Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
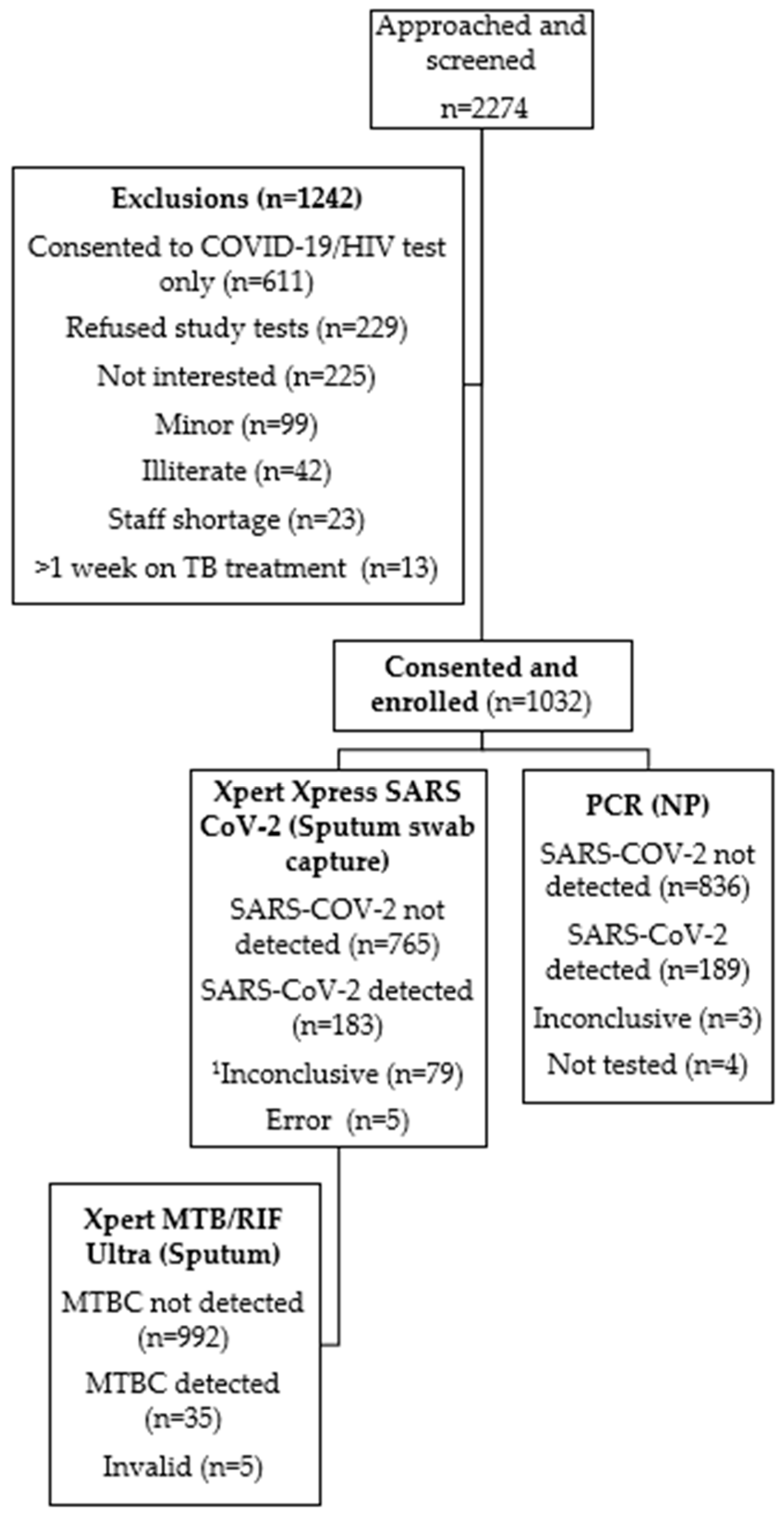
2.2. Participant Recruitment
2.3. Specimen Collection
2.4. Specimen Testing
2.4.1. Nasopharyngeal Swabs
2.4.2. Sputum
2.4.3. Saliva and Tongue Swabs
3. Results
3.1. Participant Characteristics
3.2. TB and Drug-Resistant TB
3.3. COVID-19 Routine PCR Results
3.4. Xpert Xpress SARS-CoV-2 Results
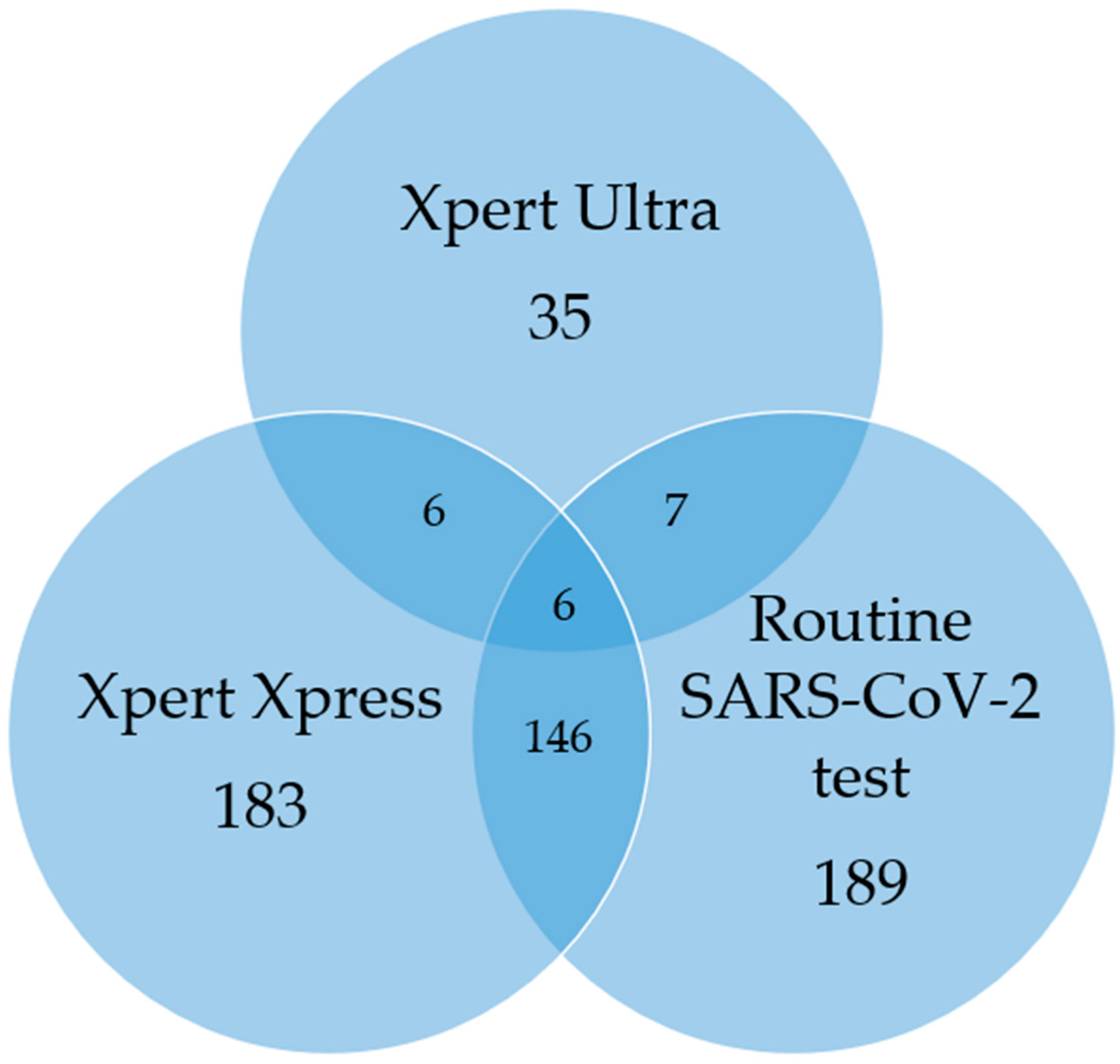
3.5. TB/SARS-CoV-2 Co-Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TB | tuberculosis |
| MTBC | Mycobacterium tuberculosis complex |
| Xpress | Xpert Xpress SARS-CoV-2 |
| COVID-19 | Coronavirus Disease 2019 |
| TUTT | Targeted Universal Testing for TB |
| PLHIV | people living with HIV |
| HCFs | health care facilities |
| W4SS | WHO-recommended four-symptom screen |
| WHO | World Health Organization |
| mWRDs | WHO-approved rapid diagnostic tests |
| NP | nasopharyngeal |
| PHCH | primary health care clinics |
| PCR | polymerase chain reaction |
| TS | tongue swab |
| ARV | antiretroviral |
References
- Global Tuberculosis Report 2021. World Health Organization. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 4 May 2022).
- Global Tuberculosis Report 2024. World Health Organization. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (accessed on 4 November 2024).
- Global Tuberculosis Report 2022. World Health Organization. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 19 November 2022).
- Global Tuberculosis Report 2023. World Health Organization. Available online: https://www.who.int/publications/i/item/9789240083851 (accessed on 19 December 2023).
- National TB Recovery Plan. 2022. Available online: https://tbthinktank.org/wp-content/uploads/2022/09/TB-Recovery-Plan-for-South-Africa-version-7.pdf (accessed on 5 November 2022).
- National TB Recovery Plan 2.0. 2023. Available online: https://tbthinktank.org/wp-content/uploads/2023/09/TB-Recovery-Plan-2.0-April-2023-March-2024.pdf (accessed on 3 September 2024).
- South African National Department of Health. National Tuberculosis Management Guidelines. Available online: https://knowledgehub.health.gov.za/elibrary/national-tuberculosis-management-guidelines (accessed on 22 May 2023).
- World Health Organization. Module 2: Systematic Screening for Tuberculosis Disease, WHO Consolidated Guidelines on TUBERCULOSIS. Available online: https://www.who.int/publications/i/item/9789240022676 (accessed on 22 November 2022).
- Frascella, B.; Richards, A.; Sossen, B.; Emery, J.; Odone, A.; Law, I.; Onozaki, I.; Esmail, H.; Houben, R.M.G.J. Subclinical tuberculosis disease—Review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin. Infect. Dis. 2021, 73, e830–e841. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.; Bryden, W.; Call, C.; McKerry, A.; Leonard, B.; Seldon, R.; Gqada, M.; Dinkele, R.; Gessner, S.; Warner, D.F.; et al. Cough-independent production of viable Mycobacterium tuberculosis in bioaerosol. Tuberculosis 2021, 126, 102038. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Prasad, R.; Gupta, A.; Das, K.; Gupta, N. Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis: Convergence can be fatal. Monaldi Arch. Chest Dis. 2020, 22, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.J. Diagnosis of pulmonary tuberculosis: Recent advances and diagnostic algorithms. Tuberc. Respir. Dis. 2015, 78, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Visca, D.; Ong, C.W.M.; Tiberi, S.; Centis, R.; D’ambrosio, L.; Chen, B.; Mueller, J.; Mueller, P.; Duarte, R.; Dalcolmo, M.; et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, Y.; Fleming, J.; Wang, T.; Shen, S.; Wang, Y.; Fan, L.; Ma, J.; Gu, Y.; Chen, Y. Severe COVID-19 cases with a history of active or latent tuberculosis. Int. J. Tuberc. Lung Dis. 2020, 24, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Sy, K.T.L.; Haw, N.H.L.; Uy, J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect. Dis. 2020, 52, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Simultaneous, Integrated Diagnostic Testing Approach to Detect COVID-19 and TB in High TB Burden Countries. Available online: http://www.stoptb.org/assets/documents/covid/COVID-TB%20Testing%20Simultaneous_March%202021.pdf (accessed on 22 October 2022).
- Glogowska, A.; Przybylski, G.; Bukowski, J.; Zebracka, R.; Krawiecka, D. TB and COVID-19 co-infection in a pulmonology hospital. Int. J. Tuberc. Lung Dis. 2023, 27, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Adusi-Poku, Y.; Wagaw, Z.A.; Frimpong-Mansoh, R.P.; Asamoah, I.O.; Sorvor, F.; Afutu, F.K.; Sarpong, C.; Amoussou-Gohoungo, L.O.; Abdulai, F.N.; Ahmedov, S. Bidirectional screening and testing for TB and COVID-19 among outpatient department attendees: Outcome of an initial intervention in Ghana. BMC Infect. Dis. 2023, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Genade, L.P.; Kahamba, T.; Scott, L.; Tempia, S.; Walaza, S.; David, A.; Stevens, W.; Hlongwane, K.; von Gottberg, A.; Du Plessis, M.; et al. Co-testing a single sputum specimen for TB and SARS-CoV-2. Int. J. Tuberc. Lung Dis. 2023, 1, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Kahamba, T.; David, A.; Martinson, N.; Nabeemeeah, F.; Zitha, J.; Moosa, F.; Du Plessis, M.; da Silva, P.; Stevens, W.; Scott, L. Dual detection of TB and SARS CoV 2 disease from sputum among in patients with pneumonia using the GeneXpert ® system. In Proceedings of the Union, 52nd World Conference on Lung Health, Virtual, 19–22 October 2021. [Google Scholar]
- Network for Genomics Surveillance in South Africa. SARS-CoV-2 Genomic Surveillance Update. Available online: https://www.nicd.ac.za/wp-content/uploads/2022/01/Update-of-SA-sequencing-data-from-GISAID-14-Jan-2022_dash_v2-Read-Only.pdf (accessed on 7 November 2022).
- Network for Genomics Surveillance in South Africa. SARS-CoV-2 Genomic Surveillance Update. Available online: https://www.nicd.ac.za/wp-content/uploads/2022/06/Update-of-SA-sequencing-data-from-GISAID-24-June-2022.pdf (accessed on 22 November 2022).
- Lacobucci, G. COVID-19: Runny Nose, Headache and Fatigue Are Commonest Symptoms of Omicron, Early Data Show. BMJ. 2021, 375, n3103. [Google Scholar] [CrossRef] [PubMed]
- Marais, G.; Hsiao, N.Y.; Iranzadeh, A.; Doolabh, D.; Enoch, A.; Chu, C.; Williamson, C.; Brink, A.; Hardie, D. Saliva swabs are the preferred sample for Omicron detection. medRxiv 2021. [Google Scholar] [CrossRef]
- Scott, L.E.; Hsiao, N.-Y.; Dor, G.; Hans, L.; Marokane, P.; da Silva, M.P.; Preiser, W.; Vreede, H.; Tsoka, J.; Mlisana, K.; et al. How South Africa Used National Cycle Threshold (Ct) Values to Continuously Monitor SARS-CoV-2 Laboratory Test Quality. Diagnostics 2023, 13, 2554. [Google Scholar] [CrossRef] [PubMed]
- South African Tuberculosis Drug Resistance Survey 2012–14: National Institute for Communicable Diseases. Available online: https://www.nicd.ac.za/assets/files/K-12750%20NICD%20National%20Survey%20Report_Dev_V11-LR.pdf (accessed on 16 February 2025).
- Wang, Q.; Cao, Y.; Liu, X.; Fu, Y.; Zhang, J.; Zhang, Y.; Zhang, L.; Wei, X.; Yang, L. Systematic review and meta-analysis of Tuberculosis and COVID-19 Co-infection: Prevalence, fatality, and treatment considerations. PLOS Neglected Trop. Dis. 2024, 18, e0012136. [Google Scholar] [CrossRef] [PubMed]
- Jassat, W.; Karim, S.S.A.; Mudara, C.; Welch, R.; Ozougwu, L.; Groome, M.J.; Govender, N.; von Gottberg, A.; Wolter, N.; Wolmarans, M.; et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: A retrospective observational study. Lancet Glob. Health 2022, 10, e961–e969. [Google Scholar] [CrossRef] [PubMed]
- Luke, E.; Swafford, K.; Shirazi, G.; Venketaraman, V. TB and COVID-19: An Exploration of the Characteristics and Resulting Complications of Co-infection. Front. Biosci. (Schol. Ed). 2022, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Karim, Q.; Baxter, C. COVID-19: Impact on the HIV and Tuberculosis Response, Service Delivery, and Research in South Africa. Curr. HIV/AIDS Rep. 2022, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zokufa, N.; Lebelo, K.; Hacking, D.; Tabo, L.; Runeyi, P.; Malabi, N.; Sibanda, S.B.; Cassidy, T.; Makanda, G.; Norman, B.; et al. Community-based TB testing as an essential part of TB recovery plans in the COVID-19 era. Int. J. Tuberc. Lung Dis. 2021, 25, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Multi-Disease Diagnostic Landscape for the Integrated Management of HIV, HCV, TB and Other Co-Infections. Available online: https://unitaid.org/assets/multi-disease-diagnostics-landscape-for-integrated-management-of-HIV-HCV-TB-and-other-coinfections-january-2018.pdf (accessed on 11 October 2024).
- Ndlovu, Z.; Fajardo, E.; Mbofana, E.; Maparo, T.; Garone, D.; Metcalf, C.; Bygrave, H.; Kao, K.; Zinyowera, S. Multidisease testing for HIV and TB using the GeneXpert platform: A feasibility study in rural Zimbabwe. PLoS ONE 2018, 2, e0193577. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All (n = 1032) | CCHC (n = 506) | MCHC (n = 526) |
|---|---|---|---|
| Demographics | |||
| Age, Mean (range), Years | 41 (18–84) | 39 (18–78) | 44 (18–84) |
| Male sex, n (%) | 459 (44.5) | 190 (37.5) | 269 (51.1) |
| HIV-related information | |||
| HIV-positive, n (%) | 248 (24.0) | 102 (20.2) | 146 (27.8) |
| HIV-negative, n (%) | 744 (72.1) | 371 (73.3) | 373 (70.9) |
| Unknown, n (%) | 40 (3.8) | 33 (6.5) | 7 (1.3) |
| Receiving ARV therapy, n/total (%) | 227/248 (91.5) | 90/102 (88.2) | 137/146 (93.8) |
| Abnormal Random glucose levels, n/total (%) | 15/1032 (1.5) | 9/506 (1.7) | 6/526 (1.1) |
| Co-morbidities | |||
| Hypertension, n (%) | 166 (16.1) | 73 (14.4) | 93 (17.7) |
| Diabetes, n (%) | 48 (4.7) | 27 (5.3) | 21 (4.0) |
| Heart disease, n (%) | 12 (1.2) | 9 (1.8) | 3 (0.6) |
| Other chronic lung diseases, n (%) | 8 (0.8) | 3 (0.6) | 5 (0.9) |
| Cancer, n (%) | 7 (0.7) | 2 (0.4) | 5 (0.9) |
| TB History | |||
| Previously diagnosed with TB, n (%) | 87 (8.4) | 34 (6.7) | 53 (10.1) |
| Contact of person diagnosed with TB, n (%) | 30 (2.9) | 21 (4.2) | 9 (1.7) |
| Clinical signs and symptoms at presentation | |||
| Symptom duration (days), Average (range) | 5.2 (1–62 days) | 3.9 (1–15 days) | 6.5 (1–62 days) |
| Cough, n/total (%) | 881/935 (94.2) | 468/482 (96.5) | 413/453 (91.2) |
| Headache, n/total (%) | 680/935 (72.7) | 405/482 (84.0) | 275/453 (60.7) |
| Sore throat, n/total (%) | 645/935 (68.9) | 377/482 (78.2) | 268/453 (59.2) |
| Fatigue, n/total (%) | 515/935 (55.1) | 240/482 (49.8) | 275/453 (60.7) |
| Nasal congestion/runny nose, n/total (%) | 424/935 (45.3) | 268/482 (55.6) | 156/453 (34.4) |
| Self-reported fever/chills, n/total (%) | 328/935 (35.1) | 242/482 (50.2) | 86/453 (18.9) |
| Muscle pain, n/total (%) | 264/935 (28.2) | 82/482 (17.0) | 183/453 (40.4) |
| Nights sweats, n/total (%) | 255/935 (27.3) | 145/482 (30.1) | 110/453 (24.3) |
| Ageusia, n/total (%) | 222/935 (23.7) | 149/482 (30.9) | 73/453 (16.1) |
| Loss of appetite, n/total (%) | 206/935 (22.0) | 134/482 (27.8) | 72/453 (15.9) |
| Shortness of breath, n/total (%) | 192/935 (20.5) | 158/482 (32.8) | 34/453 (7.5) |
| Unexplained weight loss, n/total (%) | 173/935 (18.5) | 87/482 (18.0) | 86/453 (19.0) |
| Anosmia, n/total (%) | 134/935 (14.3) | 93/482 (19.3) | 41/453 (9.1) |
| Abdominal pains, n/total (%) | 104/935 (11.1) | 52/482 (10.8) | 52/453 (11.5) |
| Other 1, n/total (%) | 101/935 (10.8) | 58/482 (12.0) | 43/453 (9.5) |
| GIT symptoms, n/total (%) | 59/935 (6.3) | 37/482 (7.7) | 22/453 (4.9) |
| COVID-19 vaccination status | |||
| Vaccinated, n/total (%) | 563/1008 (55.9) | 299/490 (61.0) | 264/518 (50.9) |
| Asymptomatic, n/total (%) | 97/1032 (9.4) | 24/506 (4.7) | 73/526 (13.9) |
| Variable | TB Xpert MTB/RIF Ultra (n = 35) | Routine PCR for SARS-CoV-2 (n = 189) | Xpert Xpress for SARS-CoV-2 (n = 183) | TB/COVID Co-Infected (n = 10) |
|---|---|---|---|---|
| Demographics | ||||
| Age, Mean (range), Years | 37 (20–74) | 42 (18–74) | 41 (18–74) | 37 (22–52) |
| Male sex, n (%) | 22 (62.8) | 62 (32.8) | 66 (36.1) | 4 (40.0) |
| HIV-related information | ||||
| HIV-positive, n (%) | 10 (28.6) | 37 (19.6) | 39 (21.3) | 5 (50.0) |
| HIV-negative, n (%) | 24 (68.6) | 135 (71.4) | 127 (69.4) | 5 (50.0) |
| Unknown, n (%) | 1 (2.9) | 33 (17.5) | 17 (9.3) | 0 (0) |
| Co-morbidities | ||||
| Other chronic lung diseases, n (%) | 1 (2.9) | 2 (1.1) | 2 (1.1) | 1 (10.0) |
| Hypertension, n (%) | 1 (2.9) | 33 (17.5) | 29 (15.9) | 1 (10.0) |
| Heart disease, n (%) | 0 (0) | 6 (3.2) | 2 (1.1) | 0 (0) |
| Diabetes, n (%) | 1 (2.9) | 11 (5.8) | 7 (3.8) | 0 (0) |
| Cancer, n (%) | 0 (0) | 1 (0.5) | 2 (1.1) | 0 (0) |
| Clinical signs and symptoms at presentation | ||||
| Symptom duration (days), Average (range) | 10.8 (3–60) | 4.6 (1–38) | 4.7 (1–38) | 9.6 (3–21) |
| Cough, n/total (%) | 31/34 (91.2) | 165/181 (91.2) | 155/171 (90.6) | 8/10 (80.0) |
| Headache, n/total (%) | 23/34 (67.6) | 145/181 (80.1) | 134/171 (78.3) | 5/8 (62.5) |
| Sore throat, n/total (%) | 23/34 (67.6) | 133/181 (73.4) | 128/171 (74.9) | 7/8 (87.5) |
| Unexplained weight loss, n/total (%) | 21/34 (61.2) | 39/181 (21.5) | 36/171 (21.1) | 8/10 (80.0) |
| Nights sweats, n/total (%) | 21/34 (61.2) | 51/181 (28.2) | 51/171 (29.8) | 8/10 (80.0) |
| Loss of appetite, n/total (%) | 20/34 (58.8) | 45/181 (24.9) | 42/171 (24.6) | 6/10 (60.0) |
| Fatigue, n/total (%) | 20/34 (58.8) | 114/181 (62.9) | 106/171 (62.0) | 5/8 (62.5) |
| Nasal congestion/runny nose, n/total (%) | 13/34 (38.2) | 82/181 (45.3) | 82/171 (48.0) | 2/8 (25.0) |
| Muscle pain, n/total (%) | 12/34 (35.3) | 63/181 (35.2) | 63/171 (36.8) | 3/8 (37.5) |
| Ageusia, n/total (%) | 11/34 (32.4) | 47/181 (26.0) | 45/171 (26.3) | 2/8 (25.0) |
| Self-reported fever/chills, n/total (%) | 7/34 (20.6) | 73/181 (40.3) | 71/171 (41.5) | 1/8 (12.5) |
| Shortness of breath, n/total (%) | 4/34 (11.8) | 35/181 (19.3) | 35/171 (20.5) | 0/8 (0.0) |
| Anosmia, n/total (%) | 4/34 (11.8) | 27/181 (15.0) | 27/171 (15.8) | 1/8 (12.5) |
| Abdominal pains, n/total (%) | 4/34 (11.8)) | 35/181 (19.3) | 33/171 (19.3) | 0/8 (0.0) |
| GIT symptoms, n/total (%) | 4/34 (11.8) | 8/181 (4.4) | 8/171 (4.7) | 1/8 (12.5) |
| Other 1, n/total (%) | 2/34 (5.9) | 11/181 (6.1) | 15/171 (8.8) | 0/8 (0.0) |
| Asymptomatic, n/total (%) | 1/35 (2.9) | 8/189 (4.2) | 12/183 (6.6) | 0/0 (0) |
| Xpert Xpress Result | |||||
|---|---|---|---|---|---|
| Routine SARS-CoV-2 result | Positive | Negative | Total | Percent agreement n/N (%) | |
| Positive | 146 | 35 | 181 | PPA, 146/181 (81%) | |
| Negative | 37 | 723 | 760 | NPA, 723/760 (95%) | |
| Total | 183 | 758 | 941 | OPA, 869/941 (92%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, A.; Genade, L.; Scott, L.E.; da Silva, M.P.; Singh, L.; Stevens, W.; Martinson, N. Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection. Diagnostics 2025, 15, 720. https://doi.org/10.3390/diagnostics15060720
David A, Genade L, Scott LE, da Silva MP, Singh L, Stevens W, Martinson N. Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection. Diagnostics. 2025; 15(6):720. https://doi.org/10.3390/diagnostics15060720
Chicago/Turabian StyleDavid, Anura, Leisha Genade, Lesley Erica Scott, Manuel Pedro da Silva, Lyndel Singh, Wendy Stevens, and Neil Martinson. 2025. "Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection" Diagnostics 15, no. 6: 720. https://doi.org/10.3390/diagnostics15060720
APA StyleDavid, A., Genade, L., Scott, L. E., da Silva, M. P., Singh, L., Stevens, W., & Martinson, N. (2025). Concurrent Tuberculosis and COVID-19 Testing from a Single Sputum Specimen for Enhanced Disease Detection. Diagnostics, 15(6), 720. https://doi.org/10.3390/diagnostics15060720






