Is the Transverse Colon Overlooked? Establishing a Comprehensive Colonoscopy Database from a Multicenter Cluster-Randomized Controlled Trial
Abstract
1. Introduction
2. Study Aim
3. Materials and Methods
3.1. Study Design
3.2. Training of Research Assistants
3.3. Data Collection
- -
- Video and coordinates: When the procedure started, the research assistant would enable the recording of the colonoscopy video along with XYZ-coordinates of the colonoscope from the Olympus ScopeGuide (UPD-3; Olympus Optical, Tokyo, Japan). CAMES has a unique research agreement with Olympus to extract these data through an Olympus receiver box (UCES-3), as described in the following study [19].
- -
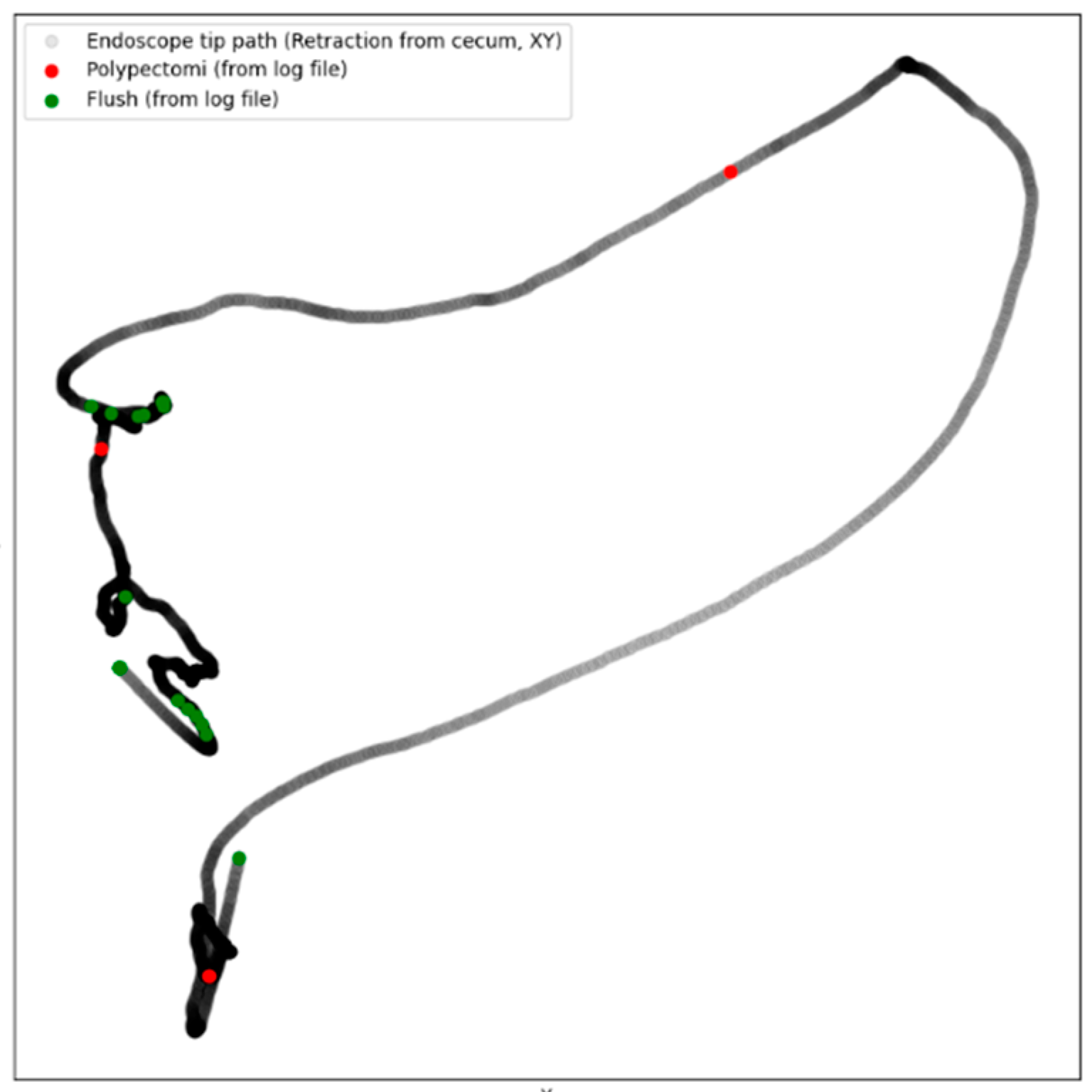
- Intraprocedure registration (Logfile): During the procedure, the research assistant would register the following through an electronic board (Figure 1, Elgato Stream Deck, Munich, Germany) by pressing a designated button: position of patient (front, back, left side, right side), anatomical landmarks (right and left flexure, caecum, terminal ileum), withdrawal started, retroflexion, flushing, polypectomy, and polyp spotted. The information was saved as a Logfile with timestamps for the whole procedure (Supplemental Material S1). Combining the Logfile with the coordinates enabled the development of a colonoscope tip and events track (Figure 2).
- -
- Patient-reported discomfort: After the procedure, the research assistant handed the patient a questionnaire about the level of pain and discomfort during the procedure.
- -
- Endoscopist description and pathological report: Four weeks after the procedure, the endoscopist’s description of the procedure was acquired through the local electronic patient journal at the Capital Region of Denmark (Sundhedsplatformen by EPIC systems, Verona, WI, USA) along with the pathological report for each polyp. The epicrisis and complete diagnosis list were also collected.
3.4. Data Processing and Establishing Database
3.5. Collaborating Partners
4. Results
5. Discussion
5.1. Procedure and Segment Durations
5.2. Patient Position, Flushing, and Retroflexion
5.3. Sedation and Patient Discomfort
5.4. Limitations and Future Directions in the Training of AI
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Hassan, C.; Piovani, D.; Spadaccini, M.; Parigi, T.; Khalaf, K.; Facciorusso, A.; Fugazza, A.; Rösch, T.; Bretthauer, M.; Mori, Y. Variability in Adenoma Detection Rate in Control Groups of Randomized Colonoscopy Trials. Gastrointest. Endosc. 2022, 97, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019, 156, 1661–1674.e1611. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Uhlmann, L.; Hoffmeister, M.; Laux, G.; Kieser, M.; Brenner, H. Identification of physicians with unusual performance in screening colonoscopy databases: A Bayesian approach. Gastrointest. Endosc. 2015, 81, 646–654.e641. [Google Scholar] [CrossRef]
- Rex, D.K.; Ponugoti, P.L. Calculating the adenoma detection rate in screening colonoscopies only: Is it necessary? Can it be gamed? Endoscopy 2017, 49, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Parasa, S.; Repici, A.; Berzin, T.; Leggett, C.; Gross, S.A.; Sharma, P. Framework and metrics for the clinical use and implementation of artificial intelligence algorithms into endoscopy practice: Recommendations from the American Society for Gastrointestinal Endoscopy Artificial Intelligence Task Force. Gastrointest. Endosc. 2023, 97, 815–824.e811. [Google Scholar] [CrossRef]
- Antonelli, G.; Libanio, D.; De Groof, A.J.; van der Sommen, F.; Mascagni, P.; Sinonquel, P.; Abdelrahim, M.; Ahmad, O.; Berzin, T.; Bhandari, P.; et al. QUAIDE—Quality assessment of AI preclinical studies in diagnostic endoscopy. Gut 2024, 74, 153–161. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Zhang, S.; Dou, Q. Foundation Model for Endoscopy Video Analysis via Large-scale Self-supervised Pre-train. arXiv 2023, arXiv:2306.16741. [Google Scholar]
- Ali, S.; Jha, D.; Ghatwary, N.; Realdon, S.; Cannizzaro, R.; Salem, O.E.; Lamarque, D.; Daul, C.; Riegler, M.A.; Anonsen, K.V. A multi-centre polyp detection and segmentation dataset for generalisability assessment. Sci. Data 2023, 10, 75. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 77–85.e76. [Google Scholar] [CrossRef] [PubMed]
- Cold, K.M.; Vamadevan, A.; Vilmann, A.S.; Svendsen, M.B.S.; Konge, L.; Bjerrum, F. Computer-aided quality assessment of endoscopist competence during colonoscopy: A systematic review. Gastrointest. Endosc. 2024, 100, 167–176.e1. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, X.; Wu, Z.; Wang, J.; Luo, C.; Chen, B.; Luo, R.; Zhang, L.; Zhang, C.; Tan, X.; et al. Effect of artificial intelligence on novice-performed colonoscopy: A multicenter randomized controlled tandem study. Gastrointest. Endosc. 2023, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-R.; Li, Z.; Shao, X.-J.; Ji, C.-R.; Ji, R.; Zhou, R.-C.; Li, G.-C.; Liu, G.-Q.; He, Y.-S.; Zuo, X.-L. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: A prospective randomized controlled study (with videos). Gastrointest. Endosc. 2020, 91, 415–424.e414. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Wu, L.; Zhang, J.; Mu, G.; Shen, L.; Liu, J.; Wang, Z.; Zhou, W.; An, P.; Huang, X. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): A randomised controlled study. Lancet Gastroenterol. Hepatol. 2020, 5, 352–361. [Google Scholar] [CrossRef]
- Plantener, E.; Deding, U.; Madsen, J.B.; Kroijer, R.; Madsen, J.S.; Baatrup, G. Using fecal immunochemical test values below conventional cut-off to individualize colorectal cancer screening. Endosc. Int. Open 2022, 10, E413–E419. [Google Scholar] [CrossRef]
- Olesen, T.B.; Jensen, H.; Møller, H.; Jensen, J.W.; Andersen, B.; Rasmussen, M. Nationwide participation in FIT-based colorectal cancer screening in Denmark during the COVID-19 pandemic: An observational study. eLife 2023, 12, e81808. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, A.S.; Svendsen, M.B.S.; Lachenmeier, C.; Søndergaard, B.; Vilmann, P.; Park, Y.S.; Svendsen, L.B.; Konge, L. Colonoscope retraction technique and predicting adenoma detection rate: A multicenter study. Gastrointest. Endosc. 2022, 95, 1002–1010. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, A.S.; Lachenmeier, C.; Svendsen, M.B.S.; Søndergaard, B.; Park, Y.S.; Svendsen, L.B.; Konge, L. Using computerized assessment in simulated colonoscopy: A validation study. Endosc. Int. Open 2020, 8, E783–E791. [Google Scholar] [CrossRef]
- Rembacken, B.; Hassan, C.; Riemann, J.; Chilton, A.; Rutter, M.; Dumonceau, J.-M.; Omar, M.; Ponchon, T. Quality in screening colonoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 2012, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, E.; Laferrere, L.; Zenon, E.; Marini, J.M.; Rainero, G.; San Roman, A.; Posadas Martinez, M.L.; Nadales, A. Timed screening colonoscopy: A randomized trial of two colonoscopic withdrawal techniques. Surg. Endosc. 2020, 34, 1200–1205. [Google Scholar] [CrossRef]
- Rath, T.; Pfeifer, L.; Neufert, C.; Kremer, A.; Leppkes, M.; Hoffman, A.; Neurath, M.F.; Zopf, S. Retrograde inspection vs standard forward view for the detection of colorectal adenomas during colonoscopy: A back-to-back randomized clinical trial. World J. Gastroenterol. 2020, 26, 1962–1970. [Google Scholar] [CrossRef]
- Wallace, M.B.; Crook, J.E.; Thomas, C.S.; Staggs, E.; Parker, L.; Rex, D.K. Effect of an endoscopic quality improvement program on adenoma detection rates: A multicenter cluster-randomized controlled trial in a clinical practice setting (EQUIP-3). Gastrointest. Endosc. 2017, 85, 538–545.e534. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Tang, R.S.; Muthusamy, V.R.; Ho, S.B.; Shah, N.K.; Wetzel, L.; Bain, A.S.; Mackintosh, E.E.; Paek, A.M.; Crissien, A.M.; et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest. Endosc. 2011, 74, 128–134. [Google Scholar] [CrossRef]
- Grover, S.C.; Walsh, C.M. Integrating artificial intelligence into endoscopy training: Opportunities, challenges, and strategies. Lancet Gastroenterol. Hepatol. 2023, 9, 11–13. [Google Scholar] [CrossRef]
- Köksal, A.; Kalkan, I.H.; Torun, S.; Taşkıran, I.; Öztaş, E.; Kayaçetin, E.; Şaşmaz, N. A simple method to improve adenoma detection rate during colonoscopy: Altering patient position. Can. J. Gastroenterol. 2013, 27, 509–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lebwohl, B.; Kastrinos, F.; Glick, M.; Rosenbaum, A.J.; Wang, T.; Neugut, A.I. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest. Endosc. 2011, 73, 1207–1214. [Google Scholar] [CrossRef]
- Parmar, R.; Martel, M.; Rostom, A.; Barkun, A.N. Validated scales for colon cleansing: A systematic review. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Heron, V.; Martel, M.; Bessissow, T.; Chen, Y.I.; Désilets, E.; Dube, C.; Lu, Y.; Menard, C.; McNabb-Baltar, J.; Parmar, R.; et al. Comparison of the Boston Bowel Preparation Scale with an Auditable Application of the US Multi-Society Task Force Guidelines. J. Can. Assoc. Gastroenterol. 2019, 2, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, A.H.; Schroy, P.C., III; Lieberman, D.A.; Logan, J.R.; Zurfluh, M.; Jacobson, B.C. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest. Endosc. 2014, 80, 269–276. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, L.; Wan, X.; Shen, L.; Liu, J.; Zhang, J.; Jiang, X.; Wang, Z.; Yu, S.; Kang, J. A novel artificial intelligence system for the assessment of bowel preparation (with video). Gastrointest. Endosc. 2020, 91, 428–435.e422. [Google Scholar] [CrossRef] [PubMed]
- Cold, K.M.; Heen, A.; Vamadevan, A.; Vilmann, A.S.; Konge, L.; Rasmussen, M.; Svendsen, M.B.S. Development and validation of the Open-Source Automatic Bowel Preparation Scale. Gastrointest. Endosc. 2024; in press. [Google Scholar] [CrossRef]
- Rex, D.K.; Vemulapalli, K.C. Retroflexion in colonoscopy: Why? Where? When? How? What value? Gastroenterology 2013, 144, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Quallick, M.R.; Brown, W.R. Rectal perforation during colonoscopic retroflexion: A large, prospective experience in an academic center. Gastrointest. Endosc. 2009, 69, 960–963. [Google Scholar] [CrossRef]
- Xiaolian, J.; Xiaolin, L.; Lan, Z.H. Effects of visual and audiovisual distraction on pain and anxiety among patients undergoing colonoscopy. Gastroenterol. Nurs. 2015, 38, 55–61. [Google Scholar] [CrossRef]
- Yang, C.; Sriranjan, V.; Abou-Setta, A.M.; Poluha, W.; Walker, J.R.; Singh, H. Anxiety Associated with Colonoscopy and Flexible Sigmoidoscopy: A Systematic Review. Am. J. Gastroenterol. 2018, 113, 1810–1818. [Google Scholar] [CrossRef]
- Trevisani, L.; Zelante, A.; Sartori, S. Colonoscopy, pain and fears: Is it an indissoluble trinomial? World J. Gastrointest. Endosc. 2014, 6, 227–233. [Google Scholar] [CrossRef]
- Vilmann, A.S.; Norsk, D.; Svendsen, M.B.S.; Reinhold, R.; Svendsen, L.B.; Park, Y.S.; Konge, L. Computerized feedback during colonoscopy training leads to improved performance: A randomized trial. Gastrointest. Endosc. 2018, 88, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Preisler, L.; Bulut, M.; Svendsen, M.S.; Svendsen, L.B.; Konge, L. An automatic measure of progression during colonoscopy correlates to patient experienced pain. Scand. J. Gastroenterol. 2018, 53, 345–349. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Varas, J.; Schwartz, T.A. Practical Guide to Machine Learning and Artificial Intelligence in Surgical Education Research. JAMA Surg. 2024, 159, 455–456. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Walsh, C.M. Artificial Intelligence in Pediatric Endoscopy: Current Status and Future Applications. Gastrointest. Endosc. Clin. N. Am. 2023, 33, 291–308. [Google Scholar] [CrossRef]
- Ahmad, H.A.; East, J.E.; Panaccione, R.; Travis, S.; Canavan, J.B.; Usiskin, K.; Byrne, M.F. Artificial Intelligence in Inflammatory Bowel Disease Endoscopy: Implications for Clinical Trials. J. Crohn’s Colitis 2023, 17, 1342–1353. [Google Scholar] [CrossRef]
- Kohli, A.; Holzwanger, E.A.; Levy, A.N. Emerging use of artificial intelligence in inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 6923–6928. [Google Scholar] [CrossRef]
- Gubatan, J.; Levitte, S.; Patel, A.; Balabanis, T.; Wei, M.T.; Sinha, S.R. Artificial intelligence applications in inflammatory bowel disease: Emerging technologies and future directions. World J. Gastroenterol. 2021, 27, 1920–1935. [Google Scholar] [CrossRef]
- Grosu, S.; Fabritius, M.P.; Winkelmann, M.; Puhr-Westerheide, D.; Ingenerf, M.; Maurus, S.; Graser, A.; Schulz, C.; Knösel, T.; Cyran, C.C.; et al. Effect of artificial intelligence-aided differentiation of adenomatous and non-adenomatous colorectal polyps at CT colonography on radiologists’ therapy management. Eur. Radiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wesp, P.; Grosu, S.; Graser, A.; Maurus, S.; Schulz, C.; Knösel, T.; Fabritius, M.P.; Schachtner, B.; Yeh, B.M.; Cyran, C.C.; et al. Deep learning in CT colonography: Differentiating premalignant from benign colorectal polyps. Eur. Radiol. 2022, 32, 4749–4759. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, J.; Chen, Y.; Sun, H.; Li, B.; Zhang, Q.; Sun, K.; Wang, Z.; Qian, X.; Zhan, T.; et al. Negative Effects of Endoscopists’ Fatigue on Colonoscopy Quality on 34,022 Screening Colonoscopies. J. Gastrointest. Liver Dis. 2021, 30, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cold, K.M.; Xie, S.; Nielsen, A.O.; Clementsen, P.F.; Konge, L. Artificial Intelligence Improves Novices’ Bronchoscopy Performance: A Randomized Controlled Trial in a Simulated Setting. Chest 2024, 165, 405–413. [Google Scholar] [CrossRef]

| Time Intervals in Seconds | With Polypectomies (n = 601) | Without Polypectomies (n = 590) | p Value |
|---|---|---|---|
| Age, years | 63.1 ± 7.59 | 63.3 ± 7.54 | 0.26 |
| Male sex | 336 (55.9%) | 327 (55.4%) | 0.71 |
| Total progression | 796 ± 478 | 823 ± 1607 | 0.70 |
| Intubation to left flexure | 359 ± 1542 | 342 ± 267 | 0.80 |
| Transverse colon (progression) | 251 ± 238 | 254 ± 235 | 0.82 |
| Right flexure to caecum | 218 ± 268 | 215 ± 259 | 0.84 |
| Total withdrawal | 1166 ± 518 | 670 ± 354 | <0.001 |
| Caecum to right flexure | 339 ± 295 | 236 ± 199 | <0.001 |
| Transverse colon (withdrawal) | 322 ± 2052 | 335 ± 2831 | 0.92 |
| Left flexure to extubation | 603 ± 379 | 228 ± 204 | <0.001 |
| Caecum | Ascending Colon | Right Flexure | Transverse Colon | Left Flexure | Descending Colon | Sigmoid Colon | Rectum | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinomas | 2 (5%) | 3 (8%) | 3 (8%) | 2 (5%) | 1 (1%) | 1 (5%) | 17 (44%) | 10 (26%) | 39 (100%) |
| Tubular adenomas | 75 (21%) | 95 (13%) | 34 (5%) | 96 (13%) | 27 (4%) | 47 (6%) | 241 (33%) | 117 (16%) | 732 (100%) |
| Sessile serrated | 25 (21%) | 37 (32%) | 9 (8%) | 23 (20%) | 4 (3%) | 3 (3%) | 13 (11%) | 3 (3%) | 117 (100%) |
| Total | 102 (11%) | 135 (15%) | 46 (5%) | 121 (14%) | 32 (4%) | 51 (6%) | 271 (31%) | 130 (15%) | 888 (100%) |
| With Polypectomies (n = 601) | Without Polypectomies (n = 590) | p Value | |
|---|---|---|---|
| * Retroflexion | 406 (0.68) | 374 (0.63) | 0.15 |
| Patient rotation (count) | 0.81 ± 1.1 | 0.70 ± 1.1 | 0.06 |
| Flushing (count) | 6.4 ± 8.7 | 3.5 ± 6.0 | <0.001 |
| Sedation | |||
| Fentanyl (µg) | 76.3 ± 48.9 | 72.7 ± 43.2 | 0.24 |
| Midazolam (mg) | 2.7 ± 10.1 | 2.2 ± 7.6 | 0.41 |
| Propofol (mg) | 5.5 ± 44.3 | 5.1 ± 33.7 | 0.95 |
| Rapifen (mg) | 83.2 ± 241.5 | 10.2 ± 69.7 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cold, K.M.; Vamadevan, A.; Heen, A.; Vilmann, A.S.; Rasmussen, M.; Konge, L.; Svendsen, M.B.S. Is the Transverse Colon Overlooked? Establishing a Comprehensive Colonoscopy Database from a Multicenter Cluster-Randomized Controlled Trial. Diagnostics 2025, 15, 591. https://doi.org/10.3390/diagnostics15050591
Cold KM, Vamadevan A, Heen A, Vilmann AS, Rasmussen M, Konge L, Svendsen MBS. Is the Transverse Colon Overlooked? Establishing a Comprehensive Colonoscopy Database from a Multicenter Cluster-Randomized Controlled Trial. Diagnostics. 2025; 15(5):591. https://doi.org/10.3390/diagnostics15050591
Chicago/Turabian StyleCold, Kristoffer Mazanti, Anishan Vamadevan, Amihai Heen, Andreas Slot Vilmann, Morten Rasmussen, Lars Konge, and Morten Bo Søndergaard Svendsen. 2025. "Is the Transverse Colon Overlooked? Establishing a Comprehensive Colonoscopy Database from a Multicenter Cluster-Randomized Controlled Trial" Diagnostics 15, no. 5: 591. https://doi.org/10.3390/diagnostics15050591
APA StyleCold, K. M., Vamadevan, A., Heen, A., Vilmann, A. S., Rasmussen, M., Konge, L., & Svendsen, M. B. S. (2025). Is the Transverse Colon Overlooked? Establishing a Comprehensive Colonoscopy Database from a Multicenter Cluster-Randomized Controlled Trial. Diagnostics, 15(5), 591. https://doi.org/10.3390/diagnostics15050591









