H. Developing Methods for Assessing Mental Activity Using Human-Smartphone Interactions: Comparative Analysis of Activity Levels and Phase Patterns in General Mental Activities, Working Mental Activities, and Physical Activities. J. Med. Internet Res. 2024, 26, e56144. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, S.; Wang, W.; Lei, X. Using actigraphy to assess chronotype: Simpler is better. Chronobiol. Int. 2024, 41, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Cribb, L.; Sha, R.; Yiallourou, S.; Grima, N.A.; Cavuoto, M.; Baril, A.-A.; Pase, M.P. Sleep regularity and mortality: A prospective analysis in the UK Biobank. eLife 2023, 12, RP88359. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.P.; Wolf, L.; Taillard, J.; Schlangen, L.J.M.; Salam, A.; Cajochen, C.; Gronfier, C. Chronic Artificial Blue-Enriched White Light is an Effective Countermeasure to Delayed Circadian Phase and Neurobehavioral Decrements. PLoS ONE 2014, 9, e102827. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry 2014, 26, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Weinert, D. Melatonin, circadian rhythms and glaucoma: Current perspective. Neural Regen. Res. 2022, 17, 1759–1760. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Malishevskaya, T.; Weinert, D.; Zakharova, E.; Astakhov, S.; Cornelissen, G. Circadian Disruption in Glaucoma: Causes, Consequences, and Countermeasures. Front. Biosci. 2024, 29, 410. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Kolomeichuk, S.N.; Weinert, D. Circadian clock precision, health, and longevity. J. Chronomed. 2021, 23, 3–15. [Google Scholar] [CrossRef]
- Dement, W.; Kleitman, N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. 1957, 9, 673–690. [Google Scholar] [CrossRef]
- Levi, F.; Halberg, F. Circaseptan (about-7-day) bioperiodicity—spontaneous and reactive—and the search for pacemakers. Ric. Clin. Lab. 1982, 12, 323–370. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Zarepour, A.; Zarrabi, A. Physiological Rhythms and Biological Variation of Biomolecules: The Road to Personalized Laboratory Medicine. Int. J. Mol. Sci. 2023, 24, 6275. [Google Scholar] [CrossRef]
- Madden, K.M.; Feldman, B. Weekly, Seasonal, and Geographic Patterns in Health Contemplations About Sundown Syndrome: An Ecological Correlational Study. JMIR Aging 2019, 2, e13302. [Google Scholar] [CrossRef] [PubMed]
- Seizer, L.; Cornélissen-Guillaume, G.; Schiepek, G.K.; Chamson, E.; Bliem, H.R.; Schubert, C. About-Weekly Pattern in the Dynamic Complexity of a Healthy Subject’s Cellular Immune Activity: A Biopsychosocial Analysis. Front. Psychiatry 2022, 13, 799214. [Google Scholar] [CrossRef]
- Cornelissen, G.; Hirota, T. Quo Vadis. In Chronobiology and ChronomedicineFrom Molecular and Cellular Mechanisms to Whole Body Interdigitating Networks; Cornelissen, G., Hirota, T., Eds.; Royal Society of Chemistry: London, UK, 2024; Volume 23, Chapter 24; pp. 648–664. [Google Scholar]
- Reinberg, A.E.; Dejardin, L.; Smolensky, M.H.; Touitou, Y. Seven-day human biological rhythms: An expedition in search of their origin, synchronization, functional advantage, adaptive value and clinical relevance. Chronobiol. Int. 2016, 34, 162–191. [Google Scholar] [CrossRef]
- Cajochen, C.; Altanay-Ekici, S.; Münch, M.; Frey, S.; Knoblauch, V.; Wirz-Justice, A. Evidence that the Lunar Cycle Influences Human Sleep. Curr. Biol. 2013, 23, 1485–1488. [Google Scholar] [CrossRef]
- Reinberg, A.; Smolensky, M.H.; Touitou, Y. The full moon as a synchronizer of circa-monthly biological rhythms: Chronobiologic perspectives based on multidisciplinary naturalistic research. Chronobiol. Int. 2016, 33, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Hartstein, L.E.; Wright, K.P.; Akacem, L.D.; Behn, C.D.; LeBourgeois, M.K. Evidence of circalunar rhythmicity in young children’s evening melatonin levels. J. Sleep Res. 2022, 32, e13635. [Google Scholar] [CrossRef] [PubMed]
- Lunsford-Avery, J.R.; Engelhard, M.M.; Navar, A.M.; Kollins, S.H. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci. Rep. 2018, 8, 14158, Erratum in Sci. Rep. 2020, 10, 2993. https://doi.org/10.1038/s41598-020-59762-1. [Google Scholar] [CrossRef] [PubMed]
- Pye, J.; Phillips, A.J.; Cain, S.W.; Montazerolghaem, M.; Mowszowski, L.; Duffy, S.; Hickie, I.B.; Naismith, S.L. Irregular sleep-wake patterns in older adults with current or remitted depression. J. Affect. Disord. 2020, 281, 431–437. [Google Scholar] [CrossRef]
- Wong, P.M.; Barker, D.; Roane, B.M.; Van Reen, E.; Carskadon, M.A. Sleep regularity and body mass index: Findings from a prospective study of first-year college students. SLEEP Adv. 2022, 3, zpac004. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Cribb, L.; Cavuoto, M.G.; Rowsthorn, E.; Nicolazzo, J.; Gibson, M.; Baril, A.-A.; Pase, M.P. Association of the Sleep Regularity Index with Incident Dementia and Brain Volume. Neurology 2024, 102, e208029. [Google Scholar] [CrossRef]
- Van Someren, E.J. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol. Int. 2000, 17, 313–354. [Google Scholar] [CrossRef]
- Gompper, B.; Bromundt, V.; Orgül, S.; Flammer, J.; Kräuchi, K. Phase relationship between skin temperature and sleep-wake rhythms in women with vascular dysregulation and controls under real-life conditions. Chronobiol. Int. 2010, 27, 1778–1796. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Circadian phase asessment by ambulatory monitoring in humans: Correlation with dim light melatonin onset. Chronobiol. Int. 2013, 31, 37–51. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef]
- Stone, J.E.; Phillips, A.J.K.; Ftouni, S.; Magee, M.; Howard, M.; Lockley, S.W.; Sletten, T.L.; Anderson, C.; Rajaratnam, S.M.W.; Postnova, S. Generalizability of A Neural Network Model for Circadian Phase Prediction in Real-World Conditions. Sci. Rep. 2019, 9, 11001. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54. [Google Scholar] [CrossRef]
- Zerbini, G.; Winnebeck, E.C.; Merrow, M. Weekly, seasonal, and chronotype-dependent variation of dim-light melatonin onset. J. Pineal Res. 2021, 70, e12723. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Cornelissen, G.; Halberg, F.; Bakken, E.E.; Singh, R.B.; Otsuka, K.; Tomlinson, B.; Delcourt, A.; Toussaint, G.; Bathina, S.; Schwartzkopff, O.; et al. 100 or 30 years after Janeway or Bartter, Healthwatch helps avoid “flying blind”. Biomed. Pharmacother. 2004, 58 (Suppl. S1), S69–S86. [Google Scholar] [CrossRef] [PubMed]
- Halberg, F.; Powell, D.; Otsuka, K.; Watanabe, Y.; Beaty, L.A.; Rosch, P.; Czaplicki, J.; Hillman, D.; Schwartzkopff, O.; Cornelissen, G. Diagnosing vascular variability anomalies, not only MESOR-hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H279–H294. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef] [PubMed]
- Bacalini, M.G.; Palombo, F.; Garagnani, P.; Giuliani, C.; Fiorini, C.; Caporali, L.; Maserati, M.S.; Capellari, S.; Romagnoli, M.; De Fanti, S.; et al. Association of rs3027178 polymorphism in the circadian clock gene PER1 with susceptibility to Alzheimer’s disease and longevity in an Italian population. GeroScience 2021, 44, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Neroev, V.; Malishevskaya, T.; Kolomeichuk, S.; Cornelissen, G.; Yuzhakova, N.; Vlasova, A.; Weinert, D. Depression scores are associated with retinal ganglion cells loss. J. Affect. Disord. 2023, 333, 290–296. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Martin, R.A.; Esser, K.A. Time for Exercise? Exercise and Its Influence on the Skeletal Muscle Clock. J. Biol. Rhythm. 2022, 37, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A.; Viggars, M.R.; Esser, K.A. Metabolism and exercise: The skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol. 2023, 19, 272–284. [Google Scholar] [CrossRef]
- Rogers-Soeder, T.S.; Blackwell, T.; Yaffe, K.; Ancoli-Israel, S.; Redline, S.; Cauley, J.A.; Ensrud, K.E.; Paudel, M.; Barrett-Connor, E.; LeBlanc, E.; et al. Rest-Activity Rhythms and Cognitive Decline in Older Men: The Osteoporotic Fractures in Men Sleep Study. J. Am. Geriatr. Soc. 2018, 66, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Masumoto, K.; Okada, S. Physical Activity Components that Determine Daily Life Satisfaction Among Older Adults: An Intensive Longitudinal Diary Study. Int. J. Behav. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Lombardi, D.A.; Marucci-Wellman, H.; Roenneberg, T. Chronotypes in the US—Influence of age and sex. PLoS ONE 2017, 12, e0178782. [Google Scholar] [CrossRef]
- Borisenkov, M.; Gubin, D.; Sergey, K. On the issue of adaptive fitness of chronotypes in high latitudes. Biol. Rhythm. Res. 2024, 55, 354–358. [Google Scholar] [CrossRef]
- Farabi, S.S.; Quinn, L.; Carley, D.W. Validity of Actigraphy in Measurement of Sleep in Young Adults with Type 1 Diabetes. J. Clin. Sleep Med. 2017, 13, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Xue, T. Circadian-independent light regulation of mammalian metabolism. Nat. Metab. 2024, 6, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D.; Gubin, D. The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Appl. Sci. 2022, 12, 9220. [Google Scholar] [CrossRef]
- Zambrano, C.; Garitaonaindia, M.T.; Salmerón, D.; Pérez-Sanz, F.; Tchio, C.; Picinato, M.C.; de Medina, F.S.; Luján, J.; Scheer, F.A.J.L.; Saxena, R.; et al. Melatonin decreases human adipose tissue insulin sensitivity. J. Pineal Res. 2024, 76, e12965. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D.; Waterhouse, J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 2006, 90, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Tranel, H.R.; Schroder, E.A.; England, J.; Black, W.S.; Bush, H.; Hughes, M.E.; Esser, K.A.; Clasey, J.L. Physical activity, and not fat mass is a primary predictor of circadian parameters in young men. Chronobiol. Int. 2015, 32, 832–841. [Google Scholar] [CrossRef]
- Rocher, S.D.; Bessot, N.; Sesboüé, B.; Bulla, J.; Davenne, D. Circadian Characteristics of Older Adults and Aerobic Capacity. J. Gerontol. Ser. A 2015, 71, 817–822. [Google Scholar] [CrossRef]
- Gubin, D.G.; Weinert, D.; Rybina, S.V.; Danilova, L.A.; Solovieva, S.V.; Durov, A.M.; Prokopiev, N.Y.; Ushakov, P.A. Activity, sleep and ambient light have a different impact on circadian blood pressure, heart rate and body temperature rhythms. Chronobiol. Int. 2017, 34, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Tserne, T.A.; Bakutova, L.A.; Gubin, D.G. Food addiction and emotional eating are associated with intradaily rest–activity rhythm variability. Eat. Weight Disord. 2022, 27, 3309–3316. [Google Scholar] [CrossRef]
- Healy, K.L.; Morris, A.R.; Liu, A.C. Circadian Synchrony: Sleep, Nutrition, and Physical Activity. Front. Netw. Physiol. 2021, 1, 732243. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.E.; May, A.M.; Halle, M.; Dorresteijn, J.A.N.; van der Meer, M.G.; Ruigrok, Y.M.; de Borst, G.J.; Geleijnse, J.M.; Visseren, F.L.J.; Koopal, C. Physical exercise volume, type, and intensity and risk of all-cause mortality and cardiovascular events in patients with cardiovascular disease: A mediation analysis. Eur. Heart J. Open 2023, 3, oead057. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Ławiński, J.; Rysz, J. The Impact of Aerobic Exercise on HDL Quantity and Quality: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4653. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Wang, K.; Zhang, T.; Yin, L.; Liang, J.; Yang, Y.; Luo, J. Time-Dependent Effects of Physical Activity on Cardiovascular Risk Factors in Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14194. [Google Scholar] [CrossRef] [PubMed]
- Schock, S.; Hakim, A. The Physiological and Molecular Links Between Physical Activity and Brain Health: A Review. Neurosci. Insights 2023, 18, 26331055231191523. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Cui, M.; Chai, H.; Chen, L.; Zhang, T.; Mi, J.; Guan, H.; Zhao, L. Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents. J. Transl. Intern. Med. 2023, 11, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.A.; Rahman, S.A.; Hilaire, M.A.S.; Grant, L.K.; Rüger, M.; Czeisler, C.A.; Lockley, S.W. Circadian lipid and hepatic protein rhythms shift with a phase response curve different than melatonin. Nat. Commun. 2022, 13, 2241. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Neroev, V.; Malishevskaya, T.; Kolomeichuk, S.; Weinert, D.; Yuzhakova, N.; Nelaeva, A.; Filippova, Y.; Cornelissen, G. Daytime Lipid Metabolism Modulated by CLOCK Gene Is Linked to Retinal Ganglion Cells Damage in Glaucoma. Appl. Sci. 2022, 12, 6374. [Google Scholar] [CrossRef]
- Wei, W.; Raun, S.H.; Long, J.Z. Molecular Insights from Multiomics Studies of Physical Activity. Diabetes 2024, 73, 162–168. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S. Physical activity for cognitive health promotion: An overview of the underlying neurobiological mechanisms. Ageing Res. Rev. 2023, 86, 101868. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Dillon, C.B.; Perry, I.J. Does replacing sedentary behaviour with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int. J. Behav. Nutr. Phys. Act. 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Ukropec, J.; Ukropcová, B.; Pai, M.-C. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. NeuroImage Clin. 2018, 17, 272–284. [Google Scholar] [CrossRef]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.-H.; Lyu, X.; Zushin, P.-J.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, H.; Hashimoto, C.; Nakao, R.; Oishi, K. Timing of food intake is more potent than habitual voluntary exercise to prevent diet-induced obesity in mice. Chronobiol. Int. 2018, 36, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Lu, C.; Qian, R. The circadian clock regulates metabolic responses to physical exercise. Chronobiol. Int. 2022, 39, 907–917. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Vamvini, M.; Nigro, P.; Ho, L.-L.; Galani, K.; Alvarez, M.; Tanigawa, Y.; Renfro, A.; Carbone, N.P.; Laakso, M.; et al. Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells. Cell Metab. 2022, 34, 1578–1593.e6. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’neill, J.S.; Reddy, A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Mannelli, L.D.C.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Esteras, N.; Blacker, T.S.; Zherebtsov, E.A.; Stelmashuk, O.A.; Zhang, Y.; Wigley, W.C.; Duchen, M.R.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol. 2023, 62, 102672. [Google Scholar] [CrossRef] [PubMed]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R.; States, U. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. eLife 2018, 7, e31656. [Google Scholar] [CrossRef] [PubMed]
- Fasipe, B.; Laher, I. Nrf2 modulates the benefits of evening exercise in type 2 diabetes. Sports Med. Health Sci. 2023, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Davies, N.A.; Moir, H.; Watkeys, L.; Ruffino, J.S.; Isa, S.A.; Butcher, L.R.; Hughes, M.G.; Morris, K.; Webb, R. Exercise-associated generation of PPARγ ligands activates PPARγ signaling events and upregulates genes related to lipid metabolism. J. Appl. Physiol. 2012, 112, 806–815. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014, 2014, 653017. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear Receptor Expression Links the Circadian Clock to Metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ma, S.; Cai, Y.; Wang, S.; Ren, J.; Yang, Y.; Ping, J.; Wang, X.; Zhang, Y.; Yan, H.; et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation 2023, 4, 100380. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef] [PubMed]
- Nebe, S.; Reutter, M.; Baker, D.H.; Bölte, J.; Domes, G.; Gamer, M.; Gärtner, A.; Gießing, C.; Gurr, C.; Hilger, K.; et al. Enhancing precision in human neuroscience. eLife 2023, 12, e85980. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef]
- Breasail, M.Ó.; Biswas, B.; Smith, M.D.; Mazhar, K.A.; Tenison, E.; Cullen, A.; Lithander, F.E.; Roudaut, A.; Henderson, E.J. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors 2021, 21, 8261. [Google Scholar] [CrossRef]
- Cote, A.C.; Phelps, R.J.; Kabiri, N.S.; Bhangu, J.S.; Thomas, K. Evaluation of Wearable Technology in Dementia: A Systematic Review and Meta-Analysis. Front. Med. 2021, 7, 501104. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer-Streit, B.; Forneris, C.A.; Morgan, L.C.; Van Noord, M.G.; Gaynes, B.N.; Greenblatt, A.; Wipplinger, J.; Lux, L.J.; Winkler, D.; Gartlehner, G. Light therapy for preventing seasonal affective disorder. Cochrane Database Syst. Rev. 2019, 2019, CD011269. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Gong, S.-Y.; Xia, S.-T.; Wang, Y.-L.; Peng, H.; Shen, Y.; Liu, C.-F. Light therapy: A new option for neurodegenerative diseases. Chin. Med. J. 2020, 134, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Na, K.-S.; Kim, Y.-K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2021, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W. Neuroprotective effects of melatonin in neurodegenerative and autoimmune central nervous system diseases. Encephalitis 2023, 3, 44–53. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, J.; Liu, Y.; Dong, G.-H.; Yang, B.-Y.; Li, N.; Wang, L.; Chen, G.; Li, S.; Guo, Y. Long-term exposure to outdoor light at night and mild cognitive impairment: A nationwide study in Chinese veterans. Sci. Total. Environ. 2022, 847, 157441. [Google Scholar] [CrossRef]
- Mazzoleni, E.; Vinceti, M.; Costanzini, S.; Garuti, C.; Adani, G.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Salemme, S.; et al. Outdoor artificial light at night and risk of early-onset dementia: A case-control study in the Modena population, Northern Italy. Heliyon 2023, 9, e17837. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Harper, D.G.; Manning, B.C.; Goldstein, R.; Satlin, A. Sundowning and Circadian Rhythms in Alzheimer’s Disease. Am. J. Psychiatry 2001, 158, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Valletta, M.; Trebbastoni, A.; Sarli, G.; D’antonio, F.; Tariciotti, L.; de Lena, C.; Bruno, G. Sundowning in Dementia: Clinical Relevance, Pathophysiological Determinants, and Therapeutic Approaches. Front. Med. 2016, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.T.; Valletta, M.; Trebbastoni, A.; D’antonio, F.; Talarico, G.; Campanelli, A.; Monti, M.S.; Salati, E.; Gasparini, M.; Buscarnera, S.; et al. Sundowning in Patients with Dementia: Identification, Prevalence, and Clinical Correlates. J. Alzheimer’s Dis. 2023, 94, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, L.; Yu, H.; Liu, M.; Zhao, W. Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer’s disease (Review). Mol. Med. Rep. 2016, 13, 3397–3400. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2015, 79, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lv, Q.-K.; Xie, W.-Y.; Gong, S.-Y.; Zhuang, S.; Liu, J.-Y.; Mao, C.-J.; Liu, C.-F. Circadian disruption and sleep disorders in neurodegeneration. Transl. Neurodegener. 2023, 12, 8. [Google Scholar] [CrossRef]
- Gracitelli, C.P.; Duque-Chica, G.L.; Roizenblatt, M.; Moura, A.L.; Nagy, B.V.; Ragot de Melo, G.; Borba, P.D.; Teixeira, S.H.; Tufik, S.; Ventura, D.F.; et al. Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology 2015, 122, 1139–1148. [Google Scholar] [CrossRef]
- Mahanna-Gabrielli, E.; Kuwayama, S.; Tarraf, W.; Kaur, S.; DeBuc, D.C.; Cai, J.; Daviglus, M.L.; Joslin, C.E.; Lee, D.J.; Mendoza-Santiesteban, C.; et al. The Effect of Self-Reported Visual Impairment and Sleep on Cognitive Decline: Results of the Hispanic Community Health Study/Study of Latinos. J. Alzheimer’s Dis. 2023, 92, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Udry, M.; El Wardani, M.; Münch, M. Can Extra Daytime Light Exposure Improve Well-Being and Sleep? A Pilot Study of Patients with Glaucoma. Front. Neurol. 2021, 11, 584479. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Bromundt, V.; Frey, S.; Schlote, T.; Goldblum, D.; Cajochen, C. Cross-sectional study of intraocular cataract lens replacement, circadian rest–activity rhythms, and sleep quality in older adults. Sleep 2022, 45, zsac027. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Boldyreva, J.; Stefani, O.; Kolomeichuk, S.; Danilova, L.; Markov, A.; Shigabaeva, A.; Cornelissen, G.; Weinert, D. Light exposure predicts COVID-19 negative status in young adults. Biol. Rhythm. Res. 2024, 55, 1–12. [Google Scholar] [CrossRef]
- Shen, B.; Ma, C.; Wu, G.; Liu, H.; Chen, L.; Yang, G. Effects of exercise on circadian rhythms in humans. Front. Pharmacol. 2023, 14, 1282357. [Google Scholar] [CrossRef]
- Weinert, D.; Schöttner, K.; Müller, L.; Wienke, A. Intensive voluntary wheel running may restore circadian activity rhythms and improves the impaired cognitive performance of arrhythmic Djungarian hamsters. Chronobiol. Int. 2016, 33, 1161–1170. [Google Scholar] [CrossRef]
- Weinert, D.; Weinandy, R.; Gattermann, R. Photic and non-photic effects on the daily activity pattern of Mongolian gerbils. Physiol. Behav. 2007, 90, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fritzsche, P.; Weinert, D. Novel object recognition of Djungarian hamsters depends on circadian time and rhythmic phenotype. Chronobiol. Int. 2014, 32, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Weinert, D. Individual recognition of social rank and social memory performance depends on a functional circadian system. Behav. Process. 2016, 132, 85–93. [Google Scholar] [CrossRef]
- Iwayama, K.; Seol, J.; Tokuyama, K. Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h. Nutrients 2023, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Ives, S.J.; Mohr, A.E.; Robinson, N.; Escudero, D.; Robinson, J.; Rose, K.; Minicucci, O.; O’brien, G.; Curran, K.; et al. Morning Exercise Reduces Abdominal Fat and Blood Pressure in Women; Evening Exercise Increases Muscular Performance in Women and Lowers Blood Pressure in Men. Front. Physiol. 2022, 13, 893783. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, N.A.; McPhillips, M.V.; Petrovsky, D.V.; Perez, A.; Talwar, S.; Gooneratne, N.; Riegel, B.; Aryal, S.; Gitlin, L.N. Timed Activity to Minimize Sleep Disturbance in People with Cognitive Impairment. Innov. Aging 2023, 8, igad132. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.; Kim, N.; Ko, K.S.; Rhee, B.D.; Park, B.J.; Han, J. Morning and evening exercise. Integr. Med. Res. 2013, 2, 139–144. [Google Scholar] [CrossRef]
- Smith, P.J.; Merwin, R.M. The Role of Exercise in Management of Mental Health Disorders: An Integrative Review. Annu. Rev. Med. 2021, 72, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase–response curves for exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Feng, H.; Chen, Y.; Jin, X.; Xue, H.; Zhou, M.; Ma, H.; Ai, S.; Wing, Y.-K.; Geng, Q.; et al. Joint association of physical activity and sleep duration with risk of all-cause and cause-specific mortality: A population-based cohort study using accelerometry. Eur. J. Prev. Cardiol. 2023, 30, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, Y.; Feng, H.; Zhou, M.; Chan, J.W.; Liu, Y.; Kong, A.P.S.; Tan, X.; Wing, Y.-K.; Liang, Y.Y.; et al. Association of accelerometer-measured sleep duration and different intensities of physical activity with incident type 2 diabetes in a population-based cohort study. J. Sport Health Sci. 2023, 13, 222–232. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Choi, E.-K.; Rhee, T.-M.; Choi, J.; Lee, K.-Y.; Kwon, S.; Lee, S.-R.; Oh, S.; Lip, G.Y.H. Accelerometer-derived physical activity and the risk of death, heart failure, and stroke in patients with atrial fibrillation: A prospective study from UK Biobank. Br. J. Sports Med. 2024, 58, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Ahmadi, M.N.; Friedenreich, C.M.; Blodgett, J.M.; Koster, A.; Holtermann, A.; Atkin, A.; Rangul, V.; Sherar, L.B.; Teixeira-Pinto, A.; et al. Vigorous Intermittent Lifestyle Physical Activity and Cancer Incidence Among Nonexercising Adults. JAMA Oncol. 2023, 9, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Al-Alusi, M.A.; Churchill, T.W.; Guseh, J.S.; Ellinor, P.T. Accelerometer-Derived “Weekend Warrior” Physical Activity and Incident Cardiovascular Disease. JAMA 2023, 330, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lin, Y.; Chen, L.; Huang, T.; Lin, T.; He, J.; Lu, X.; Chen, X.; Wang, Y.; Ye, Q.; et al. Association of physical activity pattern and risk of Parkinson’s disease. npj Digit. Med. 2024, 7, 137. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Doherty, A.; Smith-Byrne, K.; Rahimi, K.; Bennett, D.; Woodward, M.; Walmsley, R.; Dwyer, T. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank cohort study. PLoS Med. 2021, 18, e1003487, Erratum in PLoS Med. 2021, 18, e1003809. [Google Scholar] [CrossRef]
- Shreves, A.H.; Small, S.R.; Travis, R.C.; Matthews, C.E.; Doherty, A. Dose-response of accelerometer-measured physical activity, step count, and cancer risk in the UK Biobank: A prospective cohort analysis. Lancet 2023, 402 (Suppl. S1), S83. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.L.; Saint-Maurice, P.F.; Doherty, A.; Fensom, G.K.; Freeman, J.R.; Gorzelitz, J.S.; Jin, D.; McClain, K.M.; Papier, K.; Patel, S.; et al. Association of Accelerometer-Measured Physical Activity Level with Risks of Hospitalization for 25 Common Health Conditions in UK Adults. JAMA Netw. Open 2023, 6, e2256186. [Google Scholar] [CrossRef]
- Hooker, S.P.; Diaz, K.M.; Blair, S.N.; Colabianchi, N.; Hutto, B.; McDonnell, M.N.; Vena, J.E.; Howard, V.J. Association of Accelerometer-Measured Sedentary Time and Physical Activity with Risk of Stroke Among US Adults. JAMA Netw. Open 2022, 5, e2215385. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Kim, B.; Yang, J.-G.; Park, H.-J.; Jang, M.; Son, H.-E.; Kim, G.-M.; Park, H. The Relationship between Chronotype, Physical Activity and the Estimated Risk of Dementia in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 3701. [Google Scholar] [CrossRef] [PubMed]
- Soldan, A.; Alfini, A.; Pettigrew, C.; Faria, A.; Hou, X.; Lim, C.; Lu, H.; Spira, A.P.; Zipunnikov, V.; Albert, M.; et al. Actigraphy-estimated physical activity is associated with functional and structural brain connectivity among older adults. Neurobiol. Aging 2022, 116, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mahindru, A.; Patil, P.; Agrawal, V. Role of Physical Activity on Mental Health and Well-Being: A Review. Cureus 2023, 15, e33475. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yang, L.; Liang, Y.Y.; Ai, S.; Liu, Y.; Liu, Y.; Jin, X.; Lei, B.; Wang, J.; Zheng, N.; et al. Associations of timing of physical activity with all-cause and cause-specific mortality in a prospective cohort study. Nat. Commun. 2023, 14, 930. [Google Scholar] [CrossRef]
- Brito, L.C.; Marin, T.C.; Azevêdo, L.; Rosa-Silva, J.M.; Shea, S.A.; Thosar, S.S. Chronobiology of Exercise: Evaluating the Best Time to Exercise for Greater Cardiovascular and Metabolic Benefits. Compr. Physiol. 2022, 12, 3621–3639. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Bürki, C.; Westerman, K.E.; Patel, C.J. Association between timing and consistency of physical activity and type 2 diabetes: A cohort study on participants of the UK Biobank. Diabetologia 2023, 66, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Homolka, P.; Cornelissen, G.; Homolka, A.; Siegelova, J.; Halberg, F. Exercise-associated transient circadian hypertension (CHAT)? In Proceedings of the III International Conference, Civilization Diseases in the Spirit of V.I. Vernadsky, People’s Friendship University of Russia, Moscow, Russia, 10–12 October 2005; pp. 419–421. [Google Scholar]
- Bano-Otalora, B.; Martial, F.; Harding, C.; Bechtold, D.A.; Allen, A.E.; Brown, T.M.; Belle, M.D.C.; Lucas, R.J. Bright daytime light enhances circadian amplitude in a diurnal mammal. Proc. Natl. Acad. Sci. USA 2021, 118, e2100094118. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Nowozin, C.; Regente, J.; Bes, F.; De Zeeuw, J.; Hädel, S.; Wahnschaffe, A.; Kunz, D. Blue-Enriched Morning Light as a Countermeasure to Light at the Wrong Time: Effects on Cognition, Sleepiness, Sleep, and Circadian Phase. Neuropsychobiology 2016, 74, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, M.G.; Sahin, L.; Kalsher, M.; Plitnick, B.; Rea, M.S. Long-Term, All-Day Exposure to Circadian-Effective Light Improves Sleep, Mood, and Behavior in Persons with Dementia. J. Alzheimer’s Dis. Rep. 2020, 4, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Juda, M.; Liu-Ambrose, T.; Feldman, F.; Suvagau, C.; Mistlberger, R.E. Light in the Senior Home: Effects of Dynamic and Individual Light Exposure on Sleep, Cognition, and Well-Being. Clocks Sleep 2020, 2, 557–576. [Google Scholar] [CrossRef]
- Zang, L.; Liu, X.; Li, Y.; Liu, J.; Lu, Q.; Zhang, Y.; Meng, Q. The effect of light therapy on sleep disorders and psychobehavioral symptoms in patients with Alzheimer’s disease: A meta-analysis. PLoS ONE 2023, 18, e0293977. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Gubin, G.D.; Waterhouse, J.; Weinert, D. The Circadian Body Temperature Rhythm in the Elderly: Effect of Single Daily Melatonin Dosing. Chronobiol. Int. 2006, 23, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Javid, A.Z.; Zakerkish, M.; Yousefimanesh, H.A.; Haghighi-Zadeh, M.H. Effects of melatonin supplementation in patients with type 2 diabetes mellitus and chronic periodontitis under nonsurgical periodontal therapy: A double-blind randomized controlled trial. J. Res. Med. Sci. 2022, 27, 52. [Google Scholar] [CrossRef]
- Böhmer, M.N.; Oppewal, A.; Valstar, M.J.; Bindels, P.J.E.; van Someren, E.J.W.; Maes-Festen, D.A.M. Light up: An intervention study of the effect of environmental dynamic lighting on sleep–wake rhythm, mood and behaviour in older adults with intellectual disabilities. J. Intellect. Disabil. Res. 2022, 66, 756–781. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A Phase Response Curve to Single Bright Light Pulses in Human Subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Ahmed, S.; Jackson, J.M.L.; Sack, R.L. Melatonin Shifts Human Orcadian Rhythms According to a Phase-Response Curve. Chronobiol. Int. 1992, 9, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Revell, V.L.; Molina, T.A.; Eastman, C.I. Human Phase Response Curves to Three Days of Daily Melatonin: 0.5 mg Versus 3.0 mg. J. Clin. Endocrinol. Metab. 2010, 95, 3325–3331. [Google Scholar] [CrossRef]
- Partonen, T. Chronotype and Health Outcomes. Curr. Sleep Med. Rep. 2015, 1, 205–211. [Google Scholar] [CrossRef]
- Knutson, K.L.; von Schantz, M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol. Int. 2018, 35, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Paul, J.; Giardina, E.G.V.; Liao, M.; Aggarwal, B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol. Int. 2020, 37, 673–685. [Google Scholar] [CrossRef]
- Baldanzi, G.; Hammar, U.; Fall, T.; Lindberg, E.; Lind, L.; Elmståhl, S.; Theorell-Haglöw, J. Evening chronotype is associated with elevated biomarkers of cardiometabolic risk in the EpiHealth cohort: A cross-sectional study. Sleep 2021, 45, zsab226. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Burr, R.L.; Van Someren, E.J.W.; Hubbard, E.M.; Luxenberg, J.S.; Mastick, J.; Cooper, B.A. Melatonin and Bright-Light Treatment for Rest–Activity Disruption in Institutionalized Patients with Alzheimer’s Disease. J. Am. Geriatr. Soc. 2008, 56, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Riemersma-van der Lek, R.F.; Swaab, D.F.; Twisk, J.; Hol, E.M.; Hoogendijk, W.J.; Van Someren, E.J. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA 2008, 299, 2642–2655. [Google Scholar] [CrossRef]
- McCurry, S.M.; Pike, K.C.; Vitiello, M.V.; Logsdon, R.G.; Larson, E.B.; Teri, L. Increasing Walking and Bright Light Exposure to Improve Sleep in Community-Dwelling Persons with Alzheimer’s Disease: Results of a Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2011, 59, 1393–1402. [Google Scholar] [CrossRef]
- Hand, A.J.; Stone, J.E.; Shen, L.; Vetter, C.; Cain, S.W.; Bei, B.; Phillips, A.J.K. Measuring light regularity: Sleep regularity is associated with regularity of light exposure in adolescents. Sleep 2023, 46, zsad001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, H.; Sun, Q.; Li, J.; Heianza, Y.; Van Dam, R.M.; Hu, F.B.; Rimm, E.; Manson, J.E.; Qi, L. Coffee drinking timing and mortality in US adults. Eur. Heart J. 2025, ehae871. [Google Scholar] [CrossRef]
- Duncan, M.J.; Dobell, A.P.; Caygill, C.L.; Eyre, E.; Tallis, J. The effect of acute caffeine ingestion on upper body anaerobic exercise and cognitive performance. Eur. J. Sport Sci. 2018, 19, 103–111. [Google Scholar] [CrossRef]
- Xue, H.; Zou, Y.; Yang, Q.; Zhang, Z.; Zhang, J.; Wei, X.; Zhou, J.; Tao, X.L.; Zhang, C.; Xia, Y.; et al. The association between different physical activity (PA) patterns and cardiometabolic index (CMI) in US adult population from NHANES (2007–2016). Heliyon 2024, 10, e28792. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, D.; Wei, Z.; Liu, P.; Yang, Q.; Zhang, L.; Zhang, J.; Zhang, C.; Xue, H.; Xie, Z.; et al. Whether weekend warriors (WWs) achieve equivalent benefits in lipid accumulation products (LAP) reduction as other leisure-time physical activity patterns? -Results from a population-based analysis of NHANES 2007–2018. BMC Public Health 2024, 24, 1550. [Google Scholar] [CrossRef]
- Piarulli, A.; Bergamasco, M.; Thibaut, A.; Cologan, V.; Gosseries, O.; Laureys, S. EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 2016, 263, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.H.; Maloney, S.K.; Mark, P.J.; Blache, D. Episodic Ultradian Events—Ultradian Rhythms. Biology 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Baria, A.T.; Baliki, M.N.; Parrish, T.; Apkarian, A.V. Anatomical and Functional Assemblies of Brain BOLD Oscillations. J. Neurosci. 2011, 31, 7910–7919. [Google Scholar] [CrossRef]
- Chang, C.; Metzger, C.D.; Glover, G.H.; Duyn, J.H.; Heinze, H.-J.; Walter, M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage 2013, 68, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.E.; Glover, G.H. BOLD fractional contribution to resting-state functional connectivity above 0.1 Hz. NeuroImage 2015, 107, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Cornelissen, G.; Furukawa, S.; Shibata, K.; Kubo, Y.; Mizuno, K.; Aiba, T.; Ohshima, H.; Mukai, C. Unconscious mind activates central cardiovascular network and promotes adaptation to microgravity possibly anti-aging during 1-year-long spaceflight. Sci. Rep. 2022, 12, 11862. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Cornelissen, G.; Kubo, Y.; Shibata, K.; Mizuno, K.; Aiba, T.; Furukawa, S.; Ohshima, H.; Mukai, C. Methods for assessing change in brain plasticity at night and psychological resilience during daytime between repeated long-duration space missions. Sci. Rep. 2023, 13, 10909. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, U. The Pulse-Respiration Quotient: A Powerful but Untapped Parameter for Modern Studies About Human Physiology and Pathophysiology. Front. Physiol. 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Rafl, J.; Bachman, T.E.; Rafl-Huttova, V.; Walzel, S.; Rozanek, M. Commercial smartwatch with pulse oximeter detects short-time hypoxemia as well as standard medical-grade device: Validation study. Digit. Health 2022, 8, 20552076221132127. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Vollam, S.; Pimentel, M.A.; Areia, C.; Young, L.; Roman, C.; Ede, J.; Piper, P.; King, E.; Harford, M.; et al. The Use of Wearable Pulse Oximeters in the Prompt Detection of Hypoxemia and During Movement: Diagnostic Accuracy Study. J. Med. Internet Res. 2022, 24, e28890. [Google Scholar] [CrossRef]
- Charlton, P.H.; Kyriacou, P.A.; Mant, J.; Marozas, V.; Chowienczyk, P.; Alastruey, J. Wearable Photoplethysmography for Cardiovascular Monitoring. Proc. IEEE Inst. Electr. Electron. Eng. 2022, 110, 355–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charlton, P.H.; Allen, J.; Bailón, R.; Baker, S.; Behar, J.A.; Chen, F.; Clifford, G.D.; Clifton, D.A.; Davies, H.J.; Ding, C.; et al. The 2023 wearable photoplethysmography roadmap. Physiol. Meas. 2023, 44, 111001. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Lin, J. Probing depression, schizophrenia, and other psychiatric disorders using fNIRS and the verbal fluency test: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 140, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Nishi, R.; Fukumoto, T.; Asakawa, A. Possible effect of natural light on emotion recognition and the prefrontal cortex: A scoping review of near-infrared (NIR) spectroscopy. Adv. Clin. Exp. Med. 2023, 32, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Direito, B.; Lührs, M.; Castelo-Branco, M.; Sousa, T. Multimodal assessment of the spatial correspondence between fNIRS and fMRI hemodynamic responses in motor tasks. Sci. Rep. 2023, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional Magnetic Resonance Imaging and Functional Near-Infrared Spectroscopy: Insights from Combined Recording Studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Orban, C.; Kong, R.; Li, J.; Chee, M.W.L.; Yeo, B.T.T. Time of day is associated with paradoxical reductions in global signal fluctuation and functional connectivity. PLoS Biol. 2020, 18, e3000602. [Google Scholar] [CrossRef]
- Murata, E.M.; Pritschet, L.; Grotzinger, H.; Taylor, C.M.; Jacobs, E.G. Circadian rhythms tied to changes in brain morphology in a densely-sampled male. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Brothers, M.C.; DeBrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms. Acc. Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, B.; Lin, K.-C.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. A Sweat-based Wearable Enabling Technology for Real-time Monitoring of IL-1β and CRP as Potential Markers for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Lin, K.-C.; Whang, J.; Shahub, S.; Churcher, N.K.; Helmus, D.; Muthukumar, S.; Sands, B.; Prasad, S. Longitudinal monitoring of IL-6 and CRP in inflammatory bowel disease using IBD-AWARE. Biosens. Bioelectron. X 2024, 16, 100435. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Feng, S.; Gao, Z.; Chen, R.; Cai, G.; Bian, S. Wearable Microfluidic Sweat Chip for Detection of Sweat Glucose and pH in Long-Distance Running Exercise. Biosensors 2023, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Ok, J.; Park, S.; Jung, Y.H.; Kim, T. Wearable and Implantable Cortisol-Sensing Electronics for Stress Monitoring. Adv. Mater. 2023, 36, e2211595. [Google Scholar] [CrossRef] [PubMed]
- Watkins, Z.; McHenry, A.; Heikenfeld, J. Wearing the Lab: Advances and Challenges in Skin-Interfaced Systems for Continuous Biochemical Sensing. Adv. Biochem. Eng. Biotechnol. 2024, 187, 223–282. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Zhao, W.; Liu, C.; Zhou, S.; Ibrahim, O.O.; Wang, C.; Wang, Q. Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications. Nanomaterials 2024, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yang, H.; Tsujimura, S. Nature-Inspired Superhydrophilic Biosponge as Structural Beneficial Platform for Sweating Analysis Patch. Adv. Sci. 2024, 11, e2401947. [Google Scholar] [CrossRef]
- Brown, T.M. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J. Pineal Res. 2020, 69, e12655. [Google Scholar] [CrossRef] [PubMed]
- van Duijnhoven, J.; Hartmeyer, S.; Didikoğlu, A.; Stefani, O.; Houser, K.; Kalavally, V.; Spitschan, M. Overview of wearable light loggers: State-of-the-art and outlook. TechRxiv 2024. [Google Scholar] [CrossRef]
- Giménez, M.C.; Stefani, O.; Cajochen, C.; Lang, D.; Deuring, G.; Schlangen, L.J.M. Predicting melatonin suppression by light in humans: Unifying photoreceptor-based equivalent daylight illuminances, spectral composition, timing and duration of light exposure. J. Pineal Res. 2022, 72, e12786. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.Q.; Bodrogi, P.; Khanh, T.Q. Determination and Measurement of Melanopic Equivalent Daylight (D65) Illuminance (mEDI) in the Context of Smart and Integrative Lighting. Sensors 2023, 23, 5000. [Google Scholar] [CrossRef] [PubMed]
- Stampfli, J.; Schrader, B.; di Battista, C.; Häfliger, R.; Schälli, O.; Wichmann, G.; Zumbühl, C.; Blattner, P.; Cajochen, C.; Lazar, R.; et al. The Light-Dosimeter: A new device to help advance research on the non-visual responses to light. Light. Res. Technol. 2023, 55, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-Endocrinology 2012, 4, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.C.; Luxwolda, M.; Van Stipriaan, E.G.; Bollen, P.P.; Hoekman, R.L.; Koopmans, M.A.; Arany, P.R.; Krames, M.R.; Berends, A.C.; Hut, R.A.; et al. Effects of Near-Infrared Light on Well-Being and Health in Human Subjects with Mild Sleep-Related Complaints: A Double-Blind, Randomized, Placebo-Controlled Study. Biology 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, F.; Wang, T.; Wang, W.; Zhang, D. The impact of sunlight exposure on brain structural markers in the UK Biobank. Sci. Rep. 2024, 14, 10313. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Kallioğlu, M.A.; Sharma, A.; Kallioğlu, A.; Kumar, S.; Khargotra, R.; Singh, T. UV index-based model for predicting synthesis of (pre-)vitamin D3 in the mediterranean basin. Sci. Rep. 2024, 14, 3541. [Google Scholar] [CrossRef] [PubMed]
- Hacker, E.; Horsham, C.; Allen, M.; Nathan, A.; Lowe, J.; Janda, M. Capturing Ultraviolet Radiation Exposure and Physical Activity: Feasibility Study and Comparison Between Self-Reports, Mobile Apps, Dosimeters, and Accelerometers. JMIR Res. Protoc. 2018, 7, e102. [Google Scholar] [CrossRef] [PubMed]
- Kervezee, L.; Dashti, H.S.; Pilz, L.K.; Skarke, C.; Ruben, M.D. Using routinely collected clinical data for circadian medicine: A review of opportunities and challenges. PLoS Digit. Health 2024, 3, e0000511. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Zauner, J.; Tengelin, M.N.; Bouroussis, C.A.; Caspar, P.; Eloi, F. Illuminating the future of wearable light metrology: Overview of the MeLiDos Project. Measurement 2024, 235, 114909. [Google Scholar] [CrossRef]
- Spitschan, M. Selecting, implementing and evaluating control and placebo conditions in light therapy and light-based interventions. Ann. Med. 2024, 56, 2298875. [Google Scholar] [CrossRef]
- Bowman, C.; Huang, Y.; Walch, O.J.; Fang, Y.; Frank, E.; Tyler, J.; Mayer, C.; Stockbridge, C.; Goldstein, C.; Sen, S.; et al. A method for characterizing daily physiology from widely used wearables. Cell Rep. Methods 2021, 1, 100058. [Google Scholar] [CrossRef] [PubMed]
- Buekers, J.; Theunis, J.; De Boever, P.; Vaes, A.W.; Koopman, M.; Janssen, E.V.; Wouters, E.F.; Spruit, M.A.; Aerts, J.-M. Wearable Finger Pulse Oximetry for Continuous Oxygen Saturation Measurements During Daily Home Routines of Patients with Chronic Obstructive Pulmonary Disease (COPD) Over One Week: Observational Study. JMIR mHealth uHealth 2019, 7, e12866. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Li, J.; Jiang, Y.; Xie, S.; Shi, Y.; Pan, L. Skin-Attachable Sensors for Biomedical Applications. Biomed. Mater. Devices 2022, 1, 256–268. [Google Scholar] [CrossRef]
- You, Y.; Liu, J.; Li, X.; Wang, P.; Liu, R.; Ma, X. Relationship between accelerometer-measured sleep duration and Stroop performance: A functional near-infrared spectroscopy study among young adults. PeerJ 2024, 12, e17057. [Google Scholar] [CrossRef] [PubMed]
- Emish, M.; Young, S.D. Remote Wearable Neuroimaging Devices for Health Monitoring and Neurophenotyping: A Scoping Review. Biomimetics 2024, 9, 237. [Google Scholar] [CrossRef]
- Huang, Y.; Mayer, C.; Walch, O.J.; Bowman, C.; Sen, S.; Goldstein, C.; Tyler, J.; Forger, D.B. Distinct Circadian Assessments from Wearable Data Reveal Social Distancing Promoted Internal Desynchrony Between Circadian Markers. Front. Digit. Health 2021, 3, 727504. [Google Scholar] [CrossRef] [PubMed]
- Braund, T.A.; Zin, M.T.; Boonstra, T.W.; Wong, Q.J.J.; Larsen, M.E.; Christensen, H.; Tillman, G.; O’dea, B. Smartphone Sensor Data for Identifying and Monitoring Symptoms of Mood Disorders: A Longitudinal Observational Study. JMIR Ment. Health 2022, 9, e35549. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McMahon, M.; Fritz, H.; Schnyer, D.M. circadian rhythms are not captured equal: Exploring Circadian metrics extracted by differentcomputational methods from smartphone accelerometer and GPS sensors in daily life tracking. Digit. Health 2022, 8, 20552076221114201. [Google Scholar] [CrossRef]
- Chuang, H.H.; Lin, C.; Lee, L.A.; Chang, H.C.; She, G.J.; Lin, Y.H. Comparing Human-Smartphone Interactions and Actigraphy Measurements for Circadian Rhythm Stability and Adiposity: Algorithm Development and Validation Study. J. Med. Internet. Res. 2024, 26, e50149. [Google Scholar] [CrossRef]
- Innominato, P.F.; Macdonald, J.H.; Saxton, W.; Longshaw, L.; Granger, R.; Naja, I.; Allocca, C.; Edwards, R.; Rasheed, S.; Folkvord, F.; et al. Digital Remote Monitoring Using an mHealth Solution for Survivors of Cancer: Protocol for a Pilot Observational Study. JMIR Res. Protoc. 2024, 13, e52957. [Google Scholar] [CrossRef] [PubMed]
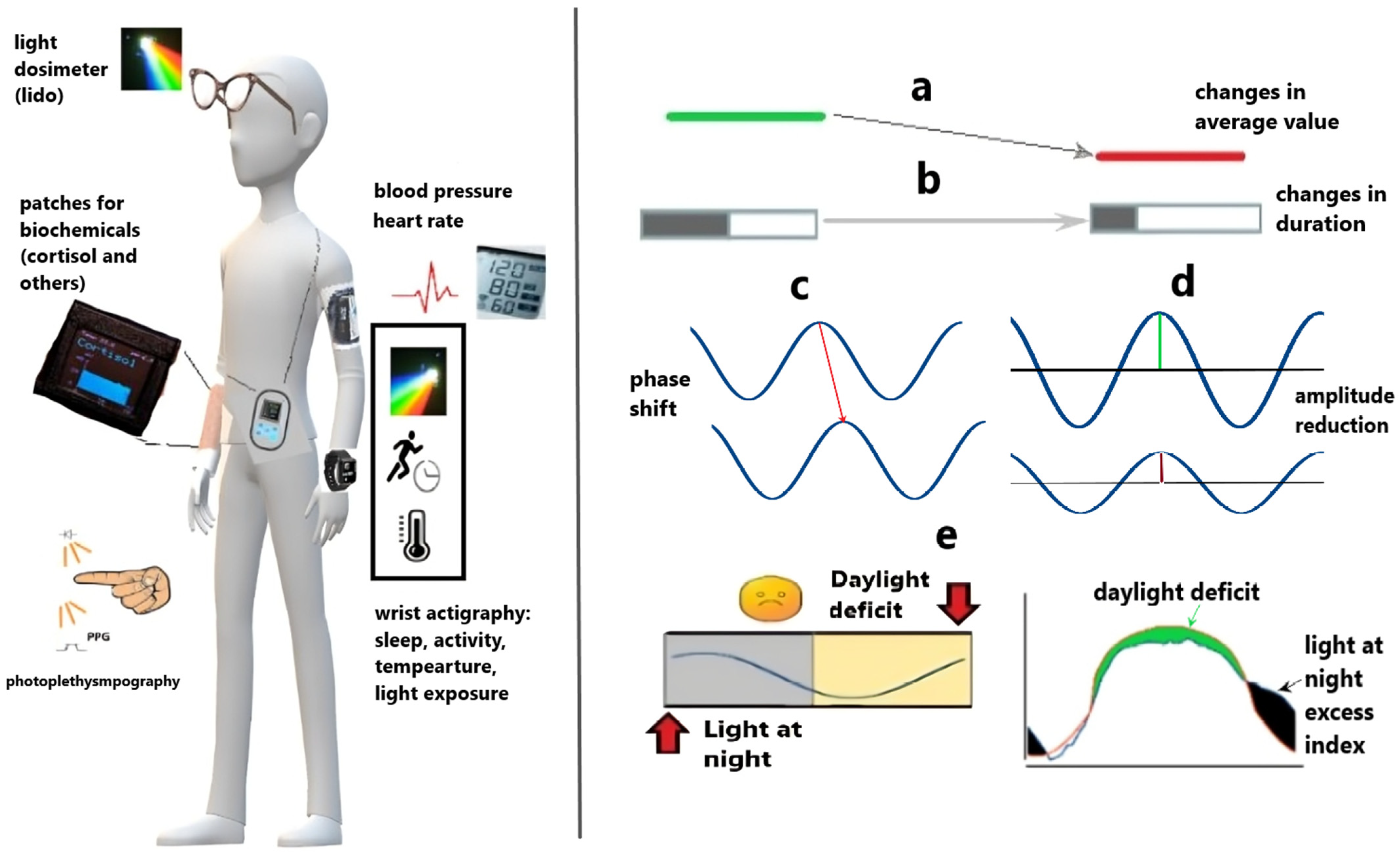
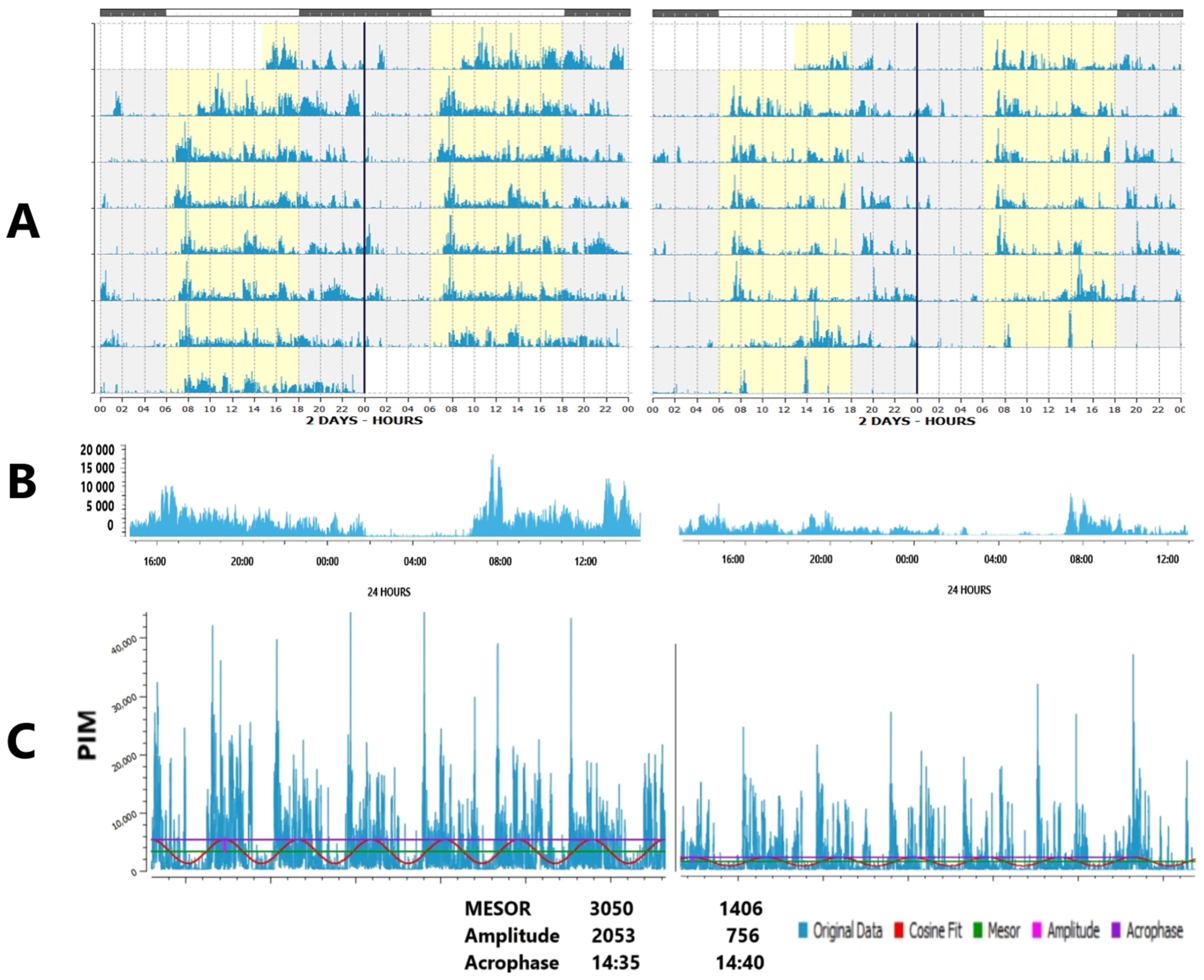

| Accelerometry-Based: | Others: |
|---|---|
| Actigraphs: usually wrist-worn or hip-worn devices that track movement to estimate sleep patterns over extended periods, some can also measure physical activity, skin and ambient temperature, and light exposure. Issues to resolve: lack of unified standards for quality of minimal sampling requirements, light sensors are distant from organ of vision and can be covered by clothes. Poor precision in assessment of sleep latency. | Heart Rate Monitors: can be used as stand-alone devices or integrated into other wearables, offering data on heart rate variability, which can correlate with cardiovascular health, stress and sleep quality. Limitation: highly variable and depends on various external and internal factors. |
| Sleep Trackers: specialized watches and bands that monitor sleep stages, and overall sleep quality. Merit: can be used more widely than professional devices. Issues include inconsistent and limited accuracy across devices, as well as reduced reliability when compared to professional actigraphs. | Blood Pressure Monitors: Blood pressure monitors can be utilized in chronomedicine to track 24 h changes in blood pressure throughout the day and night and how these changes vary from day to day. Limitation: regarded obtrusive by most users, interfere with quality of sleep. |
| Smart Watches: commercially available devices that come with built-in features to track physical activity. Merit: can be used more widely than professional devices. Issues include inconsistent and limited accuracy across devices, as well as reduced reliability when compared to professional actigraphs. | Continuous Glucose Monitors (CGMs): while not exclusively for circadian research, CGMs can provide data on metabolic changes throughout the day and night. From the viewpoint of diabetes research, glucose variability is now considered as important a biomarker as the A1c. Sensitive to meal regimens and contents, which can be both limitation and merit depending on purpose of research. However, CGMs are invaluable for health in patients with diabetes. |
| Fitness Trackers: similar to smartwatches, these devices track steps, activity, heart rate, all of which can be relevant to circadian rhythms. Merit: can be used more widely than professional devices. Issues include inconsistent and limited accuracy across devices, as well as reduced reliability when compared to professional actigraphs. | Photopletysmography (PPG) Monitors: PPG monitors can be used to measure changes in blood volume in the body, providing valuable data on heart rate variability and respiration rate. By analyzing these metrics over a 24 h period, healthcare providers can gain insight into an individual’s circadian rhythm and identify any deviations or abnormalities that may be indicative of increased risk of disease. Benefits: cost-effective, user-friendly, non-invasive, real-time monitoring of cardiovascular metrics. Limitations: sensitivity to motion artifacts, variability in accuracy based on skin characteristics. |
| Smart Rings: devices like the Oura Ring track sleep, temperature, and activity, and light providing insights into 24 h rhythms. Merits and issues are overall similar to smart watches and fitness trackers. Can be regarded as more convenient by some users. Light sensors are less dependent on clothes, while depend on gloves in location with low ambient temperature. | Wearables for Monitoring Biochemicals: wearables that can monitor biochemicals such as C-reactive protein, interleukin-1b, and cortisol can provide important information on inflammation levels, immune system function, and stress levels throughout the day and night. By tracking these biochemical markers over time, healthcare providers can better understand how individuals’ circadian rhythm may be influencing their overall health and make informed treatment decisions. Merits: offers unique opportunity to monitor biochemicals and gene expression providing unprecedented personalized insight into non-invasive health tracking. Limitations: newly evolving field, which encounters challenges of data accuracy, interpretation, privacy concerns, and the complexities of obtaining necessary approvals and compliance with health regulations. |
| Circadian Disruption Marker | Typical Deviation | Alternatives/Comments |
|---|---|---|
| Circadian rhythm measures | ||
| Amplitude | ↓ [11,12,36,38,56,61,62,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | ↑ Transient elevation of amplitude due to jet lag, or shift work, over-swinging functions such as blood pressure [84,85,86,87] |
| Phase | Delay → [61,72,79,80,81,82,88,89,90,91] | Both delay → or advance ← (optimal phase position is determined by circadian clock precision [81,91,92,93] |
| Waveform (circadian robustness) | Reduced fitness of the curve to predictable best-fitted model [36,61,62,72] | Flexibility rather than rigidity can be useful for adaptive needs, i.e., heart rate variability |
| Variability measures | ||
| Fragmentation and regularity (activity and sleep) | IV (intra-daily variability) ↑ IS (inter-daily stability) ↓ [38,51,61,62,72,74,75,77,80,94] | Can be beneficial in certain cases such as short-term adaptation requiring high vigilance [95,96] Large inter- and intra-individual differences in the duration of sleep cycles throughout the night [46,47] |
| Spectral composition | Extra-circadian dissemination (ECD): ratio between circadian and non-circadian (ultradian/infradian) amplitudes ↓ [2,10,11,12,97] | Some ultradian and infradian components are built-in and beneficial for health [47,98,99,100,101,102] |
| Alignment | Misalignment: disturbed phase relationship between circadian marker rhythms [55,56,103] | Optimal phase angles may vary depending on genotype, age, light environment: season and latitude; and meal timing if peripheral rhythms’ phases are considered. |
| Composite markers (area under curve), AUCs; function-on-scalar regression (FOSR), etc. | ↑ or ↓ [53,54,63,76,104,105,106] | Optimal reference curves may depend on genotype, age, light environment: season and latitude; and meal timing if peripheral rhythms’ phases are considered. |
| Social jet lag | ↑ [107,108,109,110,111,112,113,114] | Largely varies with age [111] and social obligations [115], may depend on the number of actual working days per week. All these factors can modify hazards of SJL for health. |
| Method | Advantages | Disadvantages |
|---|---|---|
| Moving Linear Regression Models | Easy to implement and interpret, widely accessible. Good agreement with questionnaires [148]. | Assume linear relationships. |
| Non-parametric Actigraphy Indices | Effectively capture informative features of the circadian rhythms of activity and light exposure such as their extent of irregularity; less sensitive to noise in the data. | Provide information on some features of the rhythm but do not quantify the shape of circadian rhythms. Interpretation can be subjective, when sleep patterns are irregular. Non-parametric analyses might overlook underlying multi-frequency patterns or relationships that parametric methods capture. |
| Parametric Cosinor Analysis | Effectively models periodic data and quantifies amplitude and acrophase. Optimal for oscillations that demonstrate a tied fit to the fitted model. Provides statistical tests for the presence of a rhythm and a measure of uncertainty for the estimation of its parameters, making it easier to draw conclusions about the presence and shape of rhythms. | May require the consideration of multiple harmonic terms for non-sinusoidal waveforms. |
| Approximation-Based Least-Squares Methods | Flexible for fitting various non-linear models. Can be applied to a wide range of models, including linear, polynomial, and non-linear functions. Effective in minimizing residuals, thus enabling a better model fit. | Can become complex to implement and interpret with intricate models. Require advanced computational resources. Higher-order modeling overestimates amplitude when data have gaps [138]. |
| Fourier Transformation | Provides analysis of periodic components, which is effective for analysis of periodic signals to identify dominant frequencies. Transformation of time-domain data into frequency-domain amplitudes and phases at specified frequencies simplifies the identification of cycles. | Assumption that the signal is stationary (its statistical properties do not change over time) is not always true for real-life data. Provides complex output, which may lack physiological relevance and requires additional expertise for meaningful interpretation of the results. |
| Wavelet Transformation | Decomposes a signal into wavelets, localized in both time and frequency. This method is useful for analyzing non-stationary signals. Provides information on the dominant mode of variability and how it varies over time. | Requirement of choosing the appropriate wavelet for a specific application is often not straightforward and may require trial and error, and may often lack physiological relevance. Aimed to analyze non-stationary signals, it relies on certain assumptions of signal behavior that may not hold true for physiological data from wearables, potentially leading to inaccurate conclusions. |
| Hilbert Transformation | Useful to analyze the phase of the reference signal against the phase of the target signal and measure phase relationships between signals [147]. Can be used to decipher non-linear and non-stationary signals. | While it can be applied for non-stationary signals, this method assumes that the signal is relatively smooth and continuous. Swift changes in the amplitude-phase domain, data gaps and noisy data limit the accuracy of the results |
| Artificial Neural Networks | Capable of modeling complex, non-linear relationships. Can learn from large datasets and improve over time. | Requires large databases for training. Often seen as a “black box,” making the interpretation of results challenging and less transparent. |
| Limit-Cycle Oscillator Models | Provide a dynamic representation of circadian rhythms. Can incorporate feedback mechanisms, adding depth to the modeling process. | Complex to implement and understand, which may deter some users. Require advanced computational resources and expertise, making them less accessible. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics 2025, 15, 327. https://doi.org/10.3390/diagnostics15030327
Gubin D, Weinert D, Stefani O, Otsuka K, Borisenkov M, Cornelissen G. Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics. 2025; 15(3):327. https://doi.org/10.3390/diagnostics15030327
Chicago/Turabian StyleGubin, Denis, Dietmar Weinert, Oliver Stefani, Kuniaki Otsuka, Mikhail Borisenkov, and Germaine Cornelissen. 2025. "Wearables in Chronomedicine and Interpretation of Circadian Health" Diagnostics 15, no. 3: 327. https://doi.org/10.3390/diagnostics15030327
APA StyleGubin, D., Weinert, D., Stefani, O., Otsuka, K., Borisenkov, M., & Cornelissen, G. (2025). Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics, 15(3), 327. https://doi.org/10.3390/diagnostics15030327










