Association of Polycystic Ovary Syndrome with Clinical, Physical, and Reproductive Factors: A Data-Driven Analysis
Abstract
:1. Introduction
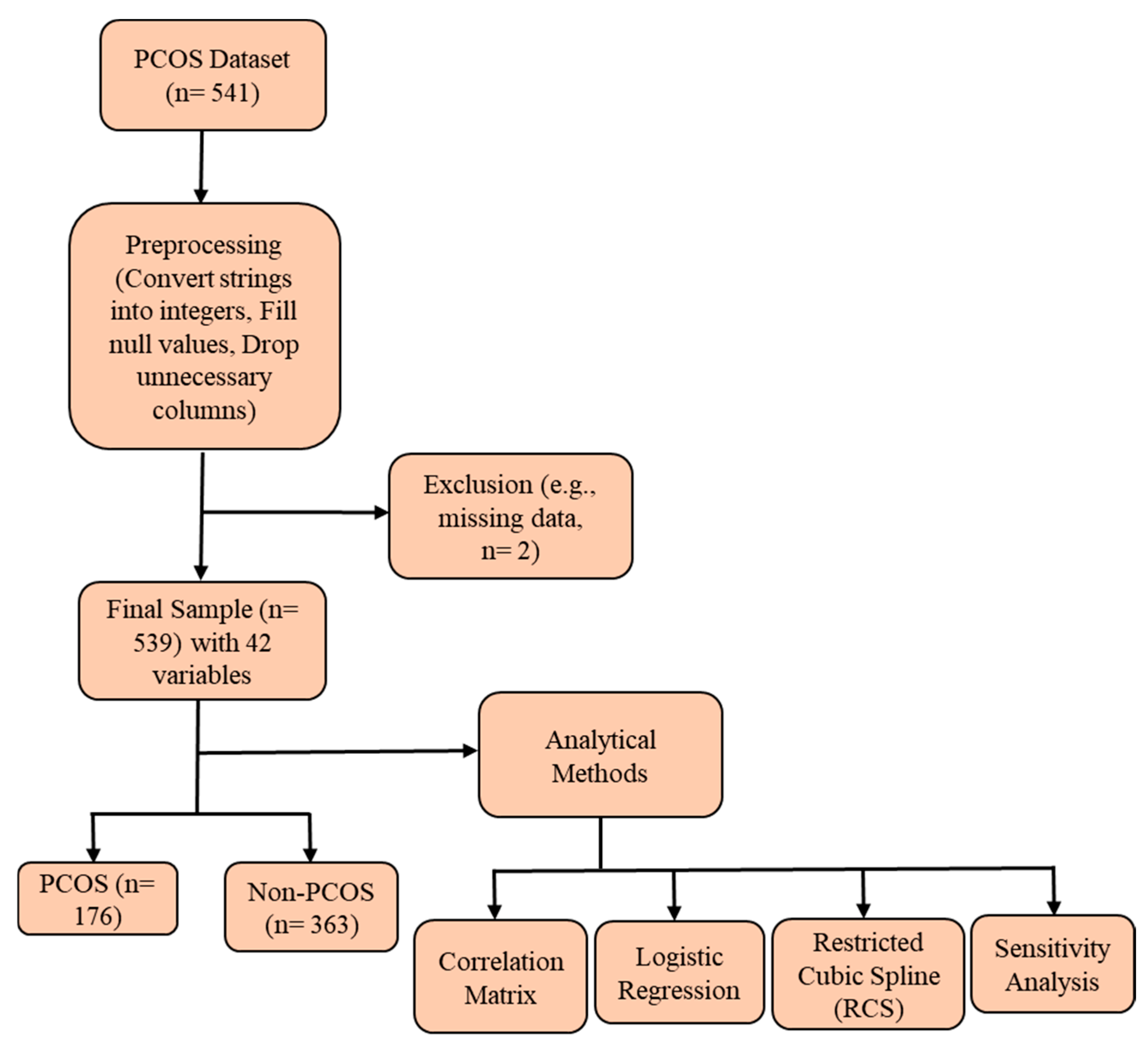
2. Methods
2.1. Survey Description
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the PCOS Group Versus the Non-PCOS Group
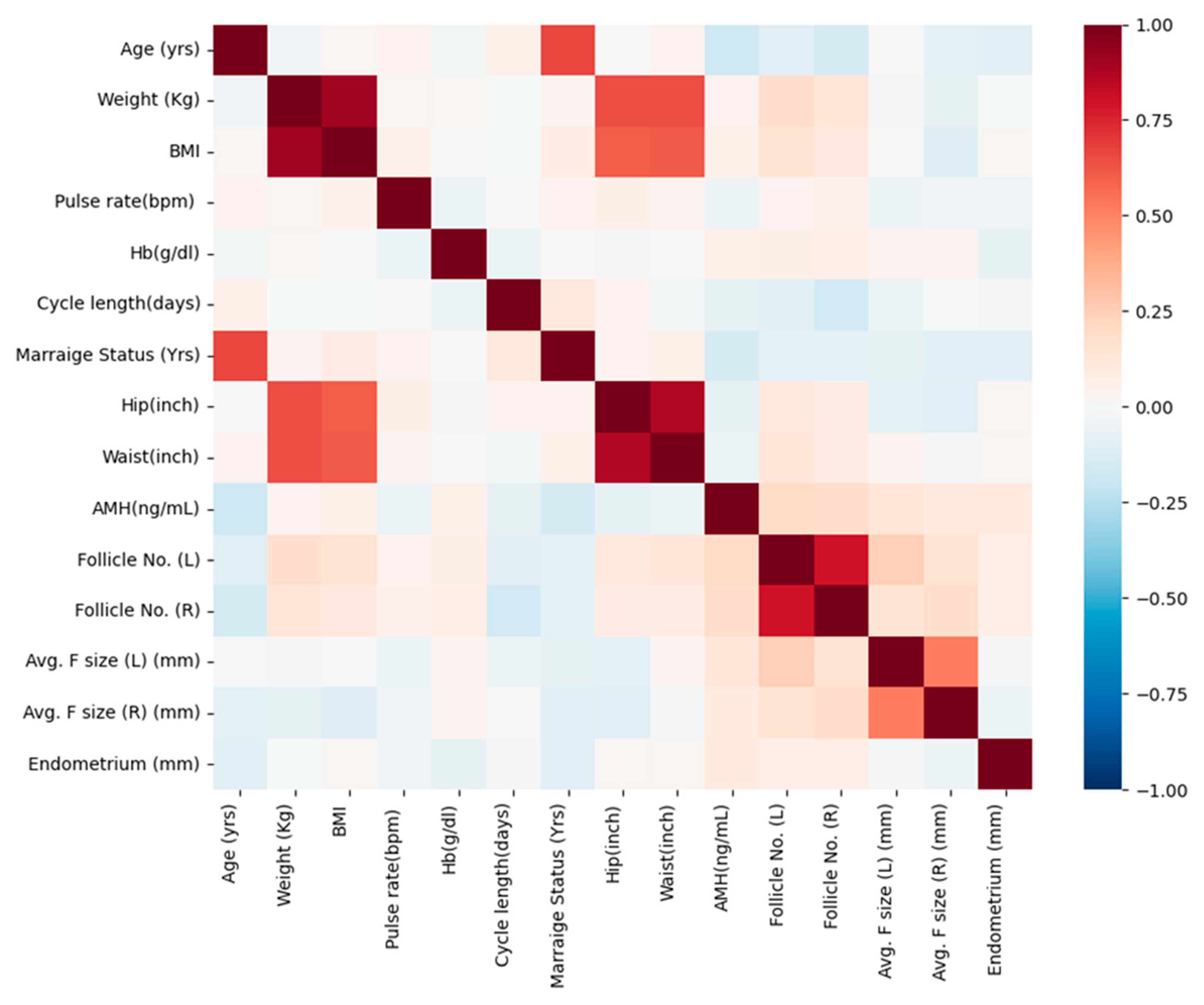
3.2. Correlation Analysis of Significant Continuous Variables in the Study Population
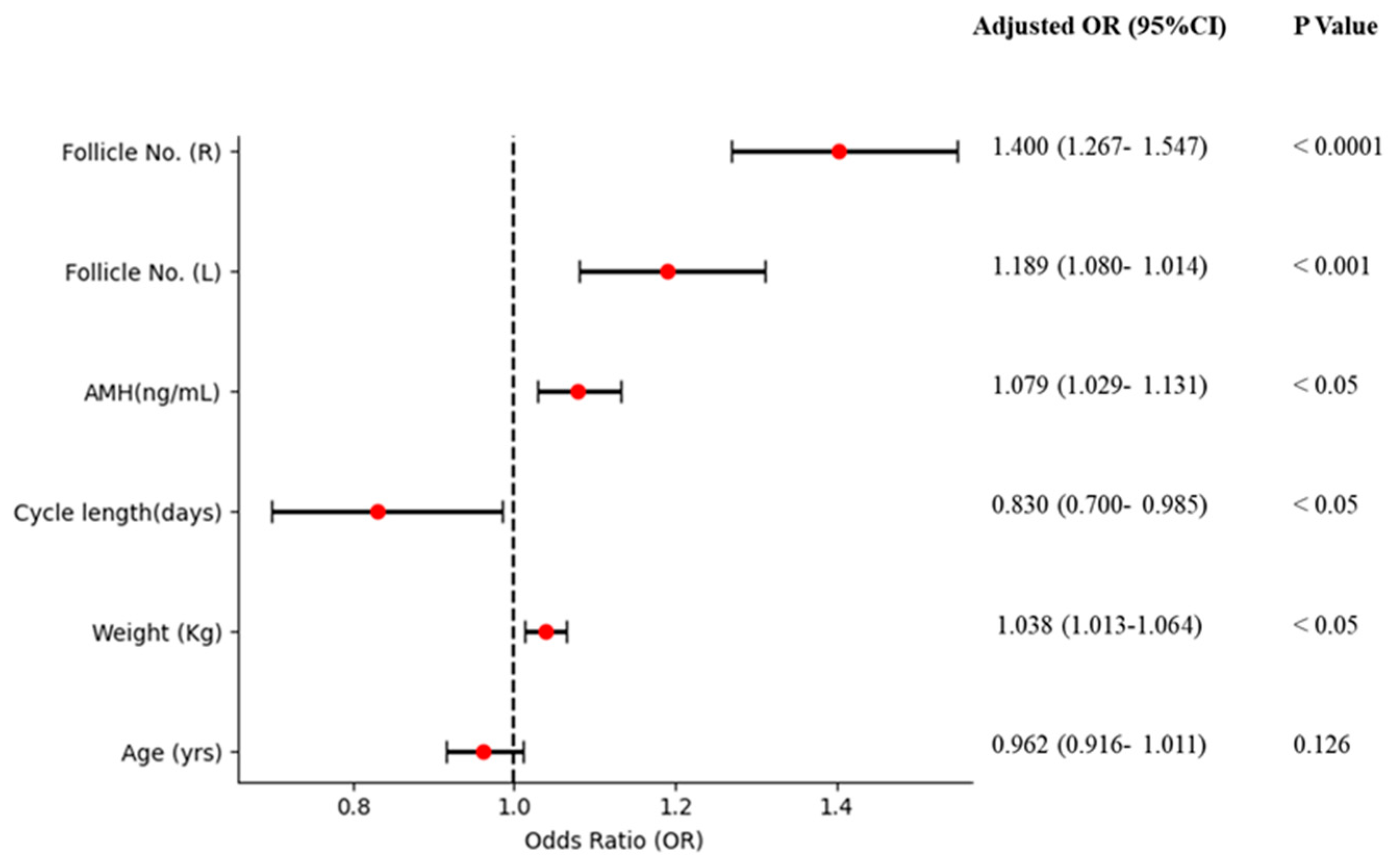
3.3. Association of AMH and Other Factors with PCOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Unraveling the Complexities of Polycystic Ovarian Syndrome: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Rosenfield, R. The Polycystic Ovary Morphology-Polycystic Ovary Syndrome Spectrum. J. Pediatr. Adolesc. Gynecol. 2015, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rather, H.A.; Kumar, R.; Basheer, M.; Reshi, M.S. A review on critical appraisal and pathogenesis of polycystic ovarian syndrome. Endocr. Metab. Sci. 2024, 14, 100162. [Google Scholar]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The prevalence of polycystic ovary syndrome: A brief systematic review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar]
- Upreti, K.; George, J.; Upreti, S.; Mahajan, S. Polycystic Ovary Syndrome Diagnosis: The Promise of Artificial Intelligence for Improved Clinical Accuracy. Biomed. Pharmacol. J. 2025, 18. Available online: https://bit.ly/4iit7Ky (accessed on 6 February 2025).
- Hussein, R.S.; Dayel, S.B.; Abahussein, O. Polycystic Ovary Syndrome and Reproductive Health: A Comprehensive Review. Clin. Exp. Obstet. Gynecol. 2024, 51, 269. [Google Scholar] [CrossRef]
- Choudhari, R.; Tayade, S.; Tiwari, A.; Satone, P.; Choudhari, R.G.; Satone, P.D. Diagnosis, Management, and Associated Comorbidities of Polycystic Ovary Syndrome: A Narrative Review. Cureus 2024, 16, e58733. [Google Scholar] [CrossRef]
- Rubin, K.H.; Glintborg, D.; Nybo, M.; Abrahamsen, B.; Andersen, M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3848–3857. [Google Scholar] [CrossRef]
- Glintborg, D.; Rubin, K.H.; Nybo, M.; Abrahamsen, B.; Andersen, M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc. Diabetol. 2018, 17, 37. [Google Scholar] [CrossRef]
- Yu, H.-F.; Chen, H.-S.; Rao, D.-P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Landin-Wilhelmsen, K.; Brannstrom, M.; Dahlgren, E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: A 21-year controlled follow-up study. J. Clin. Endocrinol. Metab. 2011, 96, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Sadeeqa, S.; Mustafa, T.; Latif, S. Polycystic ovarian syndrome–related depression in adolescent girls: A review. J. Pharm. Bioallied Sci. 2018, 10, 55–59. [Google Scholar] [CrossRef]
- Marschalek, M.L.; Marculescu, R.; Schneeberger, C.; Marschalek, J.; Dewailly, D.; Ott, J. A case-control study about markers of stress in normal-/ overweight women with polycystic ovary syndrome and in controls. Front. Endocrinol. 2023, 14, 1173422. [Google Scholar] [CrossRef]
- Behboodi Moghadam, Z.; Fereidooni, B.; Saffari, M.; Montazeri, A. Measures of health-related quality of life in PCOS women: A systematic review. Int. J. Womens Health 2018, 10, 397–408. [Google Scholar] [CrossRef]
- Sangaraju, S.L.; Yepez, D.; Grandes, X.A.; Manjunatha, R.T.; Habib, S.; Grandes, X. Cardio-Metabolic Disease and Polycystic Ovarian Syndrome (PCOS): A Narrative Review. Cureus 2022, 14, e25076. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, Y.; Wu, H.; Lin, X.; Guo, F.; Li, J.; Long, Y.; Zhou, B.; She, J.; Zhang, C.; et al. Polycystic Ovary Syndrome Fuels Cardiovascular Inflammation and Aggravates Ischemic Cardiac Injury. Circulation 2023, 148, 1958–1973. [Google Scholar] [CrossRef]
- Khomami, M.B.; Tehrani, F.R.; Hashemi, S.; Farahmand, M.; Azizi, F. Of PcOs symptoms, hirsutism has the most significant impact on the quality of life of iranian women. PLoS ONE 2015, 10, e0123608. [Google Scholar] [CrossRef]
- Hahn, S.; Janssen, O.E.; Tan, S.; Pleger, K.; Mann, K.; Schedlowski, M.; Kimmig, R.; Benson, S.; Balamitsa, E.; Elsenbruch, S. Clinical and psycho logical correlates of quality-of-life in polycystic ovary syndrome. Eur. J. Endocrinol. 2005, 153, 853–860. [Google Scholar] [CrossRef]
- Neubronner, S.A.; Indran, I.R.; Chan, Y.H.; Thu, A.W.; Yong, E.L. Effect of body mass index (BMI) on phenotypic features of poly cystic ovary syndrome (PcOs) in singapore women: A prospective cross-sectional study. BMC Womens Health 2021, 21, 135. [Google Scholar] [CrossRef]
- Sahmay, S.; Aydin, Y.; Oncul, M.; Senturk, L.M. Diagnosis of polycystic ovary syndrome: AMH in combination with clinical symptoms. J. Assist. Reprod. Genet. 2014, 31, 213–220. [Google Scholar] [PubMed]
- Di Paola, R.; Garzon, S.; Giuliani, S.; Laganà, A.S.; Noventa, M.; Parissone, F.; Zorzi, C.; Raffaelli, R.; Ghezzi, F.; Franchi, M.; et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch. Gynecol. Obst. 2018, 298, 1029–1035. [Google Scholar]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, S. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3129. [Google Scholar] [PubMed]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar]
- Oguz, S.H.; Yildiz, B.O. An Update on Contraception in Polycystic Ovary Syndrome. Endocrinol. Metab. 2021, 36, 296–311. [Google Scholar]
- Zhu, X.; Han, Y.; Feng, Y.; Shan, Y.; Liu, C.; Wang, K.; Li, X.; Zhang, S.; Han, Y. Identification of molecular characteristics in polycystic ovary syndrome using single-cell and transcriptome analysis. Sci. Rep. 2025, 15, 2970. [Google Scholar]
- Pourkhani, Z.; Jahanian Sadatmahalleh, S.; Moini, A.; Nasiri, M. The association between the follicular distribution pattern of polycystic ovaries and metabolic syndrome development in patients with polycystic ovary syndrome a prospective cohort study. Sci. Rep. 2025, 15, 5284. [Google Scholar]
- Ran, Y. The Relationship of Anti-Mullerian Hormone in Polycystic Ovary Syndrome Patients with Different Subgroups. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1419–1424. [Google Scholar]
- Wang, Q. Serum anti-Müllerian hormone as a predictor of polycystic ovarian syndrome among women of reproductive age. J. Clin. Med. 2023, 12, 493. [Google Scholar]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar]
- Osman, N. A comparison between the anti-Mullerian hormone and ovarian ultrasound for diagnosing polycystic ovary syndrome: A systematic review. GREM Gynecol. Reprod. Endocrinol. Metab. 2024, 5. [Google Scholar] [CrossRef]
- Wang, K. Androgen excess: A hallmark of polycystic ovary syndrome. Front Endocrinol. 2023, 14, 1273542. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Liu, F.T.; Xu, H.; Li, R.; Qiao, J. High antimüllerian hormone levels are associated with preterm delivery in patients with polycystic ovary syndrome. Fertil. Steril. 2020, 113, 444–452. [Google Scholar] [PubMed]
- Rosenfield, R. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar]
- Chen, W. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Bai, H.; Ding, H.; Wang, M. Polycystic Ovary Syndrome (PCOS): Symptoms, Causes, and Treatment. Clin. Exp. Obstet. Gynecol. 2024, 51, 126. [Google Scholar]
- Alomran, S.; Estrella, E. Effect of Dietary Regimen on the Development of Polycystic Ovary Syndrome: A Narrative Review. Cureus 2023, 15, e47569. [Google Scholar] [CrossRef]
- Gautam, R.; Maan, P.; Jyoti, A.; Kumar, A.; Malhotra, N.; Arora, T. The Role of Lifestyle Interventions in PCOS Management: A Systematic Review. Nutrients 2025, 17, 310. [Google Scholar] [CrossRef]
- Susan, S.M. Obesity and Polycystic Ovary Syndrome. Obes. Manag. 2007, 3, 69–73. [Google Scholar]
- Wang, Z. Irregular Cycles, Ovulatory Disorders, and Cardiometabolic Conditions. In a US-Based Digital Cohort. JAMA Netw. Open 2024, 7, e249657. [Google Scholar]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Titus, L.J.; Cramer, D.W.; Terry, K.L. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case control study. Int. J. Cancer 2017, 140, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An update of polycystic ovary syndrome: Causes Optionsoptions. Heliyon 2022, 8, e11010. [Google Scholar] [PubMed]
- He, Y.; Deng, S.; Wang, Y.; Wang, X.; Huang, Q.; Cheng, J.; Wang, D.; Lyu, G. Evaluation of ovarian stiffness and its biological mechanism using shear wave elastography in polycystic ovary syndrome. Sci. Rep. 2025, 15, 585. [Google Scholar] [CrossRef]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar]

| Characteristic | Total | PCOS | Non-PCOS | p Value |
|---|---|---|---|---|
| (n = 539) | (n = 176) | (n = 363) | ||
| Age (yrs) | 31.44 ± 5.40 | 30.16 ± 5.28 | 32.05 ± 5.36 | 0.0001 |
| Weight (kg) | 59.68 ± 11.02 | 63.12 ± 12.08 | 58.01 ± 10.06 | 0.0000 |
| Height (cm) | 156.51 ± 6.04 | 157.12 ± 6.17 | 156.21 ± 5.95 | 0.1071 |
| BMI | 24.32 ± 4.05 | 25.51 ± 4.37 | 23.74 ± 3.76 | 0.0000 |
| Blood Group (%) | 0.9570 | |||
| 11 | 20 | 19.3 | 20.4 | |
| 12 | 2.4 | 2.3 | 2.5 | |
| 13 | 25.0 | 23.9 | 25.6 | |
| 14 | 3.0 | 3.4 | 2.8 | |
| 15 | 38.2 | 37.5 | 38.6 | |
| 16 | 3.5 | 4.5 | 3.0 | |
| 17 | 7.8 | 9.1 | 7.2 | |
| 18 | 0.4 | 0.6 | 0.3 | |
| Pulse rate (bpm) | 73.24 ± 4.44 | 73.83 ± 2.74 | 72.96 ± 5.04 | 0.0099 |
| RR (breaths/min) | 19.24 ± 1.69 | 19.34 ± 1.65 | 19.20 ± 1.71 | 0.3531 |
| Hb (g/dl) | 11.16 ± 0.87 | 11.27 ± 0.83 | 11.11 ± 0.88 | 0.0347 |
| Cycle (%) | 0.0000 | |||
| R | 72.12 | 46.29 | 84.57 | |
| I | 27.88 | 53.71 | 15.43 | |
| Cycle length (days) | 4.94 ± 1.49 | 4.56 ± 1.83 | 5.13 ± 1.27 | 0.0002 |
| Marriage Status (Yrs) | 7.67 ± 4.81 | 6.89 ± 4.71 | 8.06 ± 4.82 | 0.0079 |
| Pregnant (%) | 0.6012 | |||
| Yes | 38.22 | 36.16 | 39.01 | |
| No | 61.78 | 63.84 | 60.99 | |
| No. of abortions | 0.29 ± 0.69 | 0.23 ± 0.55 | 0.32 ± 0.75 | 0.1446 |
| I beta-HCG (mIU/mL) | 666.93 ± 3354.91 | 535.05 ± 2930.93 | 730.87 ± 3544.34 | 0.4981 |
| II beta-HCG (mIU/mL) | 239.11 ± 1606.74 | 269.06 ± 1911.04 | 224.59 ± 1438.96 | 0.7847 |
| FSH (mIU/mL) | 14.64 ± 217.42 | 5.18 ± 5.76 | 19.23 ± 264.91 | 0.313 |
| LH (mIU/mL) | 6.48 ± 86.83 | 14.45 ± 151.91 | 2.62 ± 2.10 | 0.3027 |
| FSH/LH | 6.91 ± 60.80 | 5.34 ± 25.09 | 7.68 ± 72.03 | 0.5796 |
| Hip (inch) | 37.98 ± 3.96 | 38.88 ± 4.17 | 37.55 ± 3.78 | 0.0004 |
| Waist (inch) | 33.83 ± 3.60 | 34.67 ± 3.76 | 33.43 ± 3.45 | 0.0003 |
| Waist/Hip Ratio | 0.89 ± 0.05 | 0.89 ± 0.05 | 0.89 ± 0.05 | 0.6918 |
| TSH (mIU/L) | 2.96 ± 3.72 | 2.93 ± 2.83 | 2.97 ± 4.09 | 0.8994 |
| AMH (ng/mL) | 5.60 ± 5.86 | 7.78 ± 7.76 | 4.54 ± 4.29 | 0.0000 |
| PRL (ng/mL) | 24.38 ± 14.96 | 24.48 ± 13.91 | 24.33 ± 15.47 | 0.9067 |
| Vit D3 (ng/mL) | 49.99 ± 346.85 | 92.71 ± 605.64 | 29.27 ± 12.38 | 0.1664 |
| PRG (ng/mL) | 0.61 ± 3.82 | 0.37 ± 0.17 | 0.73 ± 4.65 | 0.1414 |
| RBS (mg/dl) | 99.85 ± 18.59 | 101.19 ± 23.69 | 99.20 ± 15.53 | 0.3122 |
| Weight gain (%) | 0.0000 | |||
| Yes | 37.66 | 68.36 | 22.80 | |
| No | 62.34 | 31.64 | 77.20 | |
| Hair growth (%) | 0.0000 | |||
| Yes | 27.27 | 57.06 | 12.91 | |
| No | 72.73 | 42.94 | 87.09 | |
| Skin darkening (%) | 0.0000 | |||
| Yes | ||||
| No | 30.61 | 62.15 | 15.38 | |
| 69.39 | 37.85 | 84.62 | ||
| Hair loss (%) | 0.0001 | |||
| Yes | 45.27 | 57.62 | 39.29 | |
| No | 54.73 | 42.94 | 60.71 | |
| Pimples (%) | 0.0000 | |||
| Yes | 48.98 | 69.49 | 39.01 | |
| No | 51.02 | 30.51 | 60.99 | |
| Fast food (%) | 0.0000 | |||
| Yes | 51.39 | 78.53 | 38.19 | |
| No | 48.61 | 21.47 | 61.81 | |
| Reg. Exercise (%) | 0.1718 | |||
| Yes | 24.49 | 28.81 | 22.80 | |
| No | 75.51 | 71.19 | 77.20 | |
| BP_Systolic (mmHg) | 114.68 ± 7.37 | 114.83 ± 5.55 | 114.61 ± 8.11 | 0.7083 |
| BP_Diastolic (mmHg) | 76.95 ± 5.57 | 77.27 ± 4.47 | 76.80 ± 6.03 | 0.3057 |
| Follicle No. (L) | 6.13 ± 4.23 | 9.78 ± 4.32 | 4.35 ± 2.82 | 0.0000 |
| Follicle No. (R) | 6.63 ± 4.44 | 10.75 ± 4.17 | 4.64 ± 2.93 | 0.0000 |
| Avg. F size (L) (mm) | 15.01 ± 3.57 | 15.68 ± 2.73 | 14.68 ± 3.87 | 0.0006 |
| Avg. F size (R) (mm) | 15.45 ± 3.32 | 15.91 ± 3.10 | 15.22 ± 3.41 | 0.0202 |
| Endometrium (mm) | 8.47 ± 2.16 | 8.79 ± 1.91 | 8.32 ± 2.26 | 0.0133 |
| Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|
| Parameter | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
| Total population (n = 541) | ||||
| Age (yrs) | 0.934 (0.901–0.968) | <0.001 | 0.962 (0.916–1.011) | 0.126 |
| Weight (kg) | 1.044 (1.026–1.062) | <0.0001 | 1.038 (1.013–1.064) | <0.05 |
| Cycle length (days) | 0.750 (0.654–0.862) | <0.0001 | 0.830 (0.700–0.985) | <0.05 |
| AMH (ng/mL) | 1.108 (1.069–1.149) | <0.0001 | 1.079 (1.029–1.131) | <0.05 |
| Follicle No. (L) | 1.533 (1.422–1.652) | <0.0001 | 1.189 (1.080–1.014) | <0.001 |
| Follicle No. (R) | 1.605 (1.481–1.739) | <0.0001 | 1.400 (1.267–1.547) | <0.0001 |
| Women Without Regular Exercise (n = 406) | ||||
| Age (yrs) | 0.955 (0.918–0.994) | <0.05 | 0.993 (0.938–1.051) | 0.802 |
| Weight (kg) | 1.047 (1.027–1.067) | <0.0001 | 1.036 (1.009–1.064) | <0.05 |
| Cycle length (days) | 0.678 (0.573–0.803) | <0.0001 | 0.774 (0.631–0.949) | <0.05 |
| AMH (ng/mL) | 1.110 (1.065–1.159) | <0.0001 | 1.060 (1.005–1.119) | <0.05 |
| Follicle No. (L) | 1.549 (1.417–1.694) | <0.0001 | 1.205 (1.075–1.351) | <0.001 |
| Follicle No. (R) | 1.589 (1.450–1.740) | <0.0001 | 1.369 (1.220–1.535) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, I.A.; Hosen, A.S.M.S.; Ghimire, D.; Park, M.J. Association of Polycystic Ovary Syndrome with Clinical, Physical, and Reproductive Factors: A Data-Driven Analysis. Diagnostics 2025, 15, 711. https://doi.org/10.3390/diagnostics15060711
Begum IA, Hosen ASMS, Ghimire D, Park MJ. Association of Polycystic Ovary Syndrome with Clinical, Physical, and Reproductive Factors: A Data-Driven Analysis. Diagnostics. 2025; 15(6):711. https://doi.org/10.3390/diagnostics15060711
Chicago/Turabian StyleBegum, Ismat Ara, A. S. M. Sanwar Hosen, Deepak Ghimire, and Mi Jin Park. 2025. "Association of Polycystic Ovary Syndrome with Clinical, Physical, and Reproductive Factors: A Data-Driven Analysis" Diagnostics 15, no. 6: 711. https://doi.org/10.3390/diagnostics15060711
APA StyleBegum, I. A., Hosen, A. S. M. S., Ghimire, D., & Park, M. J. (2025). Association of Polycystic Ovary Syndrome with Clinical, Physical, and Reproductive Factors: A Data-Driven Analysis. Diagnostics, 15(6), 711. https://doi.org/10.3390/diagnostics15060711








