Contrast Volume Reduction in Oncologic Body Imaging Using Dual-Energy CT: A Comparison with Single-Energy CT
Abstract
:1. Introduction
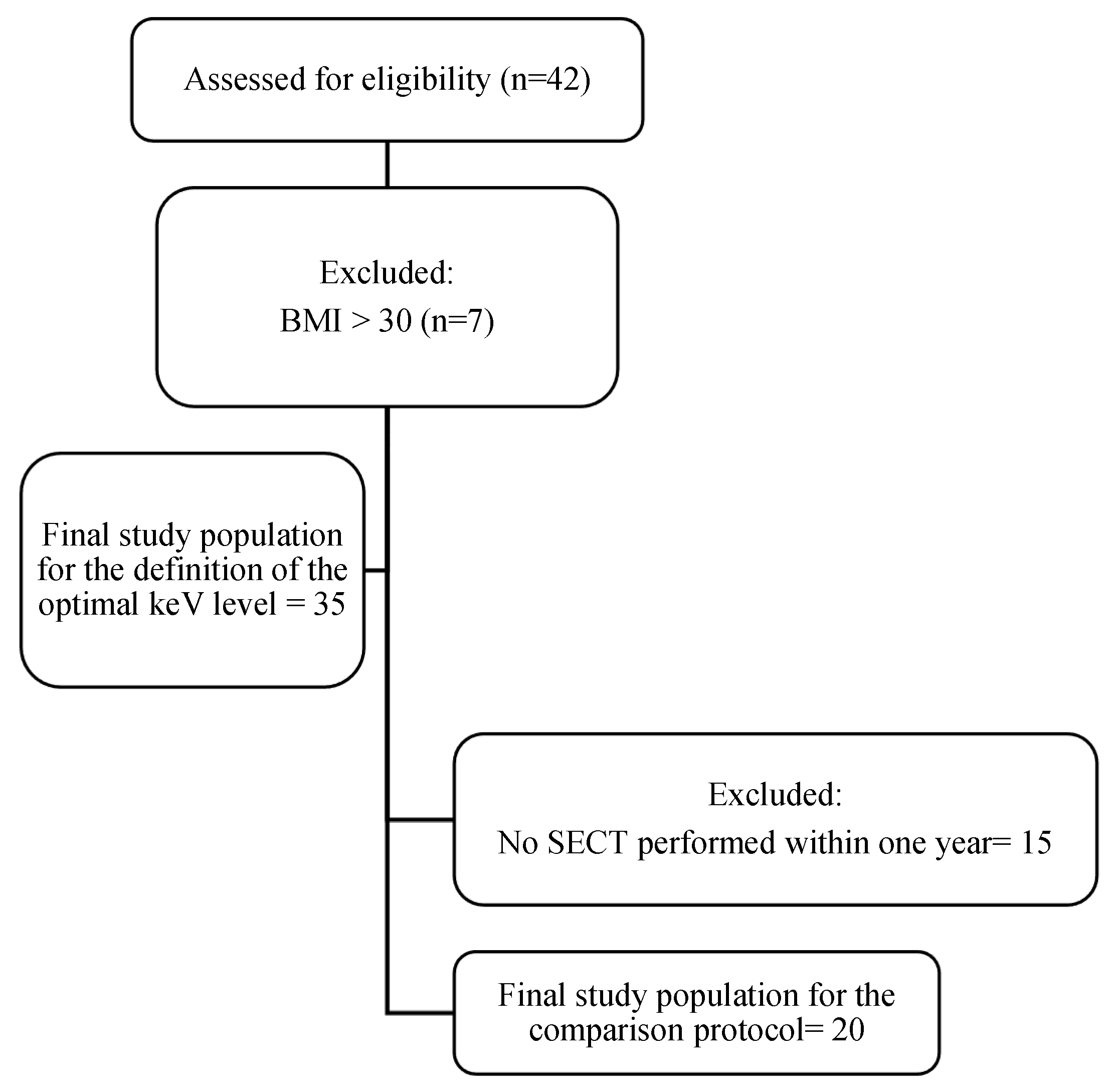
2. Materials and Methods
2.1. Study Design
2.2. Imaging Acquisition
2.3. Definition of the Optimal VMI Reconstruction
2.3.1. Qualitative Image Analysis
2.3.2. Quantitative Image Analysis
2.4. Comparison of DECT and SECT
2.5. Radiation Dose Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Optimal VMI Reconstruction Results
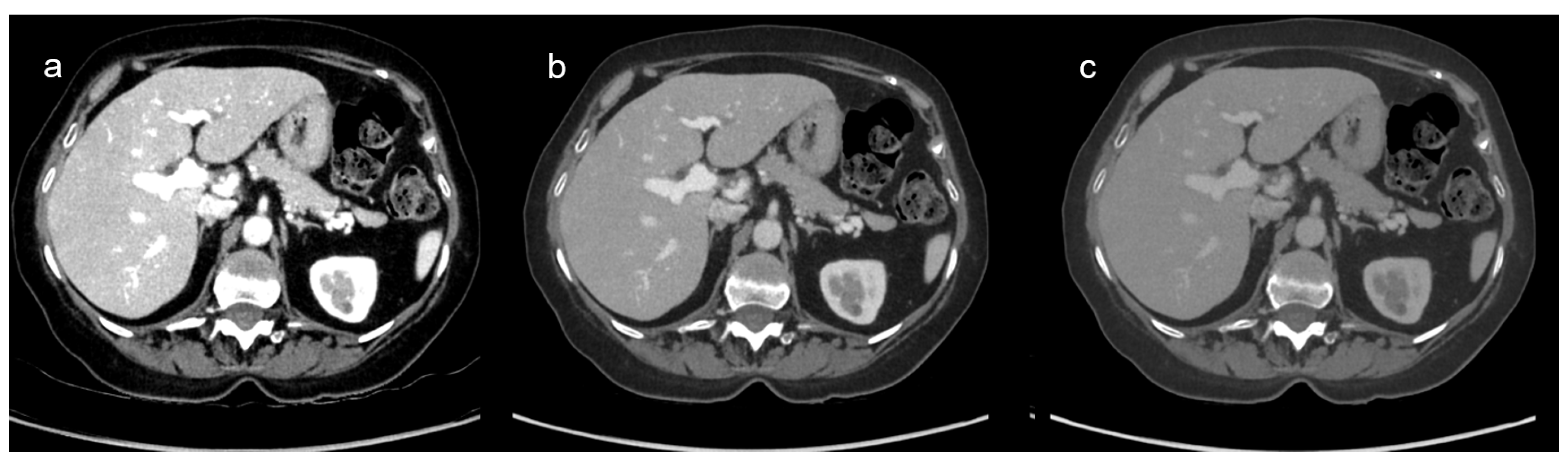
3.2.1. VMI Comparison: Qualitative Results
3.2.2. VMI Comparison: Quantitative Analysis Results
3.3. Comparison of DECT and SECT Results
3.3.1. DECT and SECT Comparison: Qualitative Results
3.3.2. DECT and SECT Comparison: Quantitative Analysis Results
3.4. Radiation Exposure Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CT | Computed Tomography |
| DECT | Dual-energy Computed Tomography |
| keV | Kiloelectronvolts |
| kV | Kilovolts |
| ICM | Iodinated Contrast Medium |
| PV | Portal Vein |
| SD | Standard Deviation |
| SECT | Single-energy Computed Tomography |
| VMI | Virtual Monochromatic Image |
References
- Pomerantz, B.J. Imaging and Interventional Radiology for Cancer Management. Surg. Clin. N. Am. 2020, 100, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Fanni, S.C.; Morganti, R.; Febi, M.; Ambrosini, I.; De Gori, C.; D’Amore, C.A.; Bruni, L.; D’Agostino, G.; Milazzo, A.; et al. Looking for appropriateness in follow-up CT of oncologic patients: Results from a cross-sectional study. Eur. J. Radiol. 2023, 167, 111080. [Google Scholar] [CrossRef]
- Park, C.; Gruber-Rouh, T.; Leithner, D.; Zierden, A.; Albrecht, M.H.; Wichmann, J.L.; Bodelle, B.; Elsabaie, M.; Scholtz, J.-E.; Kaup, M.; et al. Single-source chest-abdomen-pelvis cancer staging on a third generation dual-source CT system: Comparison of automated tube potential selection to second generation dual-source CT. Cancer Imaging 2016, 16, 33. [Google Scholar] [CrossRef]
- Davenport, M.S.; Khalatbari, S.; Cohan, R.H.; Ellis, J.H. Contrast medium-induced nephrotoxicity risk assessment in adult inpatients: A comparison of serum creatinine level- and estimated glomerular filtration rate-based screening methods. Radiology 2013, 269, 92–100. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, E.A.; Schuster, K.M.; Kaplan, L.J.; Maung, A.A.; Lui, F.Y.; Maerz, L.L.; Johnson, D.C.; Davis, K.A. Contrast-induced nephropathy in elderly trauma patients. J. Trauma 2010, 68, 294–297. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Mor, M.K.; Resnick, A.L.; Hartwig, K.C.; Palevsky, P.M.; Fine, M.J. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin. J. Am. Soc. Nephrol. 2008, 3, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Kalva, S.P.; Gunnarsson, C.; Ryan, M.P.; Baker, E.R.; Mehta, R.L. Risk of renal events following intravenous iodinated contrast material administration among inpatients admitted with cancer a retrospective hospital claims analysis. Cancer Imaging 2018, 18, 30. [Google Scholar] [CrossRef]
- European Society of Urogenital Radiology. ESUR Guidelines on Contrast Agents 2018; European Society of Urogenital Radiology: Rome, Italy, 2018. [Google Scholar]
- Beer, M.; Schuler, J.; Kraus, E.; Zhou, S.; Stanzel, S.; Lorenz, S.; Rygula, A.; Schmidt, S.A.; Niederste-Hollenberg, J. Discharge of iodine-containing contrast media into the environment problem analysis and implementation of measures to reduce discharge by means of separation toilets experience from a pilot project. Rofo 2023, 195, 1122–1127. [Google Scholar] [CrossRef]
- Dekker, H.M.; Stroomberg, G.J.; Prokop, M. Tackling the increasing contamination of the water supply by iodinated contrast media. Insights Imaging 2022, 13, 30. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: Minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Fox, B. GE Healthcare’s Gemstone Spectral Imaging Emerging as a “Must Have” Diagnostic Tool. Available online: https://www.ge.com/news/press-releases/ge-healthcares-gemstone-spectral-imaging-emerging-must-have-diagnostic-tool (accessed on 27 February 2024).
- Hickethier, T.; Kroeger, J.R.; Lennartz, S.; Doerner, J.; Maintz, D.; Chang, D.H. Venous-phase chest CT with reduced contrast medium dose: Utilization of spectral low keV monoenergetic images improves image quality. Eur. J. Radiol. 2020, 122, 108756. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, J.M.; Kim, S.W.; Park, S.; Han, S.; Yoon, J.H.; Joo, I.; Hong, H. Low-contrast-dose liver CT using low monoenergetic images with deep learning–based denoising for assessing hepatocellular carcinoma: A randomized controlled noninferiority trial. Eur. Radiol. 2022, 33, 4344–4354. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Mujtaba, B.; Jensen, C.; Aslam, R.; Elsayes, A.K.; Kuchana, V.; Bhosale, P. Feasibility of half the recommended dose of IV contrast in DECT: Image quality evaluation and diagnostic acceptability in cancer patients. Clin. Imaging 2022, 88, 59–65. [Google Scholar] [CrossRef]
- Gwet, K.L. Handbook of Inter-Rater Reliability: The Definitive Guide to Measuring the Extent of Agreement Among Raters, 4th ed.; Advances Analytics, LLC: Gaithersburg, MD, USA, 2014; ISBN 978-0-9708062-8-4. [Google Scholar]
- Gao, K.; Ma, Z.-P.; Zhang, T.-L.; Liu, Y.-W.; Zhao, Y.-X. Comparative study of abdominal CT enhancement in overweight and obese patients based on different scanning modes combined with different contrast medium concentrations. J. X-Ray Sci. Technol. 2024, 32, 1–13. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, Z.; Liu, J.; Chai, Y.; Zhao, H.; Guo, H.; Marin, D.; Gao, J. Can virtual monochromatic images from dual-energy CT replace low-kVp images for abdominal contrast-enhanced CT in small- and medium-sized patients? Eur. Radiol. 2019, 29, 2878–2889. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Ichikawa, T.; Morisaka, H.; Akai, H.; Izuka, K.; Ueno, T.; Abe, O.; Tsushima, Y. Early detection of hypervascularization in hepatocellular carcinoma (≤2 cm) on hepatic arterial phase with virtual monochromatic imaging: Comparison with low-tube voltage CT. Medicine 2023, 102, e34774. [Google Scholar] [CrossRef]
- Greffier, J.; Si-Mohamed, S.; Dabli, D.; De Forges, H.; Hamard, A.; Douek, P.; Beregi, J.P.; Frandon, J. Performance of four dual-energy CT platforms for abdominal imaging: A task-based image quality assessment based on phantom data. Eur. Radiol. 2021, 31, 5324–5334. [Google Scholar] [CrossRef]
- Cester, D.; Eberhard, M.; Alkadhi, H.; Euler, A. Virtual monoenergetic images from dual-energy CT: Systematic assessment of task-based image quality performance. Quant. Imaging Med. Surg. 2022, 12, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Z.-C.; Zhao, Y.-X.; Zhang, T.-L.; Zuo, Z.-W. A feasibility study of different GSI noise indexes and concentrations of contrast medium in hepatic CT angiography of overweight patients: Image quality, radiation dose, and iodine intake. Jpn. J. Radiol. 2023, 41, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Alexander, L.; Allen, B.; Berland, L.; Borhani, A.; Mileto, A.; Moreno, C.; Morgan, D.; Sahani, D.; Shuman, W.; et al. Dual-energy CT workflow: Multi-institutional consensus on standardization of abdominopelvic MDCT protocols. Abdom. Radiol. 2017, 42, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Lin, X.Z.; Chen, K.; Gao, J. Spectral CT in patients with small HCC: Investigation of image quality and diagnostic accuracy. Eur. Radiol. 2012, 22, 2117–2124. [Google Scholar] [CrossRef]
- Bae, K.T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef]
- Heiken, J.P.; Brink, J.A.; McClennan, B.L.; Sagel, S.S.; Crowe, T.M.; Gaines, M.V. Dynamic incremental CT: Effect of volume and concentration of contrast material and patient weight on hepatic enhancement. Radiology 1995, 195, 353–357. [Google Scholar] [CrossRef]
- Pearce, F. Energy Hogs: Can World’s Huge Data Centers Be Made More Efficient? Yale Environment 360; Yale School of the Environment: New Haven, CT, USA, 2018. [Google Scholar]
- Mariampillai, J.; Rockall, A.; Manuellian, C.; Cartwright, S.; Taylor, S.; Deng, M.; Sheard, S. The green and sustainable radiology department. Die Radiologie 2023, 63, 21–26. [Google Scholar] [CrossRef]
- Noda, Y.; Kawai, N.; Kawamura, T.; Kobori, A.; Miyase, R.; Iwashima, K.; Kaga, T.; Miyoshi, T.; Hyodo, F.; Kato, H.; et al. Radiation and iodine dose reduced thoraco-abdomino-pelvic dual-energy CT at 40 keV reconstructed with deep learning image reconstruction. Br. J. Radiol. 2022, 95, 20211163. [Google Scholar] [CrossRef]
- Tsang, D.S.; Merchant, T.E.; Merchant, S.E.; Smith, H.; Yagil, Y.; Hua, C.-H. Quantifying potential reduction in contrast dose with monoenergetic images synthesized from dual-layer detector spectral CT. Br. J. Radiol. 2017, 90, 20170290. [Google Scholar] [CrossRef]
- Nagayama, Y.; Nakaura, T.; Oda, S.; Utsunomiya, D.; Funama, Y.; Iyama, Y.; Taguchi, N.; Namimoto, T.; Yuki, H.; Kidoh, M.; et al. Dual-layer DECT for multiphasic hepatic CT with 50 percent iodine load: A matched-pair comparison with a 120 kVp protocol. Eur. Radiol. 2018, 28, 1719–1730. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Morcos, S.K.; Thomsen, H.S.; Webb, J.A.W. Contrast-media-induced nephrotoxicity: A consensus report. Eur. Radiol. 1999, 9, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Faucon, A.-L.; Bobrie, G.; Clément, O. Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. Eur. J. Radiol. 2019, 116, 231–241. [Google Scholar] [CrossRef]
- Van Der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.-F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-contrast acute kidney injury Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Kanal, K.M.; Butler, P.F.; Sengupta, D.; Bhargavan-Chatfield, M.; Coombs, L.P.; Morin, R.L.U.S. Diagnostic Reference Levels and Achievable Doses for 10 Adult CT Examinations. Radiology 2017, 284, 120–133. [Google Scholar] [CrossRef]
- Nakaura, T.; Awai, K.; Maruyama, N.; Takata, N.; Yoshinaka, I.; Harada, K.; Uemura, S.; Yamashita, Y. Abdominal Dynamic CT in Patients with Renal Dysfunction: Contrast Agent Dose Reduction with Low Tube Voltage and High Tube Current–Time Product Settings at 256–Detector Row CT. Radiology 2011, 261, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, J.M.; Yoon, J.H.; Joo, I.; Bae, J.S.; Yoo, J.; Kim, J.H.; Ahn, C.; Kim, J.H. Deep learning–based image reconstruction of 40-keV virtual monoenergetic images of dual-energy CT for the assessment of hypoenhancing hepatic metastasis. Eur. Radiol. 2022, 32, 6407–6417. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Kojima, T.; Shirasaka, T.; Kondo, M.; Kato, T. Characteristics of the deep learning-based virtual monochromatic image with fast kilovolt-switching CT: A phantom study. Radiol. Phys. Technol. 2023, 16, 77–84. [Google Scholar] [CrossRef]
- Gulizia, M.; Ding, S.; Sá Dos Reis, C.; Jaques, C.; Dromain, C. Adjustments of iodinated contrast media using lean body weight for abdominopelvic computed tomography: A systematic review and meta-analysis. Eur. J. Radiol. 2024, 178, 111631. [Google Scholar] [CrossRef]

| CT Parameters | DECT | SECT |
|---|---|---|
| Tube voltage | 80–140 kVp | 120 kVp |
| Automatic tube current modulation (mA) | 145–515 | 130–400 |
| Pitch | 0.992 | 1.2 |
| Collimation (mm) | 80 × 0.625 | 80 × 0.625 |
| SFOV (mm) | 500 | 500 |
| Matrix size (pixels) | 512 × 512 | 512 × 512 |
| Gantry rotation time (s/rot) | 0.6 | 0.28 |
| Slice thickness (mm) | 2.5 | 2.5 |
| Slice increment (mm) | 2 | 2 |
| Kernel | Standard | Standard |
| Reconstruction method | ASIR-V 50% | ASIR-V 50% |
| Patients (n = 35) | |
|---|---|
| Sex M/F | 21/14 |
| Age (years) | 64.6 ± 9.5 |
| Body weight (kg) | 71.4 ± 12.7 |
| Body Height (cm) | 169 ± 9.0 |
| BMI | 25.0 ± 3.7 |
| Clinical indications | 16/35 Lung cancers 9/35 Urological cancers 6/35 Gynecological cancers 4/35 Digestive cancers 3/35 Skin cancers 2/35 Hematological cancers 1/35 Brain cancer 1/35 Breast cancer |
| keV | Image Overall Quality (Range) | Contrast Enhancement (Range) | Image Noise (Range) |
|---|---|---|---|
| 40 | 3.22 (3–5) | 4.91 (4–5) | 3.24 (3–5) |
| 45 | 3.37 (3–5) | 4.87 (4–5) | 3.23 (3–5) |
| 50 | 3.87 (3–5) | 4.57 (4–5) | 3.44 (3–5) |
| 55 | 4.36 (3–5) | 4.31 (3–5) | 3.76 (3–5) |
| 60 | 4.61 (3–5) | 4.06 (3–5) | 4.00 (3–5) |
| 65 | 4.51 (3–5) | 3.86 (3–5) | 4.40 (3–5) |
| 70 | 3.99 (2–5) | 3.51 (2–5) | 4.76 (4–5) |
| 75 | 3.41 (2–5) | 3.10 (2–5) | 4.96 (4–5) |
| 80 Gwet’s AC | 3.03 (2–4) AC = 0.864 | 2.94 (2–4) AC = 0.94 | 4.96 (4–5) AC = 0.63 |
| HULP | CNRLP | SNRLP | HUPV | CNRPV | SNRPV | Image Noise | |
|---|---|---|---|---|---|---|---|
| 40 | 199.74 ± 24.67 | 6.90 ± 2.18 | 11.49 ± 2.04 | 379.13 ± 29.15 | 17.59 ± 5.36 | 13.53 ± 3.61 | 17.21 ± 4.01 |
| 45 | 174.01 ± 17.98 | 6.79 ± 1.96 | 11.80 ± 1.90 | 312.89 ± 24.29 | 16.40 ± 4.70 | 13.36 ± 3.44 | 14.75 ± 3.35 |
| 50 | 150.86 ± 14.84 | 6.53 ± 1.83 | 11.95 ± 1.94 | 260.55 ± 43.08 | 15.28 ± 4.33 | 13.22 ± 3.23 | 12.76 ± 2.89 |
| 55 | 133.15 ± 12.62 | 6.34 ± 1.70 | 12.06 ± 1.86 | 220.24 ± 34.94 | 14.27 ± 3.96 | 13.04 ± 3.01 | 11.14 ± 2.42 |
| 60 | 119.30 ± 11.08 | 6.09 ± 1.65 | 12.27 ± 1.92 | 190.85 ± 33.73 | 13.38 ± 4.14 | 13.01 ± 3.21 | 9.96 ± 2.17 |
| 65 | 108.1 ± 10.01 | 5.75 ± 1.57 | 12.35 ± 1.93 | 163.33 ± 23.61 | 11.86 ± 3.40 | 12.67 ± 2.70 | 9.15 ± 2.01 |
| 70 | 99.50 ± 9.20 | 5.55 ± 1.49 | 12.61 ± 1.94 | 143.58 ± 19.63 | 10.87 ± 3.06 | 12.40 ± 2.46 | 8.33 ± 1.78 |
| 75 | 92.42 ± 8.76 | 5.32 ± 1.46 | 12.29 ± 2.69 | 127.69 ± 16.70 | 9.90 ± 2.87 | 12.20 ± 2.31 | 7.73 ± 1.67 |
| 80 | 86.93 ± 8.49 | 5.17 ± 1.44 | 12.80 ± 2.05 | 114.78 ± 14.19 | 9.02 ± 2.66 | 11.96 ± 2.18 | 7.22 ± 1.59 |
| DECT at 60 keV | SECT | p Value | |
|---|---|---|---|
| Image overall quality (range) | 3.95 (3–5) AC = 0.769 | 3.83 ± 0.7 (2–5) AC = 0.54 | 0.287 |
| Contrast enhancement (range) | 4.08 (3–5) AC = 0.83 | 3.35 ± 0.7 (2–5) AC = 0.23 | <0.001 |
| Image noise (range) | 4.55 ± 0.6 (3–5) AC = 0.83 | 3.58 ± 0.8 (2–5) AC = 0.40 | <0.001 |
| DECT at 60 keV | SECT | p Value | |
|---|---|---|---|
| aHULP | 119.30 ± 11.1 | 109.8 ± 9.0 | <0.001 |
| CNRLP | 6.59 ± 1.5 | 4.04 ± 1.5 | <0.001 |
| SNRLP | 12.95 ± 1.8 | 7.64 ± 1.7 | <0.001 |
| HUPV | 200.01 ± 37.0 | 149.6 ± 16.6 | <0.001 |
| CNRPV | 14.47 ± 4.3 | 6.9 ± 2.2 | <0.001 |
| SNRPV | 13.44 ± 3.6 | 10.43 ± 2.6 | <0.001 |
| Image Noise | 9.93 ± 1.7 | 13.98 ± 2.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulizia, M.; Viry, A.; Jreige, M.; Fahrni, G.; Marro, Y.; Manasseh, G.; Chevallier, C.; Dromain, C.; Vietti-Violi, N. Contrast Volume Reduction in Oncologic Body Imaging Using Dual-Energy CT: A Comparison with Single-Energy CT. Diagnostics 2025, 15, 707. https://doi.org/10.3390/diagnostics15060707
Gulizia M, Viry A, Jreige M, Fahrni G, Marro Y, Manasseh G, Chevallier C, Dromain C, Vietti-Violi N. Contrast Volume Reduction in Oncologic Body Imaging Using Dual-Energy CT: A Comparison with Single-Energy CT. Diagnostics. 2025; 15(6):707. https://doi.org/10.3390/diagnostics15060707
Chicago/Turabian StyleGulizia, Marianna, Anais Viry, Mario Jreige, Guillaume Fahrni, Yannick Marro, Gibran Manasseh, Christine Chevallier, Clarisse Dromain, and Naik Vietti-Violi. 2025. "Contrast Volume Reduction in Oncologic Body Imaging Using Dual-Energy CT: A Comparison with Single-Energy CT" Diagnostics 15, no. 6: 707. https://doi.org/10.3390/diagnostics15060707
APA StyleGulizia, M., Viry, A., Jreige, M., Fahrni, G., Marro, Y., Manasseh, G., Chevallier, C., Dromain, C., & Vietti-Violi, N. (2025). Contrast Volume Reduction in Oncologic Body Imaging Using Dual-Energy CT: A Comparison with Single-Energy CT. Diagnostics, 15(6), 707. https://doi.org/10.3390/diagnostics15060707






