Morphometry of the Scapular Notch and Its Clinical Implication in Suprascapular Nerve Entrapment
Abstract
1. Introduction
2. Materials and Methods
2.1. Classification of Scapular Notches
2.2. Morphometric Analysis of Scapular Notches
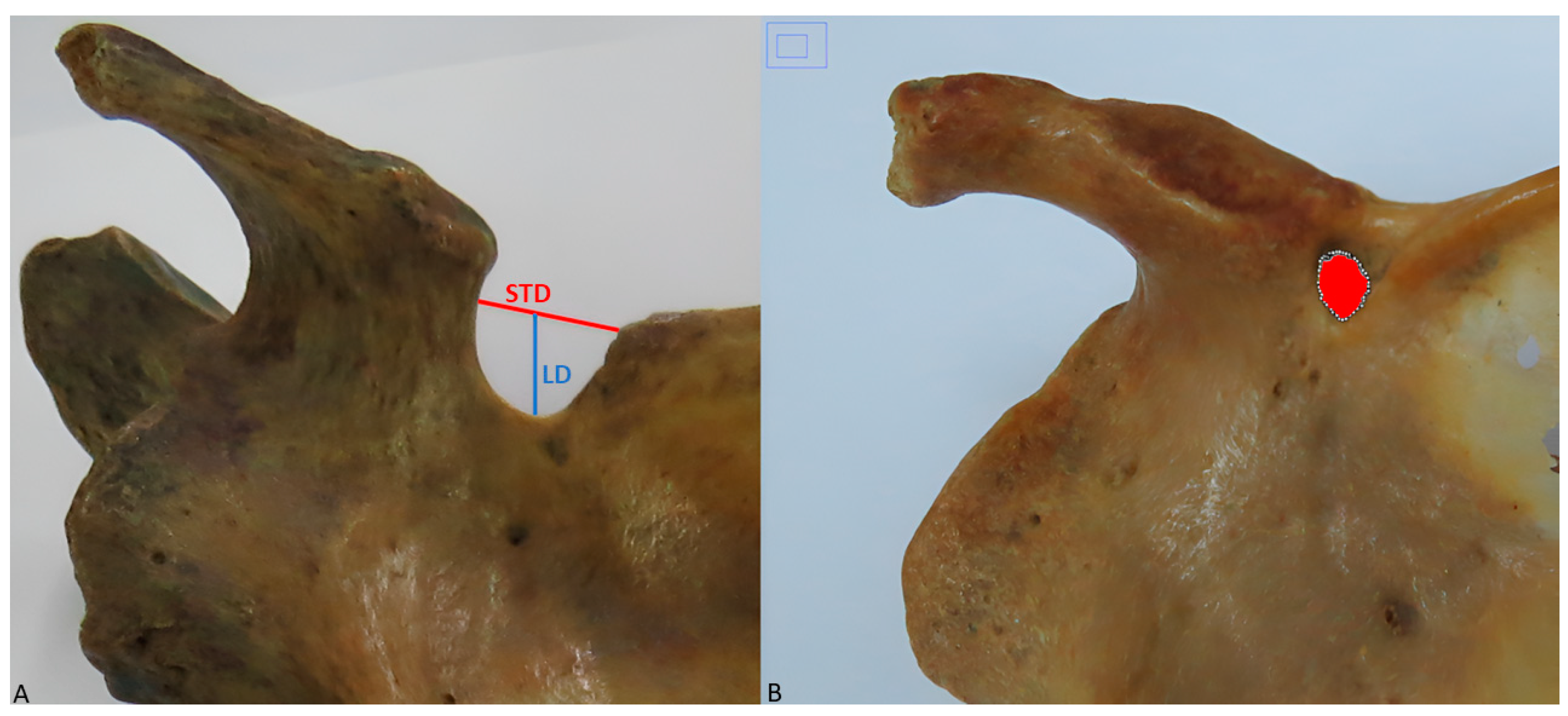
- Superior transverse diameter (STD): maximum horizontal distance between the corners of the SN (Figure 2A).
- Longitudinal diameter (LD): maximum length of the SN, from the upper limit of the SN to its deepest point (Figure 2A).
- Area of the SN: maximum extension of the SN considering its limits (Figure 2B).
2.3. Suprascapular Nerve Processing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duque-Parra, J.E.; Vásquez, B.; Duque, J.; Del Sol, M. Foramen escapular osificado en una muestra poblacional colombo-chilena. Int. J. Morphol. 2022, 40, 1395–1399. [Google Scholar] [CrossRef]
- Thoomes-de Graaf, M.; Scholten-Peeters, G.G.; Duijn, E.; Karel, Y.H.; van den Borne, M.P.; Beumer, A.; Ottenheijm, R.P.; Dinant, G.J.; Tetteroo, E.; Lucas, C.; et al. Inter-professional agreement of ultrasound-based diagnoses in patients with shoulder pain between physical therapists and radiologists in the Netherlands. Man. Ther. 2014, 19, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Amiri, Z.; Masouleh, N.A.; Kashfi, P.; Panjizadeh, F.; Hajilo, Z.; Shanayii, S.; Khodakarim, S.; Rahnama, L. Shoulder pain prevalence and risk factors in middle-aged women: A cross-sectional study. J. Bodyw. Mov. Ther. 2019, 23, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Rengachary, S.S.; Burr, D.; Lucas, S.; Hassanein, K.M.; Mohn, M.P.; Matzke, H. Suprascapular entrapment neuropathy: A clinical, anatomical, and comparative study. Part 2: Anatomical study. Neurosurgery. 1979, 5, 447–451. [Google Scholar] [CrossRef]
- Polguj, M.; Sibiński, M.; Grzegorzewski, A.; Waszczykowski, M.; Majos, A.; Topol, M. Morphological and radiological study of ossified superior transverse scapular ligament as potential risk factor of suprascapular nerve entrapment. BioMed Res. Int. 2014, 2014, 613601. [Google Scholar] [CrossRef]
- Duparc, F.; Coquerel, D.; Ozeel, J.; Noyon, M.; Gerometta, A.; Michot, C. Anatomical basis of the suprascapular nerve entrapment, and clinical relevance of the supraspinatus fascia. Surg. Radiol. Anat. 2010, 32, 277–284. [Google Scholar] [CrossRef]
- Łabętowicz, P.; Synder, M.; Wojciechowski, M.; Orczyk, K.; Jezierski, H.; Topol, M.; Polguj, M. Protective and Predisposing Morphological Factors in Suprascapular Nerve Entrapment Syndrome: A Fundamental Review Based on Recent Observations. BioMed Res. Int. 2017, 2017, 4659761. [Google Scholar] [CrossRef]
- Natsis, K.; Totlis, T.; Tsikaras, P.; Appell, H.J.; Skandalakis, P.; Koebke, J. Proposal for classification of the suprascapular notch: A study on 423 dried scapulas. Clin. Anat. 2007, 20, 135–139. [Google Scholar] [CrossRef]
- Polguj, M.; Sibiński, M.; Grzegorzewski, A.; Grzelak, P.; Majos, A.; Topol, M. Variation in morphology of suprascapular notch as a factor of suprascapular nerve entrapment. Int. Orthop. 2013, 37, 2185–2192. [Google Scholar] [CrossRef]
- Jezierski, H.; Podgórski, M.; Wysiadecki, G.; Olewnik, Ł.; De Caro, R.; Macchi, V.; Polguj, M. Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol. J. Clin. Med. 2018, 7, 491. [Google Scholar] [CrossRef]
- Antoniadis, G.; Richter, H.P.; Rath, S.; Braun, V.; Moese, G. Suprascapular nerve entrapment: Experience with 28 cases. J. Neurosurg. 1996, 85, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Moen, T.C.; Babatunde, O.M.; Hsu, S.H.; Ahmad, C.S.; Levine, W.N. Suprascapular neuropathy: What does the literature show? J. Shoulder. Elbow Surg. 2012, 21, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, F.; Alabau-Rodriguez, S.; Barrera-Ochoa, S.; Ateschrang, A.; Schreiner, A.J.; Monllau, J.C.; Perelli, S. Suprascapular Neuropathy around the Shoulder: A Current Concept Review. J. Clin. Med. 2020, 9, 2331. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.D.; Scully, T.B.; Shapiro, S.A.; Worth, R.M. Suprascapular nerve entrapment. A series of 27 cases. J. Neurosurg. 1991, 74, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Dines, D.M.; Moorman, C.T. Familial calcification of the superior transverse scapular ligament causing neuropathy. Clin. Orthop. Relat. Res. 1997, 134, 131–135. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Nechtman, C.; D’Antoni, A.V.; Shoja, M.M.; Mortazavi, M.M.; Loukas, M.; Rozzelle, C.J.; Spinner, R.J. Ossification of the suprascapular ligament: A risk factor for suprascapular nerve compression? Int. J. Shoulder Surg. 2013, 7, 19–22. [Google Scholar] [CrossRef]
- Tomaszewski, K.; Henry, B.; Kumar Ramakrishnan, P.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, S.; Walocha, J. Development of the Anatomical Quality Assurance (AQUA) Checklist: Guidelines for reporting original anatomical studies. Clin. Anat. 2017, 30, 14–20. [Google Scholar] [CrossRef]
- Kaledzera, T.; Matundu, B.; Adefolaju, G.A.; Manda, J.; Mwakikunga, A. Morphometric study of the suprascapular notch and scapular dimensions in adult Malawian cadavers and implications of completely ossified superior transverse scapular ligament. Pan. Afr. Med. J. 2022, 41, 324. [Google Scholar] [CrossRef]
- Inoue, K.; Suenaga, N.; Oizumi, N.; Sakamoto, Y.; Sakurai, G.; Miyoshi, N.; Taniguchi, N.; Tanaka, Y. Suprascapular notch variations: A 3DCT study. J. Orthop. Sci. 2014, 19, 920–924. [Google Scholar] [CrossRef]
- Albino, P.; Carbone, S.; Candela, V.; Arceri, V.; Vestri, A.R.; Gumina, S. Morphometry of the suprascapular notch: Correlation with scapular dimensions and clinical relevance. BMC Musculoskelet. Disord. 2013, 14, 172. [Google Scholar] [CrossRef]
- Boyan, N.; Ozsahin, E.; Kizilkanat, E.; Soames, R.W.; Oguz, O. Assessment of scapular morphometry. Int. J. Morphol. 2018, 36, 1035–1039. [Google Scholar] [CrossRef]
- Duque-Colorado, J.; García-Orozco, L.; Riveros, A.; del Sol, M. Scapular notch, spinoglenoid notch and scapular dimensions: Implications on the safe zone of the suprascapular nerve. Anat. Cell Biol. 2024, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Tawada, K.; Sugimoto, K.; Goto, H.; Tsuchiya, A.; Takenaga, T.; Takeuchi, S.; Takaba, K.; Murakami, H.; Yoshida, M. Bilateral suprascapular notches are asymmetrically shaped in a third of the Asian population. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Sangam, M.R.; Sarada-Devi, S.S.; Krupadanam, K.; Anasuya, K. A study on the morphology of the suprascapular notch and its distance from the glenoid cavity. J. Clin. Diagn. Res. 2013, 7, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Büyükmumcu, M.; Seker, M.; Ozbek, O.; Akin, D.; Koc, O.; Aydin, A.D.; Salbacak, A. Complete ossification of the superior transverse scapular ligament in an Turkish male adult. Int. J. Morphol. 2013, 31, 590–593. [Google Scholar] [CrossRef]
- Ohnishi, A.; Offord, K.; Dyck, P.J. Studies to improve fixation of human nerves. Part 1. Effect of duration of glutaraldehyde fixation on peripheral nerve morphometry. J. Neurol. Sci. 1974, 23, 223–226. [Google Scholar] [CrossRef]
- Fix, A.S.; Garman, R.H. Practical Aspects of Neuropathology: A Technical Guide for Working with the Nervous System. Toxicol. Pathol. 2000, 28, 122–131. [Google Scholar] [CrossRef]
- Rask, M.R. Suprascapular nerve entrapment. A report of two cases treated wilh suprascapular notch resection. Clin. Orthop. 1977, 123, 73–75. [Google Scholar]
- Kostretzis, L.; Theodoroudis, I.; Boutsiadis, A.; Papadakis, N.; Papadopoulos, P. Suprascapular Nerve Pathology: A Review of the Literature. Open Orthop. J. 2017, 11, 140–153. [Google Scholar] [CrossRef]
- Iwanaga, J.; Singh, V.; Ohtsuka, A.; Hwang, Y.; Kim, H.J.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clin. Anat. 2021, 34, 2–4. [Google Scholar] [CrossRef]

| SN Type | n | % |
|---|---|---|
| Type I | 56 | 34.0 |
| Type II | 37 | 22.4 |
| Type III | 42 | 25.5 |
| Type IV | 17 | 10.3 |
| Type V | 8 | 4.8 |
| Type VI | 5 | 3.0 |
| Total | 165 | 100 |
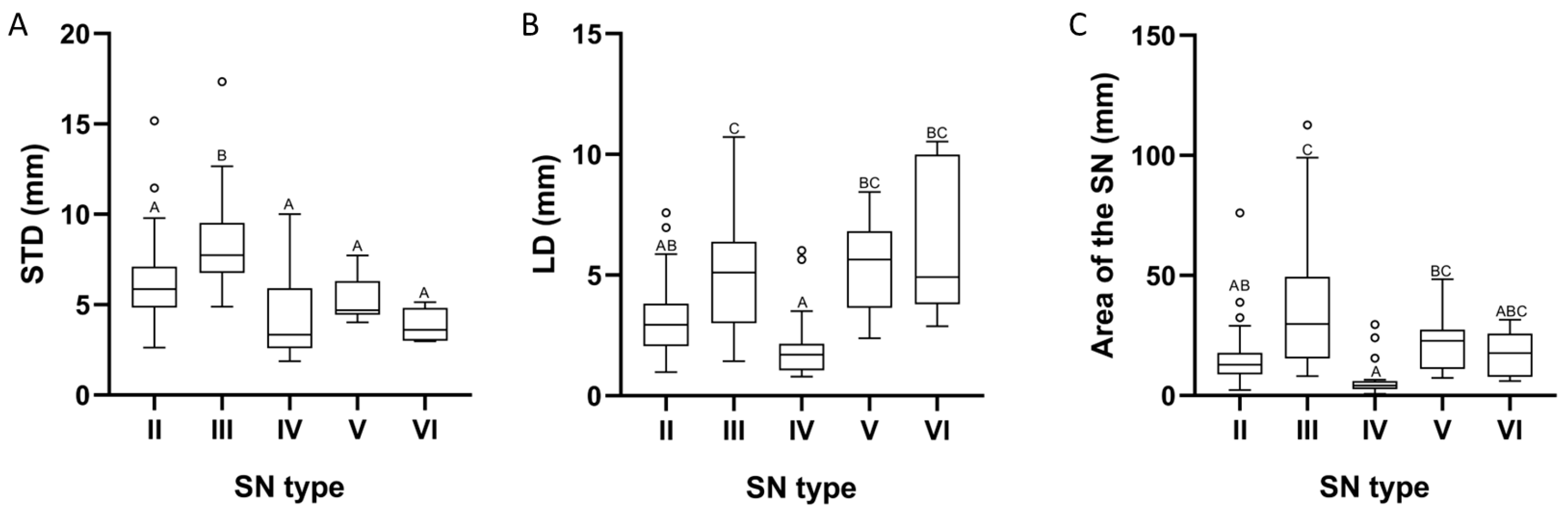
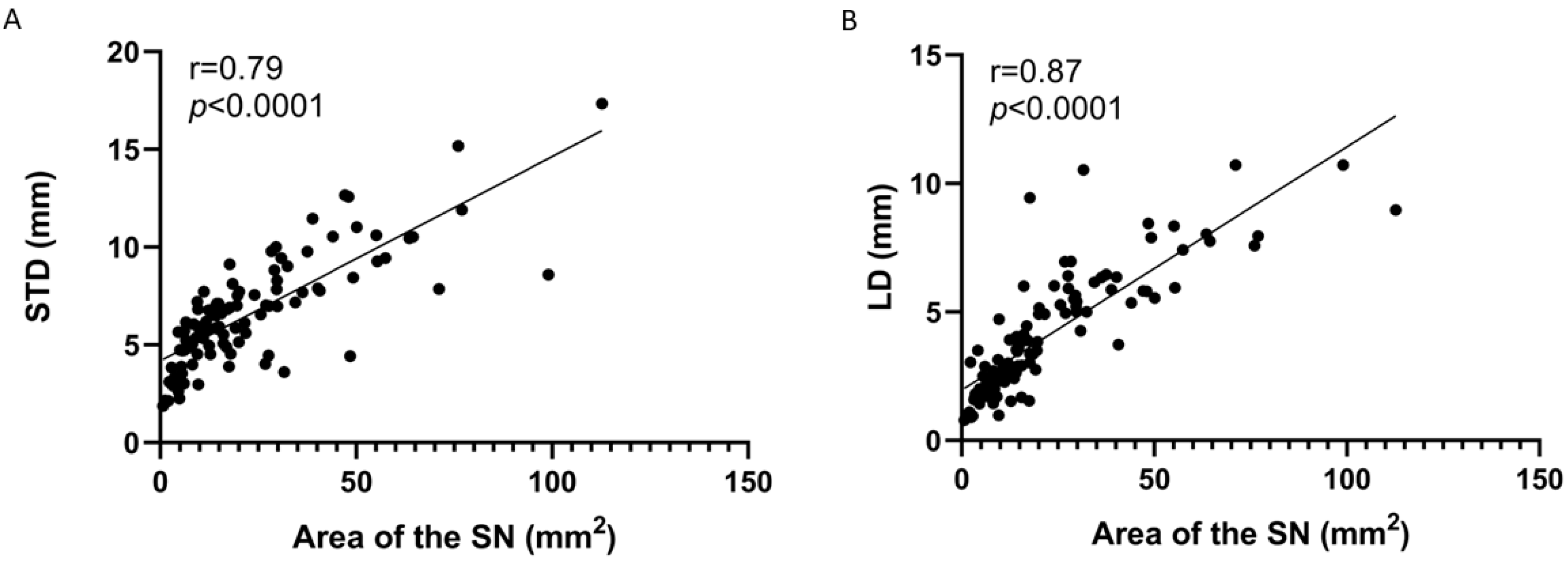
| Parameter | Type II | Type III | Type IV | Type V | Type VI | Value p |
|---|---|---|---|---|---|---|
| STD | 5.86 | 7.73 | 3.34 | 4.69 | 3.61 | 1.68 × 10−8 |
| LD | 2.94 | 5.11 | 1.71 | 5.64 | 4.92 | 1.17 × 10−7 |
| Area of the SN | 12.81 | 29.86 | 4.24 | 22.84 | 17.70 | 1.33 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Colorado, J.; Alzate-Mejia, O.A.; del Sol, M. Morphometry of the Scapular Notch and Its Clinical Implication in Suprascapular Nerve Entrapment. Diagnostics 2025, 15, 346. https://doi.org/10.3390/diagnostics15030346
Duque-Colorado J, Alzate-Mejia OA, del Sol M. Morphometry of the Scapular Notch and Its Clinical Implication in Suprascapular Nerve Entrapment. Diagnostics. 2025; 15(3):346. https://doi.org/10.3390/diagnostics15030346
Chicago/Turabian StyleDuque-Colorado, Jhonatan, Oscar Andrés Alzate-Mejia, and Mariano del Sol. 2025. "Morphometry of the Scapular Notch and Its Clinical Implication in Suprascapular Nerve Entrapment" Diagnostics 15, no. 3: 346. https://doi.org/10.3390/diagnostics15030346
APA StyleDuque-Colorado, J., Alzate-Mejia, O. A., & del Sol, M. (2025). Morphometry of the Scapular Notch and Its Clinical Implication in Suprascapular Nerve Entrapment. Diagnostics, 15(3), 346. https://doi.org/10.3390/diagnostics15030346





