A Systematic Review: State of the Science on Diagnostics of Hidden Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Summary of Selection
| Author (Year) | Reason for Exclusion from Review |
|---|---|
| Valderrama et al. (2018) [34] | Evaluation of a correlational relationship between lifetime noise exposure and ABR, rather than two distinct at-risk and control groups. The study was also released prior to 2019. |
| Guest et al. (2019) [35] | Assessed MEMR thresholds exclusively in subjects with tinnitus, rather than noise exposure or age. The presence of previously identified otologic conditions such as tinnitus merit exclusion. |
| Kramerer et al. (2019) [36] | The study sought to identify the effects of cognitive capacity in influencing physiologic and behavioral measures of CS. It focused on the assessment of variance in measuring synaptopathy and identifying confounding variables rather than quantifying synaptopathy between groups. |
| Prendergast (2019) [37] | This study included the use of a regression model to determine relative contributions of age and noise exposure on synaptopathy. There was a lack of clearly defined at-risk and control groups. |
| Marmel et al. (2020) [38] | Assessed CS in tinnitus sufferers only. Not all participants demonstrated normal audiograms. |
| Megarbane and Fuente (2020) [39] | The sample included participants who did not have occupational noise exposure; no stratification of groups by relative risk of CS. |
| Mepani et al. (2020) [40] | Assessment of purely normal-hearing subjects with no stratification of group by risk, with correlational analyses. |
| Nam et al. (2020) [41] | The use of acute noise exposure to identify at-risk patients, rather than long-term or lifetime noise exposure. |
| Bal and Derinsu (2021) [42] | Several participants in this study did not display normal pure-tone audiograms. |
| Chen et al. (2021) [43] | The study assessed the presence of CS in patients already diagnosed with presbycusis, rather than participants with no previously identified ear pathology. |
| Wang et al. (2021) [44] | Measurements of CS occurred following acute noise exposure (attendance of a music festival) rather than long-term noise exposure. |
| Carcagno and Plack (2022) [45] | The study assessed whether variance in masked-speech reception tasks can be explained by the variance in ABR and FFR. No assessment of diagnostic value for CS. |
| Kaf et al. (2022) [46] | Use of acute noise exposure (6 months) to stratify high- and low-risk groups, rather than long-term exposure. |
| Lobdell et al. (2022) [47] | Correlational study design assessing the reliability of MEMR between laboratory and clinical measures. |
| Goodman et al. (2023) [48] | The study provides a recommended study design and protocol for using ECochG to measure CS to reduce variability in measurements. |
| Haggerty et al. (2023) [49] | Use of animal models (gerbils) to assess CS rather than human subjects. |
| Shehabi et al. (2023) [50] | Association study design determining the relationship between MEMR and binaural temporal coding performance. Lack of defined at-risk and control groups. |
| Couth et al. (2024) [51] | Cumulative levels of noise exposure between the control and at-risk group were similar, indicating no difference in susceptibility in measuring CS. |
| De Poortere et al. (2024) [52] | The study measured intra-subject variability in measures of CS to assess the reliability of these measures and possible learning effects. Lack of diagnostic measurements for CS. |
| Ding et al. (2024) [53] | Assessed correlation between pure-tone audiometry and DPOAEs. Participants with abnormal audiograms were also included. |
| Fujihira et al. (2024) [54] | Correlational study design with lack of control and at-risk groups. |
| Kamerer et al. (2024) [55] | The study assessed the percentage of variance in hearing thresholds explained by physiological and behavioral measures of HHL. |
| Liu et al. (2024) [56] | The study involved the development of a novel model quantifying perceptual hearing consequences due to cochlear deafferentation. Lack of assessment of CS in human subjects. |
| McFarlane and Sanchez (2024) [57] | Correlational study design with no clearly defined at-risk and control groups. |
| Saade et al. (2024) [58] | Correlational study design assessing extended high-frequency thresholds with subjective audiologic symptoms. |
| Schirmer et al. (2024) [59] | The study did not exclude participants with abnormal audiograms. |
| Temboury-Gutierrez (2024) [60] | Participants included in this study showed normal audiograms between 125 Hz and 4 kHz, rather than up to 8 kHz. |

| Author (Year) | Diagnostic Method | Subject Population | Behavioral Correlate (If Applicable) | Key Findings |
|---|---|---|---|---|
| Bhatt and Wang (2019) [62] | Click-evoked auditory brainstem response (ABR) | 32 females; 18 with high noise exposure background (NEB) and 14 with low NEB. | Dichotic listening performance, speech-in-noise (SiN) performance | No significant difference in ABR wave I, III, and V amplitude between high and low NEB groups. No significant difference in speech-in-noise performance. Higher NEB participants demonstrated worse dichotic listening performance. |
| Carcagno and Plack (2020) [63] | Ratio of ABR wave I amplitude at high and low click levels (WIH/WIL), and difference in frequency-following response (FFR) at shallow and deep-modulated tones (FFRS-FFRD). Utilized high-pass masking to exclude response from high- frequency cochlear regions. | 102 individuals, separated into three age groups: young (n = 34), middle-aged (n = 34), and older (n = 34). | None | WIH/WIL ratio declined with age without high-pass masking, though it did not decrease in the masking condition. FFR components including temporal fine structure (TFS) and envelope (ENV) decreased with age. However, no age-related decreases of FFRS-FFRD were found with and without masking. Electrophysiological measures assessing purely low-frequency regions may not be a valuable measure of cochlear synaptopathy. |
| Dewey et al. (2020) [64] | Click-evoked auditory brainstem response (ABR), and fMRI of ascending auditory pathway. | Individuals aged 25–40, separated by lifetime noise exposure: high (n = 32) and low (n = 30). | None | Participants in the high noise exposure group had significantly enhanced fMRI responses to broadband noise compared with the low exposure group, suggesting central hyperactivity. Sustained responses did not differ among groups. No significant group differences were found in the ABR response between groups, including amplitudes of wave I and V, as well as the ratio of wave I/V. |
| Kikidis et al. (2020) [65] | ABR response amplitudes and latencies for waves I, II, and V, measured at 3 click rates (11/s, 33/s, and 44/s) | Two groups, aged 18–41: 24 musicians with occupational noise exposure and 24 healthy controls. | Speech in bubble audiometry | Wave I amplitude was significantly lower among the musician experimental group at all 3 click rates. Also, the experimental group demonstrated a significant reduction in the wave I/V ratio for the 33/s click rate. |
| Bal and Derinsu (2021) [42] | Tympanometry, electrocochleography (EcochG), ABR | 50 healthy young adults stratified by total noise level from personal devices. High-risk (n = 25) and low-risk (n = 25) groups. | Matrix Test | The action potential (AP) amplitude was significantly decreased in EcochG in high-risk compared with low-risk subjects. Wave V of ABR was also significantly decreased between groups. High-risk participants performed worse on the matrix test. |
| Bramhall et al. (2021) [66] | ABR wave I amplitude and envelope following response (EFR) | Young individuals (aged 19–35). There were 2 groups, consisting of veterans with a history of noise exposure and healthy non- veterans. | None | There was a reduction in the EFR magnitude and ABR Wave I amplitude among the Veteran population compared to the non-Veterans. |
| Megha et al. (2021) [67] | ABR, distortion product otoacoustic emissions (DPOAEs), contralateral suppression of OAE (CSOAE) | 60 adult males. Group 1: 20 subjects with no noise exposure. Group 2: 20 subjects aged 45–65 with no occupational noise exposure. Group 3: 20 subjects < 35 years old exposed to occupational noise. | None | Group 1 demonstrated greater CSOAEs compared with Groups 2 and 3. Groups 2 and 3 demonstrated a significant reduction in wave I amplitude and increase in wave V/I ratio on ABRs compared with Group 1. No significant difference in latency in ABRs. No significant difference in DEOAEs among groups. |
| Suresh and Krishnan (2021) [68] | DPOAEs and ABRs (2 experiments):
| 56 total participants. There were 28 normal-hearing adults who participated in a marching band for 5 years and 28 adults as part of a low-risk control group with no history of occupational exposure. | Conventional and high-frequency audiometry | No significant difference between groups in DPOAEs or PTA. In Experiment 1, the high-risk group showed smaller wave I amplitudes at moderate and high sounds compared with the low-risk group. Wave III and V amplitudes were similar, though the wave V/I ratio was enhanced in the high-risk group. Experiment 2 also demonstrated a smaller wave I amplitude for the high-risk group, though the relationship was less pronounced than in Experiment 1. Wave V amplitude and latency was similar between groups. |
| Bhatt et al. (2022) [69] | MEMR, DPOAE, ABR | 30 healthy adults aged 18–35; 15 with high NEB and 15 with low NEB. | Conventional and high-frequency audiometry, SiN. | The high NEB group showed significantly reduced DPOAEs and ABR wave I amplitude compared with the low NEB group. The high-risk group also performed significantly worse on the SiN assessment. MEMR was not significantly different between groups, though it was associated with NEB. |
| Bramhall et al. (2022) [70] | MEMR | 184 total participants, containing 92 young veterans and 92 non-veterans. | None | A 25% reduction in MEMR magnitude was found in the veteran compared with the non-veteran population. The reduction in MEMR is consistent with the decrease in wave I amplitude and EFR magnitude in the veteran population. |
| Cildir et al. (2022) [71] | ABR | 2 groups separated based on lifetime noise exposure. High-risk (n = 39) and low-risk (n = 30). | Dichotic digit test, matrix sentence test, amplitude modulation detection test, loudness adaptation test | The high-risk group had a significantly lowered wave I amplitude compared with the low-risk group with 70 and 80 db stimuli. Compared with the low-risk group, the high-risk group demonstrated a significantly more rapid adaptation to stimuli in the presence of background noise (120 s vs. 40 s). Amplitude modulation detection performance was higher in the high-risk group. There was a significant difference in matrix sentence test performance between groups only in the right ear, though the authors do not deem this result significant. |
| Lai and Bidelman (2022) [72] | EcochG: measuring summating potentials (SPs) with paired versus single clicks | 18 young adult subjects, between 23 and 33 years. Low- (n = 9) and high-risk (n = 9) groups demonstrated normal and elevated high-frequency thresholds, respectively. | Conventional and high-frequency audiometry, SiN, and Listening Effort and Noise Sensitivity Questionaire (LENS-Q) | High-risk subjects demonstrated significant increases in SP amplitudes in response to paired versus single clicks compared with the low-risk subjects, which had more consistent SP amplitudes. This finding was particularly relevant for paired click stimuli with short inter-click intervals. Larger SP ratios were correlated with a worse SiN performance and subjective hearing acuity. |
| Pinsonnault-Skavernina et al. (2022) [73] | ABR | 80 total subjects, including 40 young adults with occupational noise exposure and 40 non-exposed young adults. | SiN | No significant difference in ABR measures between the noise-exposed and control group, including wave 1 amplitude, wave I/V ratio, and wave V latency shift. However, the noise-exposed group performed significantly worse on the SiN assessment. |
| Pinsonnault-Skavernina et al. (2022) [74] | ABR, EcochG, equivalent rectangular bandwidth (ERB) | 40 total subjects, including 27 military recruits exposed to firearm/artillery noise and 13 non-exposed controls. | SiN | Compared with the control group, military recruits performed worse on the SiN test, had a higher ERB at 4 kHz, reduced wave I amplitude at 75 dB, and delayed wave V latency. No significant difference in ABR wave I/V ratio or EcochG measures, including summating potentials (SP), action potentials (AP), or SP/AP ratio. |
| Suresh and Krishnan (2022) [75] | Frequency-following response (FFR) of envelope periodicity (FFRENV) and temporal fine structure (FFRTFS). Responses were evaluated in quiet and with background speech. | 48 young adults aged 18–30. The high-risk group (n = 24) participated in a marching band for at least 5 years, and the low-risk group (n = 24) had low-exposure noise history. | None | No difference In neural encoding between groups for FFRENV or the F1 formant of FFRTFS in noise and quiet conditions. Paradoxically, the high-risk group demonstrated enhanced representation of F2 harmonics in FFRTFS, though the authors suspect this may be due to music experience-dependent plasticity. |
| Aedo-Sanchez et al. (2023) [76] | ABR | 45 total subjects, divided into risk groups by age: the young group (n = 27) and the adult group (n = 18). | Conventional and high-frequency audiometry, SiN | The adult group recorded significantly lower wave I and V amplitudes than the young group at suprathreshold levels (80 dB). Adults also exhibited delayed wave V latencies at threshold and supra-threshold levels. In terms of behavioral measures, adults had significantly worse tonal thresholds at high frequencies and worse performance on the SiN assessment. |
| Sabzinasab et al. (2023) [77] | ABR wave V latency with masking | A total of 38 males, with n = 20 experiencing occupational noise exposure and n = 18 without. | None | There was no significant difference between groups for wave V latency with masking conditions. However, there were significant differences within groups. |
| Vasudevamurthy and Kumar (2023) [78] | MEMR threshold and strength | 50 total subjects; n = 25 individuals who were exposed to occupational noise for >1 year and 25 controls with no exposure. | None | MEMR strength was reduced in the noise exposure group compared with the control group. The MEMR threshold remained similar in both groups. |
| Yuan et al. (2023) [79] | DPOAEs, EcochG, ABR | 101 young adults divided into high risk (n = 51) and low risk (n = 50) based on noise exposure. | Conventional and high-frequency audiometry, SiN | There were several differences between the high-risk and low-risk group. The high-risk group recorded lower DPOAEs at 8 kHz and 10 kHz, lower amplitudes of SPs and APs in EcochG, and a higher wave III amplitude. Speech discrimination scores were also significantly worse in the high-risk group. |
| Jamos and Rickman (2024) [80] | MEMR, EcochG, DPOAEs | 21 young adult participants. High-risk (n = 11) and low-risk (n = 10) groups assigned based on noise exposure history. | SiN | The high-risk group exhibited worse performance on the SiN test, as well as smaller AP amplitude and greater SP/AP amplitude ratio in EcochG. The high-risk group also exhibited a higher probability for an elevated/absent MEMR threshold. There were no significant differences in hearing thresholds or DPOAEs at any tested frequency between groups. |
| Yaşar et al. (2025) [81] | EcochG: SP/AP ratio | 68 people aged between 18–65 years. A symptomatic group (n = 35) of patients with a 2-month history of difficulty understanding speech in noisy environments and a control group (n = 33) of healthy volunteers. | None | The symptomatic group registered a statistically higher SP/AP ratio compared with the healthy control group. |
3. Results
3.1. Criteria for Risk of HHL
3.2. Participant Characteristics
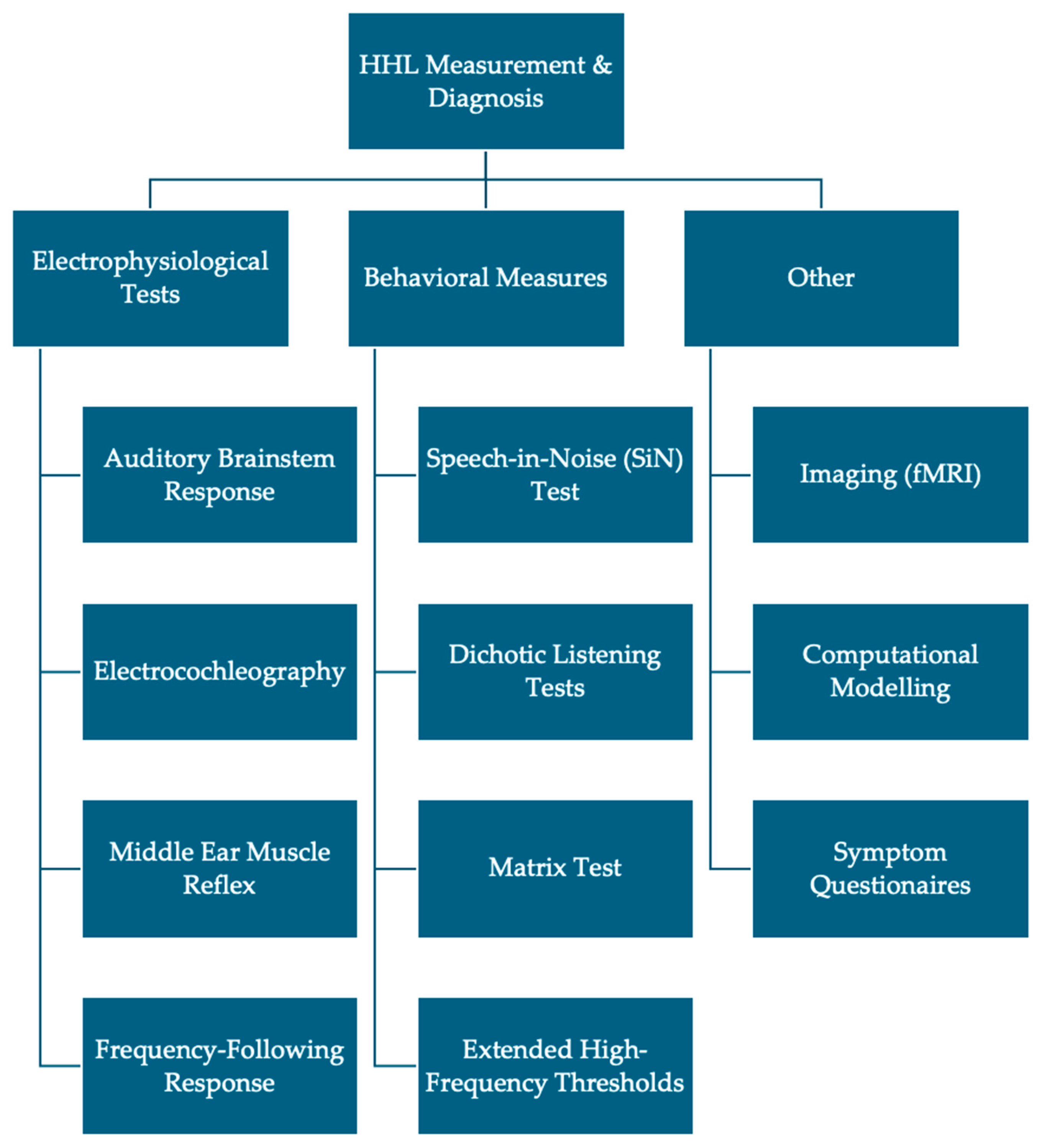
3.3. Electrophysiological Measures
3.4. Behavioral Measures
3.5. Study Characteristics and Findings
4. Discussion
4.1. Auditory Brainstem Response
4.2. Electrocochleography
4.3. Middle Ear Muscle Reflex
4.4. Frequency-Following Response
4.5. Other Synaptopathy Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HL | Hearing loss |
| HHL | Hidden hearing loss |
| SGC | Spiral ganglion cell |
| ABR | Auditory brainstem response |
| PTA | Pure-tone audiometry |
| SNHL | Sensorineural hearing loss |
| SiN | Speech-in-noise |
| EcochG | Electrocochleography |
| SP | Summating potential |
| AP | Action potential |
| MEMR | Middle ear muscle reflex |
| FFR | Frequency-following response |
| TFS | Temporal fine structure |
| EFR | Envelope following response |
| DPOAEs | Distortion product otoacoustic emissions |
| NEB | Noise exposure background |
| SR | Spontaneous rate |
| EPSPs | Excitatory postsynaptic potentials |
References
- Lin, F.R.; Niparko, J.K.; Ferrucci, L. Hearing loss prevalence in the United States. Arch. Intern. Med. 2011, 171, 1851–1852. [Google Scholar] [CrossRef] [PubMed]
- Raviv, D.; Dror, A.A.; Avraham, K.B. Hearing loss: A common disorder caused by many rare alleles. Ann. N. Y. Acad. Sci. 2010, 1214, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Besser, J.; Stropahl, M.; Urry, E.; Launer, S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear. Res. 2018, 369, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Hearing Loss and Associated Comorbidities: What Do We Know? The Hearing Review. 30 November 2017. Available online: https://hearingreview.com/hearing-loss/hearing-loss-prevention/risk-factors/hearing-loss-associated-comorbidities-know (accessed on 25 December 2024).
- GBD 2019 USA Hearing Loss Collaborators; Haile, L.M.; Orji, A.U.; Reavis, K.M.; Briant, P.S.; Lucas, K.M.; Alahdab, F.; Baernighausen, T.W.; Bell, A.W.; Cao, C.; et al. Hearing Loss Prevalence, Years Lived with Disability, and Hearing Aid Use in the United States from 1990 to 2019: Findings from the Global Burden of Disease Study. Ear Hear. 2024, 45, 257. [Google Scholar] [CrossRef]
- Walker, J.J.; Cleveland, L.M.; Davis, J.L.; Seales, J.S. Audiometry Screening and Interpretation. Am. Fam. Physician 2013, 87, 41–47. [Google Scholar]
- Salmon, M.K.; Brant, J.; Hohman, M.H.; Leibowitz, D. Audiogram Interpretation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- How to Read an Audiogram|Iowa Head and Neck Protocols. Available online: https://medicine.uiowa.edu/iowaprotocols/how-read-audiogram (accessed on 25 December 2024).
- Scarpa, A.; Ralli, M.; Cassandro, C.; Gioacchini, F.M.; Greco, A.; Di Stadio, A.; Cavaliere, M.; Troisi, D.; de Vincentiis, M.; Cassandro, E. Inner-Ear Disorders Presenting with Air-Bone Gaps: A Review. J. Int. Adv. Otol. 2020, 16, 111–116. [Google Scholar] [CrossRef]
- Bajin, M.D.; Dahm, V.; Lin, V.Y.W. Hidden hearing loss: Current concepts. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 321–325. [Google Scholar] [CrossRef]
- Bharadwaj, H.M.; Masud, S.; Mehraei, G.; Verhulst, S.; Shinn-Cunningham, B.G. Individual Differences Reveal Correlates of Hidden Hearing Deficits. J. Neurosci. 2015, 35, 2161–2172. [Google Scholar] [CrossRef]
- Liu, J.; Stohl, J.; Overath, T. Hidden hearing loss: Fifteen years at a glance. Hear. Res. 2024, 443, 108967. [Google Scholar] [CrossRef]
- Plack, C.J.; Barker, D.; Prendergast, G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014, 18, 2331216514550621. [Google Scholar] [CrossRef]
- Kohrman, D.C.; Wan, G.; Cassinotti, L.; Corfas, G. Hidden Hearing Loss: A Disorder with Multiple Etiologies and Mechanisms. Cold Spring Harb. Perspect. Med. 2020, 10, a035493. [Google Scholar] [CrossRef]
- Grinn, S.K.; Le Prell, C.G. Evaluation of hidden hearing loss in normal-hearing firearm users. Front. Neurosci. 2022, 16, 1005148. [Google Scholar] [CrossRef] [PubMed]
- Karl. Noise-Induced “Hidden Hearing Loss” Mechanism—Nerve Fiber Loss—Presented at ASA Meeting. The Hearing Review. 13 May 2014. Available online: https://hearingreview.com/hearing-loss/hearing-disorders/noise-induced-hidden-hearing-loss-mechanism-nerve-fiber-loss-discussed-asa-meeting (accessed on 25 December 2024).
- Pryce, H.; Wainwright, D. Help-seeking for medically unexplained hearing difficulties: A qualitative study. Int. J. Ther. Rehabil. 2008, 15, 343–349. [Google Scholar] [CrossRef]
- Tremblay, K.L.; Pinto, A.; Fischer, M.E.; Klein, B.E.K.; Klein, R.; Levy, S.; Tweed, T.S.; Cruickshanks, K.J. Self-Reported Hearing Difficulties Among Adults with Normal Audiograms: The Beaver Dam Offspring Study. Ear Hear. 2015, 36, e290–e299. [Google Scholar] [CrossRef]
- Chen, D.; Jia, G.; Ni, Y.; Chen, Y. Hidden hearing loss: Current perspectives and potential therapies. J. Bio-X Res. 2019, 02, 62–67. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ushio, M.; Yamasoba, T. Time course of apoptotic cell death in guinea pig cochlea following intratympanic gentamicin application. Acta Otolaryngol. 2008, 128, 724–731. [Google Scholar] [CrossRef]
- Sugawara, M.; Corfas, G.; Liberman, M.C. Influence of Supporting Cells on Neuronal Degeneration After Hair Cell Loss. J. Assoc. Res. Otolaryngol. 2005, 6, 136–147. [Google Scholar] [CrossRef]
- Gillespie, L.N.; Shepherd, R.K. Clinical application of neurotrophic factors: The potential for primary auditory neuron protection. Eur. J. Neurosci. 2005, 22, 2123–2133. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef]
- Stamataki, S.; Francis, H.W.; Lehar, M.; May, B.J.; Ryugo, D.K. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear. Res. 2006, 221, 104–118. [Google Scholar] [CrossRef]
- Michanski, S.; Smaluch, K.; Steyer, A.M.; Chakrabarti, R.; Setz, C.; Oestreicher, D.; Fischer, C.; Möbius, W.; Moser, T.; Vogl, C.; et al. Mapping developmental maturation of inner hair cell ribbon synapses in the apical mouse cochlea. Proc. Natl. Acad. Sci. USA 2019, 116, 6415–6424. [Google Scholar] [CrossRef]
- Fernandez, K.A.; Guo, D.; Micucci, S.; De Gruttola, V.; Liberman, M.C.; Kujawa, S.G. Noise-induced Cochlear Synaptopathy with and Without Sensory Cell Loss. Neuroscience 2020, 427, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, C.H.; Barth, J.L.; Darbelli, L.; Xing, Y.; Zhang, J.; Li, H.; Noble, K.V.; Liu, T.; Brown, L.N.; Schulte, B.A.; et al. Noise-Induced Dysregulation of Quaking RNA Binding Proteins Contributes to Auditory Nerve Demyelination and Hearing Loss. J. Neurosci. 2018, 38, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Mehraei, G.; Hickox, A.E.; Bharadwaj, H.M.; Goldberg, H.; Verhulst, S.; Liberman, M.C.; Shinn-Cunningham, B.G. Auditory Brainstem Response Latency in Noise as a Marker of Cochlear Synaptopathy. J. Neurosci. 2016, 36, 3755–3764. [Google Scholar] [CrossRef]
- Shehorn, J.; Strelcyk, O.; Zahorik, P. Associations between speech recognition at high levels, the middle ear muscle reflex and noise exposure in individuals with normal audiograms. Hear. Res. 2020, 392, 107982. [Google Scholar] [CrossRef]
- Billings, C.J.; Olsen, T.M.; Charney, L.; Madsen, B.M.; Holmes, C.E. Speech-in-Noise Testing: An Introduction for Audiologists. Semin. Hear. 2023, 45, 55–82. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, J.T.; Beach, E.F.; Yeend, I.; Sharma, M.; Van Dun, B.; Dillon, H. Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hear. Res. 2018, 365, 36–48. [Google Scholar] [CrossRef]
- Guest, H.; Munro, K.J.; Plack, C.J. Acoustic Middle-Ear-Muscle-Reflex Thresholds in Humans with Normal Audiograms: No Relations to Tinnitus, Speech Perception in Noise, or Noise Exposure. Neuroscience 2019, 407, 75–82. [Google Scholar] [CrossRef]
- Kamerer, A.M.; AuBuchon, A.; Fultz, S.E.; Kopun, J.G.; Neely, S.T.; Rasetshwane, D.M. The Role of Cognition in Common Measures of Peripheral Synaptopathy and Hidden Hearing Loss. Am. J. Audiol. 2019, 28, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.; Couth, S.; Millman, R.E.; Guest, H.; Kluk, K.; Munro, K.J.; Plack, C.J. Effects of Age and Noise Exposure on Proxy Measures of Cochlear Synaptopathy. Trends Hear. 2019, 23, 2331216519877301. [Google Scholar] [CrossRef] [PubMed]
- Marmel, F.; Cortese, D.; Kluk, K. The ongoing search for cochlear synaptopathy in humans: Masked thresholds for brief tones in Threshold Equalizing Noise. Hear. Res. 2020, 392, 107960. [Google Scholar] [CrossRef] [PubMed]
- Megarbane, L.; Fuente, A. Association between speech perception in noise and electrophysiological measures: An exploratory study of possible techniques to evaluate cochlear synaptopathy in humans. Int. J. Audiol. 2020, 59, 427–433. [Google Scholar] [CrossRef]
- Mepani, A.M.; Kirk, S.A.; Hancock, K.E.; Bennett, K.; de Gruttola, V.; Liberman, M.C.; Maison, S.F. Middle Ear Muscle Reflex and Word Recognition in “Normal-Hearing” Adults: Evidence for Cochlear Synaptopathy? Ear Hear. 2020, 41, 25–38. [Google Scholar] [CrossRef]
- Nam, G.S.; Kim, J.Y.; Hong, S.A.; Kim, S.G.; Son, E.J. Limitation of Conventional Audiometry in Identifying Hidden Hearing Loss in Acute Noise Exposure. Yonsei Med. J. 2021, 62, 615–621. [Google Scholar] [CrossRef]
- Bal, N.; Derinsu, U. The possibility of cochlear synaptopathy in young people using a personal listening device. Auris Nasus Larynx 2021, 48, 1092–1098. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhang, J.; Zhou, R.; Zhong, Z.; Wei, C.; Chen, J.; Liu, Y. Cochlear Synaptopathy: A Primary Factor Affecting Speech Recognition Performance in Presbycusis. Biomed. Res. Int. 2021, 2021, 6667531. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Qian, M.; Hong, Y.; Wang, X.; Huang, Z.; Wu, H. Acute Recreational Noise-Induced Cochlear Synaptic Dysfunction in Humans with Normal Hearing: A Prospective Cohort Study. Front. Neurosci. 2021, 15, 659011. [Google Scholar] [CrossRef]
- Carcagno, S.; Plack, C.J. Relations between speech-reception, psychophysical temporal processing, and subcortical electrophysiological measures of auditory function in humans. Hear. Res. 2022, 417, 108456. [Google Scholar] [CrossRef]
- Kaf, W.A.; Turntine, M.; Jamos, A.; Smurzynski, J. Examining the Profile of Noise-Induced Cochlear Synaptopathy Using iPhone Health App Data and Cochlear and Brainstem Electrophysiological Responses to Fast Clicks Rates. Semin. Hear. 2022, 43, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Lobdell, A.; Trujillo, T.; Jennings, S.G. Assessment of Cochlear Synaptopathy with Standard Clinical Equipment. J. Am. Acad. Audiol. 2022, 33, 466–473. [Google Scholar] [CrossRef]
- Goodman, S.S.; Lichtenhan, J.T.; Jennings, S.G. Minimum Detectable Differences in Electrocochleography Measurements: Bayesian-Based Predictions. J. Assoc. Res. Otolaryngol. 2023, 24, 217–237. [Google Scholar] [CrossRef]
- Haggerty, R.A.; Hutson, K.A.; Riggs, W.J.; Brown, K.D.; Pillsbury, H.C.; Adunka, O.F.; Buchman, C.A.; Fitzpatrick, D.C. Assessment of cochlear synaptopathy by electrocochleography to low frequencies in a preclinical model and human subjects. Front. Neurol. 2023, 14, 1104574. [Google Scholar] [CrossRef]
- Shehabi, A.M.; Prendergast, G.; Guest, H.; Plack, C.J. Binaural temporal coding and the middle ear muscle reflex in audiometrically normal young adults. Hear. Res. 2023, 427, 108663. [Google Scholar] [CrossRef] [PubMed]
- Couth, S.; Prendergast, G.; Guest, H.; Munro, K.J.; Moore, D.R.; Plack, C.J.; Ginsborg, J.; Dawes, P. A longitudinal study investigating the effects of noise exposure on behavioural, electrophysiological and self-report measures of hearing in musicians with normal audiometric thresholds. Hear. Res. 2024, 451, 109077. [Google Scholar] [CrossRef]
- De Poortere, N.; Keshishzadeh, S.; Keppler, H.; Dhooge, I.; Verhulst, S. Intrasubject variability in potential early markers of sensorineural hearing damage. J. Acoust. Soc. Am. 2024, 156, 3480–3495. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Li, D.; Hu, R.; Tian, Z.; Xie, Q. Correlation between pure tone audiometry at all frequencies and distortion product otoacoustic emission of patients with hidden hearing loss. Biotechnol. Genet. Eng. Rev. 2024, 40, 4250–4261. [Google Scholar] [CrossRef]
- Fujihira, H.; Yamagishi, S.; Furukawa, S.; Kashino, M. Auditory brainstem response to paired clicks as a candidate marker of cochlear synaptopathy in humans. Clin. Neurophysiol. 2024, 165, 44–54. [Google Scholar] [CrossRef]
- Kamerer, A.M.; Harris, S.E.; Wichman, C.S.; Rasetshwane, D.M.; Neely, S.T. The relationship and interdependence of auditory thresholds, proposed behavioural measures of hidden hearing loss, and physiological measures of auditory function. Int. J. Audiol. 2024, 64, 11–24. [Google Scholar] [CrossRef]
- Liu, J.; Stohl, J.; Lopez-Poveda, E.A.; Overath, T. Quantifying the Impact of Auditory Deafferentation on Speech Perception. Trends Hear. 2024, 28, 23312165241227818. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, K.A.; Sanchez, J.T. Effects of Temporal Processing on Speech-in-Noise Perception in Middle-Aged Adults. Biology 2024, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Saade, M.; Fernandez, K.; Little, C.; Schwam, Z.G.; Cosetti, M. Utility of Extended High-Frequency Audiograms in Clinical Practice. Laryngoscope 2024, 134, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, J.; Wolpert, S.; Dapper, K.; Rühle, M.; Wertz, J.; Wouters, M.; Eldh, T.; Bader, K.; Singer, W.; Gaudrain, E.; et al. Neural Adaptation at Stimulus Onset and Speed of Neural Processing as Critical Contributors to Speech Comprehension Independent of Hearing Threshold or Age. J. Clin. Med. 2024, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Temboury-Gutierrez, M.; Märcher-Rørsted, J.; Yde, J.; Encina-Llamas, G.; Hjortkjær, J.; Dau, T. Electrocochleographic frequency-following responses as a potential marker of age-related cochlear neural degeneration. Hear. Res. 2024, 446, 109005. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bhatt, I.S.; Wang, J. Evaluation of dichotic listening performance in normal-hearing, noise-exposed young females. Hear. Res. 2019, 380, 10–21. [Google Scholar] [CrossRef]
- Carcagno, S.; Plack, C.J. Effects of age on electrophysiological measures of cochlear synaptopathy in humans. Hear. Res. 2020, 396, 108068. [Google Scholar] [CrossRef]
- Dewey, R.S.; Francis, S.T.; Guest, H.; Prendergast, G.; Millman, R.E.; Plack, C.J.; Hall, D.A. The association between subcortical and cortical fMRI and lifetime noise exposure in listeners with normal hearing thresholds. Neuroimage 2020, 204, 116239. [Google Scholar] [CrossRef]
- Kikidis, D.; Vardonikolaki, A.; Zachou, Z.; Razou, A.; Pantos, P.; Bibas, A. ABR findings in musicians with normal audiogram and otoacoustic emissions: Evidence of cochlear synaptopathy? Hear. Balance Commun. 2020, 18, 36. [Google Scholar] [CrossRef]
- Bramhall, N.F.; McMillan, G.P.; Kampel, S.D. Envelope following response measurements in young veterans are consistent with noise-induced cochlear synaptopathy. Hear. Res. 2021, 408, 108310. [Google Scholar] [CrossRef]
- Megha, K.N.; Kappadi, S.; Kaverappa, G.M.; Konadath, S. Effects of Aging Versus Noise Exposure on Auditory System in Individuals with Normal Audiometric Thresholds. J. Int. Adv. Otol. 2021, 17, 335–342. [Google Scholar] [CrossRef]
- Suresh, C.H.; Krishnan, A. Search for Electrophysiological Indices of Hidden Hearing Loss in Humans: Click Auditory Brainstem Response Across Sound Levels and in Background Noise. Ear Hear. 2021, 42, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.S.; Washnik, N.; Torkamani, A. Suprathreshold Auditory Measures for Detecting Early-Stage Noise-Induced Hearing Loss in Young Adults. J. Am. Acad. Audiol. 2022, 33, 185–195. [Google Scholar] [CrossRef]
- Bramhall, N.F.; Reavis, K.M.; Feeney, M.P.; Kampel, S.D. The Impacts of Noise Exposure on the Middle Ear Muscle Reflex in a Veteran Population. Am. J. Audiol. 2022, 31, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Cildir, B.; Tokgoz-Yilmaz, S.; Türkyilmaz, M.D. Cochlear synaptopathy causes loudness perception impairment without hearing loss. Noise Health 2022, 24, 49–60. [Google Scholar] [CrossRef]
- Lai, J.; Bidelman, G.M. Relative changes in the cochlear summating potentials to paired-clicks predict speech-in-noise perception and subjective hearing acuity. JASA Express Lett. 2022, 2, 102001. [Google Scholar] [CrossRef] [PubMed]
- Pinsonnault-Skvarenina, A.; Moïn-Darbari, K.; Zhao, W.; Zhang, M.; Qiu, W.; Fuente, A. No effect of occupational noise exposure on auditory brainstem response and speech perception in noise. Front. Neurosci. 2022, 16, 915211. [Google Scholar] [CrossRef]
- Pinsonnault-Skvarenina, A.; Soucy, W.; Noël, J.; Doucet, F.; Lévesque, É.; Fuente, A.; Leroux, T. Supra-threshold deficits in normal hearing military recruits exposed to impulse noise. J. Acoust. Soc. Am. 2022, 152, 2419. [Google Scholar] [CrossRef]
- Suresh, C.H.; Krishnan, A. Frequency-Following Response to Steady-State Vowel in Quiet and Background Noise Among Marching Band Participants with Normal Hearing. Am. J. Audiol. 2022, 31, 719–736. [Google Scholar] [CrossRef]
- Aedo-Sanchez, C.; Oliveros, J.; Aranguiz, C.; Muñoz, C.; Lazo-Maturana, C.; Aguilar-Vidal, E. Subclinical hearing loss associated with aging. J. Otol. 2023, 18, 111–117. [Google Scholar] [CrossRef]
- Sabzinasab, Z.; Rouzbahani, M.; Toufan, R.; Maarefvand, M. Evaluation of Synaptopathy by Use of Latency Shift of Wave V Auditory Brainstem Response in the Presence of Ipsilateral Noise. Audit. Vestib. Res. 2023, 32, 47–53. [Google Scholar] [CrossRef]
- Vasudevamurthy, S.; Kumar, A.U. Middle Ear Muscle Reflex in Normal-Hearing Individuals with Occupational Noise Exposure. Noise Health 2023, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, P.W.; Chen, J.W.; Wang, W.L.; Gao, W.; Lu, P.H.; Ding, X.R.; Lun, Y.Q.; Lu, L.J. Development of an audiological assessment and diagnostic model for high occupational noise exposure. Eur. Arch. Otorhinolaryngol. 2023, 280, 2763–2772. [Google Scholar] [CrossRef]
- Jamos, A.M.; Rickman, R. Stimulus Rate Effect on Electrocochleogram Components in Adults with High Risk for Noise Exposure. J. Am. Acad. Audiol. 2024, 35, 13–23. [Google Scholar] [CrossRef]
- Yaşar, M.; Öner, F.; Atalay, F.; Anbar, S.S. Cochlear Synaptopathy Evaluation with Electrocochleography in Patients with Hearing Difficulty in Noise Despite Normal Hearing Levels. Clin. Otolaryngol. 2025, 50, 75–81. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, M.Y.; Choi, J.E.; Jung, J.Y. Auditory Brainstem Response to Paired Click Stimulation as an Indicator of Peripheral Synaptic Health in Noise-Induced Cochlear Synaptopathy. Front. Neurosci. 2020, 14, 596670. [Google Scholar] [CrossRef]
- Prendergast, G.; Tu, W.; Guest, H.; Millman, R.E.; Kluk, K.; Couth, S.; Munro, K.J.; Plack, C.J. Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hear. Res. 2018, 364, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.T.; Hutson, K.A.; Trecca, E.M.C.; Hamby, M.; Fitzpatrick, D.C. Neural Contributions to the Cochlear Summating Potential: Spiking and Dendritic Components. J. Assoc. Res. Otolaryngol. 2022, 23, 351–363. [Google Scholar] [CrossRef]
- Grant, K.J.; Mepani, A.M.; Wu, P.; Hancock, K.E.; De Gruttola, V.G.; Liberman, M.C.; Maison, S.F. Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. J. Neurophysiol. 2020, 124, 418–431. [Google Scholar] [CrossRef]
- Valero, M.D.; Hancock, K.E.; Liberman, M.C. The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear. Res. 2016, 332, 29–38. [Google Scholar] [CrossRef]
- Ananthakrishnan, S.; Krishnan, A.; Bartlett, E. Human Frequency Following Response: Neural Representation of Envelope and Temporal Fine Structure in Listeners with Normal Hearing and Sensorineural Hearing Loss. Ear Hear. 2016, 37, e91–e103. [Google Scholar] [CrossRef] [PubMed]
- Vasilkov, V.; Garrett, M.; Mauermann, M.; Verhulst, S. Enhancing the sensitivity of the envelope-following response for cochlear synaptopathy screening in humans: The role of stimulus envelope. Hear. Res. 2021, 400, 108132. [Google Scholar] [CrossRef]
- Bowling, T.; Wen, H.; Meenderink, S.W.F.; Dong, W.; Meaud, J. Intracochlear distortion products are broadly generated by outer hair cells but their contributions to otoacoustic emissions are spatially restricted. Sci. Rep. 2021, 11, 13651. [Google Scholar] [CrossRef]
- Hickox, A.E.; Liberman, M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014, 111, 552–564. [Google Scholar] [CrossRef]
- Mehraei, G.; Gallardo, A.P.; Shinn-Cunningham, B.G.; Dau, T. Auditory brainstem response latency in forward masking, a marker of sensory deficits in listeners with normal hearing thresholds. Hear. Res. 2017, 346, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Suthakar, K.; Liberman, M.C. Noise Masking in Cochlear Synaptopathy: Auditory Brainstem Response vs. Auditory Nerve Response in Mouse. J. Neurophysiol. 2022, 127, 1574–1585. [Google Scholar] [CrossRef]
- Valero, M.D.; Hancock, K.E.; Maison, S.F.; Liberman, M.C. Effects of cochlear synaptopathy on middle-ear muscle reflexes in unanesthetized mice. Hear. Res. 2018, 363, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Märcher-Rørsted, J.; Encina-Llamas, G.; Dau, T.; Liberman, M.C.; Wu, P.Z.; Hjortkjær, J. Age-related reduction in frequency-following responses as a potential marker of cochlear neural degeneration. Hear. Res. 2022, 414, 108411. [Google Scholar] [CrossRef]
- Ohlms, L.A.; Lonsbury-Martin, B.L.; Martin, G.K. Acoustic-distortion products: Separation of sensory from neural dysfunction in sensorineural hearing loss in human beings and rabbits. Otolaryngoly Head Neck Surg. 1991, 104, 159–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenoy, S.; Bhatt, K.; Yazdani, Y.; Rahimian, H.; Djalilian, H.R.; Abouzari, M. A Systematic Review: State of the Science on Diagnostics of Hidden Hearing Loss. Diagnostics 2025, 15, 742. https://doi.org/10.3390/diagnostics15060742
Shenoy S, Bhatt K, Yazdani Y, Rahimian H, Djalilian HR, Abouzari M. A Systematic Review: State of the Science on Diagnostics of Hidden Hearing Loss. Diagnostics. 2025; 15(6):742. https://doi.org/10.3390/diagnostics15060742
Chicago/Turabian StyleShenoy, Sunil, Khushi Bhatt, Yalda Yazdani, Helia Rahimian, Hamid R. Djalilian, and Mehdi Abouzari. 2025. "A Systematic Review: State of the Science on Diagnostics of Hidden Hearing Loss" Diagnostics 15, no. 6: 742. https://doi.org/10.3390/diagnostics15060742
APA StyleShenoy, S., Bhatt, K., Yazdani, Y., Rahimian, H., Djalilian, H. R., & Abouzari, M. (2025). A Systematic Review: State of the Science on Diagnostics of Hidden Hearing Loss. Diagnostics, 15(6), 742. https://doi.org/10.3390/diagnostics15060742








