AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring
Abstract
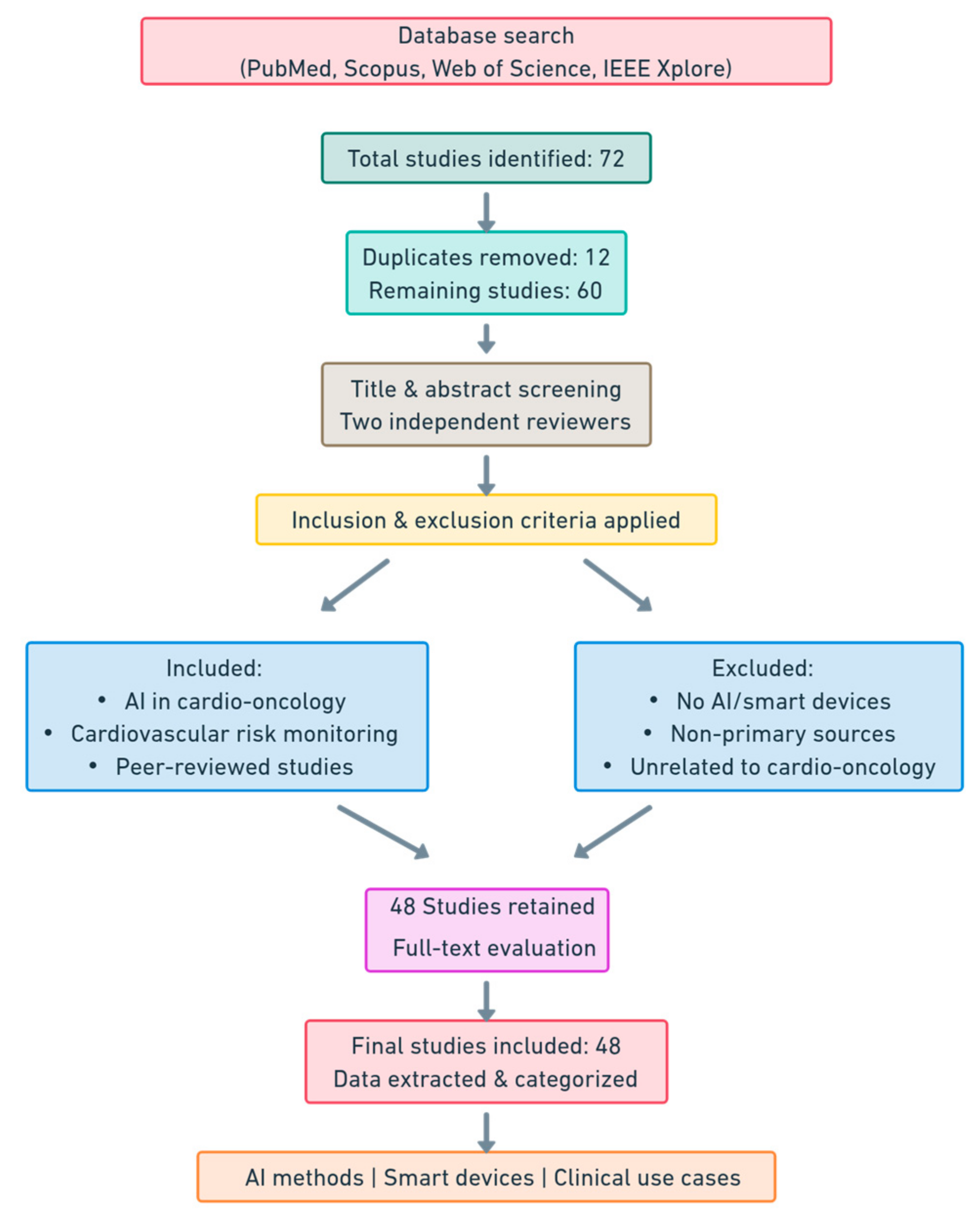
1. Introduction
2. Methods and Materials
3. Artificial Intelligence in Cardio-Oncology
3.1. Introduction to AI
- Cardiotoxicity risk prediction: ML models can analyze clinical and imaging data to estimate the likelihood of cardiovascular complications before, during, or after oncology treatment [34];
- Real-time patient monitoring: Smart wearables and AI systems integrated into remote monitoring allow for the early detection of relevant physiological changes [23];
- Personalization of treatment: AI helps to adjust doses and choose the most appropriate therapeutic regimens for each patient, minimizing the risk of cardiac toxicity without compromising the efficacy of oncology treatment [22];
- Automated imaging analysis: DL algorithms can analyze echocardiograms, cardiac MRIs, and other medical images to identify structural or functional changes in the myocardium [35];
3.2. AI Technologies and Algorithms Utilized in Cardio-Oncology
3.2.1. Machine Learning (ML)
3.2.2. Deep Learning (DL)
- Convolutional Neural Networks (CNNs): these are mainly used for analyzing medical images such as echocardiograms, cardiac MRIs, and chest CT scans. These networks are specifically designed to detect spatially relevant features such as structural changes in the myocardium. In cardio-oncology, CNNs can identify early signs of chemotherapy-induced cardiotoxicity by automatically analyzing ultrasound or MRI images [44].
- Recurrent Neural Networks (RNNs) and Long Short-Term Memory (LSTM): these networks are used for analyzing time series, such as ECG signals or variations of serum biomarkers over time. Unlike classical NNs, RNNs can learn from data sequences, considering the temporal context. In cardio-oncology, RNN or LSTM-based models can monitor changes in patients’ cardiac parameters and predict the risk of heart failure or arrhythmias associated with oncologic treatment [45]. Automated imaging analysis: DL algorithms can analyze echocardiograms, cardiac MRIs, and other medical images to identify structural or functional changes in the myocardium;
- Autoencoding and Generative Adversarial Neural Networks (GANs): these models are used to generate synthetic data or to improve the quality of existing data. For example, autoencoders can be used to reduce noise in ECG data or medical images, improving the accuracy of automated analysis. GANs can be used to generate synthetic ultrasound images that can help train other AI models [46].
3.3. Study Limitations and Methodological Challenges
3.4. Clinical Applications of AI in Cardio-Oncology
3.4.1. Cardiovascular Toxicity Risk Stratification Before Oncological Treatment
3.4.2. Prevention and Monitoring of Cardiovascular Complications During Therapy
3.4.3. Diagnosis and Management of Acute and Subacute Cardiovascular Toxicity
3.4.4. AI in Long-Term Cardiovascular Risk Identification and Chronic Complication Management in Cancer Survivors
4. Implantable Cardiac Electronic Devices Used in Cardio-Oncology
4.1. Introduction to Implantable Devices
4.2. Types of Implantable Devices in Cardio-Oncology
4.2.1. Cardiac Pacemakers
4.2.2. Clinical Impact of AI on ICDs
4.2.3. Clinical Impact of AI on CRTs
4.2.4. Clinical Impact of AI on Pulmonary Artery Pressure Monitoring Devices
4.3. Integration of AI with Implantable Devices
4.3.1. Advancements in AI-Integrated Implantable Devices
4.3.2. Clinical Impact of AI in ICEDs
5. Wearable Smart Cardiac Devices Used in Cardio-Oncology
5.1. Introduction to Wearable Devices
- Inertial Measurement Units (IMUs) for motion tracking and activity recognition [95];
- Photoplethysmography (PPG) sensors for heart rate and oxygen saturation monitoring [94];
- Electromyography (EMG) and Electroencephalography (EEG) sensors for muscle and neural activity analysis [96];
- Galvanic Skin Response (GSR) sensors for stress and emotional state assessment [97].
5.2. Advances in Wearable Cardiac Monitoring
5.3. Clinical Applications in Cardio-Oncology
6. Perspectives, Future Directions, and Conclusions
6.1. Emerging Trends and Technological Developments
6.2. Research and Multidisciplinary Collaboration
6.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AI | Artificial Intelligence |
| AI-CTRCD | AI-Based Cancer Therapy-Related Cardiac Dysfunction Model |
| AI-ECG | AI-Enhanced Electrocardiogram |
| AI-EF | AI-Based Ejection Fraction Model |
| ANN | Artificial Neural Network |
| AUROC | Area Under Receiver Operating Characteristic Curve |
| BLE | Bluetooth Low Energy |
| CAD | Coronary Artery Disease |
| CardioMEMS | Wireless Hemodynamic Monitoring System |
| CNN | Convolutional Neural Network |
| CRT | Cardiac Resynchronization Therapy |
| CTRCD | Cancer Therapy-Related Cardiac Dysfunction |
| DL | Deep Learning |
| ECG | Electrocardiogram |
| EEG | Electroencephalography |
| EHR | Electronic Health Record |
| EHRA | European Heart Rhythm Association |
| EMG | Electromyography |
| GSR | Galvanic Skin Response |
| GANs | Generative Adversarial Networks |
| HF | Heart Failure |
| HUD | Handheld Ultrasound Device |
| ICD | Implantable Cardioverter Defibrillator |
| ICEDs | Implantable Cardiac Electronic Devices |
| IMU | Inertial Measurement Unit |
| KNN | K-Nearest Neighbors |
| LD | Linear Dichroism |
| LPWAN | Low Power Wide Area Network |
| LSTM | Long Short-Term Memory |
| LVEF | Left Ventricular Ejection Fraction |
| MCU | Microcontroller Unit |
| MI | Myocardial Infarction |
| ML | Machine Learning |
| MLP | Multilayer Perceptron |
| NLP | Natural Language Processing |
| PA | Pulmonary Artery |
| PCA | Principal Component Analysis |
| PET | Positron Emission Tomography |
| PPG | Photoplethysmography |
| RF | Random Forest |
| RNN | Recurrent Neural Network |
| SVM | Support Vector Machine |
| SoC | System-on-Chip |
| VEGF | Vascular Endothelial Growth Factor |
| XGBoost | Extreme Gradient Boosting |
References
- Jhawar, N.; Mcpherson, A.; Chirila, R.; Ray, J. Cardio-Oncology for the Primary Care Provider. Rom. J. Intern. Med. 2023, 61, 127–134. [Google Scholar] [CrossRef]
- Petek, B.J.; Greenman, C.; Herrmann, J.; Ewer, M.S.; Jones, R.L. Cardio-Oncology: An Ongoing Evolution. Future Oncol. 2015, 11, 2059–2066. [Google Scholar] [CrossRef]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-Oncology—Strategies for Management of Cancer-Therapy Related Cardiovascular Disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukis, A.; Ntalianis, A.; Repasos, E.; Kastritis, E.; Dimopoulos, M.A.; Paraskevaidis, I. Cardio-oncology: A Focus on Cardiotoxicity. Eur. Cardiol. 2018, 13, 64–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choksey, A.; Timm, K.N. Cancer Therapy-Induced Cardiotoxicity—A Metabolic Perspective on Pathogenesis, Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 23, 441. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Russell, R.R.; Schwartz, R.G.; Panjrath, G.S.; Aronow, W. Cardiac Complications of Cancer Therapy: Pathophysiology, Identification, Prevention, Treatment, and Future Directions. Curr. Cardiol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Jain, D.; Aronow, W. Cardiotoxicity of Cancer Chemotherapy in Clinical Practice. Hosp. Pract. 2019, 47, 6–15. [Google Scholar] [CrossRef]
- Mondal, P.; Jain, D.; Aronow, W.S.; Frishman, W.H. Cardiotoxicity of Cancer Therapies. Cardiol. Rev. 2019, 27, 230–235. [Google Scholar] [CrossRef]
- Madanat, L.; Gupta, R.; Weber, P.; Kumar, N.; Chandra, R.; Ahaneku, H.; Bansal, Y.; Anderson, J.; Bilolikar, A.; Jaiyesimi, I. Cardiotoxicity of Biological Therapies in Cancer Patients: An In-depthReview. Curr. Cardiol. Rev. 2023, 19, e310522205428. [Google Scholar] [CrossRef]
- Truong, L.-L.; Scott, L.; Pal, R.S.; Jalink, M.; Gunasekara, S.; Wijeratne, D.T. Cancer and Cardiovascular Disease: Can Understanding the Mechanisms of Cardiovascular Injury Guide Us to Optimise Care in Cancer Survivors? Ecancer 2022, 16, 1430. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Han, X.; Sun, J.; Li, C.; Adhikari, B.K.; Zhang, J.; Miao, X.; Chen, Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022, 9, 727487. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Sfendouraki, E.; Markousis-Mavrogenis, G.; Rigopoulos, A.; Noutsias, M.; Kolovou, G.; Angeli, C.; Tousoulis, D. Cardio-Oncology, the Myth of Sisyphus, and Cardiovascular Disease in Breast Cancer Survivors. Heart Fail. Rev. 2019, 24, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Florido, R.; Daya, N.R.; Ndumele, C.E.; Koton, S.; Russell, S.D.; Prizment, A.; Blumenthal, R.S.; Matsushita, K.; Mok, Y.; Felix, A.S.; et al. Cardiovascular Disease Risk Among Cancer Survivors. J. Am. Coll. Cardiol. 2022, 80, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamin, M.; Salim, S.; Azizi, M.S.; Rusdi, L.; Sudoyo, A.W.; Putri, A.A. Advancing The Cardiovascular Care in Cancer Patients on Chemotherapy. Acta Med. Indones. 2023, 55, 494–501. [Google Scholar] [PubMed]
- Martinez, D.S.-L.; Noseworthy, P.A.; Akbilgic, O.; Herrmann, J.; Ruddy, K.J.; Hamid, A.; Maddula, R.; Singh, A.; Davis, R.; Gunturkun, F.; et al. Artificial Intelligence Opportunities in Cardio-Oncology: Overview with Spotlight on Electrocardiography. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 15, 100129. [Google Scholar] [CrossRef]
- Jain, S.S.; Elias, P.; Poterucha, T.; Randazzo, M.; Lopez Jimenez, F.; Khera, R.; Perez, M.; Ouyang, D.; Pirruccello, J.; Salerno, M.; et al. Artificial Intelligence in Cardiovascular Care—Part 2: Applications. J. Am. Coll. Cardiol. 2024, 83, 2487–2496. [Google Scholar] [CrossRef]
- Jacobs, J.E.J.; Greason, G.; Mangold, K.E.; Wildiers, H.; Willems, R.; Janssens, S.; Noseworthy, P.; Lopez-Jimenez, F.; Voigt, J.-U.; Friedman, P.; et al. Artificial Intelligence Electrocardiogram as a Novel Screening Tool to Detect a Newly Abnormal Left Ventricular Ejection Fraction after Anthracycline-Based Cancer Therapy. Eur. J. Prev. Cardiol. 2024, 31, 560–566. [Google Scholar] [CrossRef]
- Matheson, M.B.; Kato, Y.; Baba, S.; Cox, C.; Lima, J.A.C.; Ambale-Venkatesh, B. Cardiovascular Risk Prediction Using Machine Learning in a Large Japanese Cohort. Circ. Rep. 2022, 4, 595–603. [Google Scholar] [CrossRef]
- Maddula, R.; MacLeod, J.; McLeish, T.; Painter, S.; Steward, A.; Berman, G.; Hamid, A.; Abdelrahim, M.; Whittle, J.; Brown, S.A. The Role of Digital Health in the Cardiovascular Learning Healthcare System. Front. Cardiovasc. Med. 2022, 9, 1008575. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Habibian, M.; Lyon, A.R. Monitoring the Heart during Cancer Therapy. Eur. Heart J. Suppl. 2019, 21, M44–M49. [Google Scholar] [CrossRef]
- Patel, R.A.; Klasnja, P.; Hartzler, A.; Unruh, K.T.; Pratt, W. Probing the benefits of real-time tracking during cancer care. AMIA Annu. Symp. Proc. 2012, 2012, 1340–1349. [Google Scholar] [PubMed] [PubMed Central]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart Wearable Devices in Cardiovascular Care: Where We Are and How to Move Forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring. J. Am. Coll. Cardiol. 2020, 75, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Thompson, C.; Peterson, S.; Mandrola, J.; Beg, M.S. The Future of Wearable Technologies and Remote Monitoring in Health Care. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 115–121. [Google Scholar] [CrossRef]
- Masoumian Hosseini, M.; Masoumian Hosseini, S.T.; Qayumi, K.; Hosseinzadeh, S.; Sajadi Tabar, S.S. Smartwatches in Healthcare Medicine: Assistance and Monitoring; a Scoping Review. BMC Med. Inf. Decis. Mak. 2023, 23, 248. [Google Scholar] [CrossRef]
- Stuijt, D.G.; Van Doeveren, E.E.M.; Kos, M.; Eversdijk, M.; Bosch, J.J.; Bins, A.D.; Bak, M.A.R.; Van Oijen, M.G.H. Remote Patient Monitoring Using Mobile Health Technology in Cancer Care and Research: Patients’ Views and Preferences. JCO Clin. Cancer Inf. 2024, 8, e2400092. [Google Scholar] [CrossRef]
- Kappel, C.; Rushton-Marovac, M.; Leong, D.; Dent, S. Pursuing Connectivity in Cardio-Oncology Care—The Future of Telemedicine and Artificial Intelligence in Providing Equity and Access to Rural Communities. Front. Cardiovasc. Med. 2022, 9, 927769. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial Intelligence in Healthcare: Transforming the Practice of Medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Echefu, G.; Shah, R.; Sanchez, Z.; Rickards, J.; Brown, S. Artificial intelligence: Applications in cardio-oncology and potential impact on racial disparities. Am. Heart J. Plus Cardiol. Res. Pract. 2024, 48, 100479. [Google Scholar] [CrossRef] [PubMed]
- Nechita, L.C.; Nechita, A.; Voipan, A.E.; Voipan, D.; Debita, M.; Fulga, A.; Fulga, I.; Musat, C.L. AI-Enhanced ECG Applications in Cardiology: Comprehensive Insights from the Current Literature with a Focus on COVID-19 and Multiple Cardiovascular Conditions. Diagnostics 2024, 14, 1839. [Google Scholar] [CrossRef] [PubMed]
- Coviello, J.S. Cardiovascular and Cancer Risk: The Role of Cardio-oncology. J. Adv. Pract. Oncol. 2018, 9, 160–176. [Google Scholar] [PubMed] [PubMed Central]
- Musat, C.L.; Mereuta, C.; Nechita, A.; Tutunaru, D.; Voipan, A.E.; Voipan, D.; Mereuta, E.; Gurau, T.V.; Gurău, G.; Nechita, L.C. Diagnostic Applications of AI in Sports: A Comprehensive Review of Injury Risk Prediction Methods. Diagnostics 2024, 14, 2516. [Google Scholar] [CrossRef]
- Santamato, V.; Tricase, C.; Faccilongo, N.; Iacoviello, M.; Marengo, A. Exploring the Impact of Artificial Intelligence on Healthcare Management: A Combined Systematic Review and Machine-Learning Approach. Appl. Sci. 2024, 14, 10144. [Google Scholar] [CrossRef]
- Luțenco, V.; Țocu, G.; Guliciuc, M.; Moraru, M.; Candussi, I.L.; Dănilă, M.; Luțenco, V.; Dimofte, F.; Mihailov, O.M.; Mihailov, R. New Horizons of Artificial Intelligence in Medicine and Surgery. J. Clin. Med. 2024, 13, 2532. [Google Scholar] [CrossRef]
- Staszak, K.; Tylkowski, B.; Staszak, M. From Data to Diagnosis: How Machine Learning Is Changing Heart Health Monitoring. Int. J. Environ. Res. Public Health 2023, 20, 4605. [Google Scholar] [CrossRef]
- Barbierato, E.; Gatti, A. The Challenges of Machine Learning: A Critical Review. Electronics 2024, 13, 416. [Google Scholar] [CrossRef]
- Talaei Khoei, T.; Kaabouch, N. Machine Learning: Models, Challenges, and Research Directions. Future Internet 2023, 15, 332. [Google Scholar] [CrossRef]
- Budisteanu, E.-A.; Mocanu, I.G. Combining Supervised and Unsupervised Learning Algorithms for Human Activity Recognition. Sensors 2021, 21, 6309. [Google Scholar] [CrossRef] [PubMed]
- Sivamayil, K.; Rajasekar, E.; Aljafari, B.; Nikolovski, S.; Vairavasundaram, S.; Vairavasundaram, I. A Systematic Study on Reinforcement Learning Based Applications. Energies 2023, 16, 1512. [Google Scholar] [CrossRef]
- Abut, S.; Okut, H.; Kallail, K.J. Paradigm shift from Artificial Neural Networks (ANNs) to deep Convolutional Neural Networks (DCNNs) in the field of medical image processing. Expert. Syst. Appl. 2023, 244, 122983. [Google Scholar] [CrossRef]
- Luca, A.R.; Ursuleanu, T.F.; Gheorghe, L.; Grigorovici, R.; Iancu, S.; Hlusneac, M.; Grigorovici, A. Impact of quality, type and volume of data used by deep learning models in the analysis of medical images. Inform. Med. Unlocked 2022, 29, 100911. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Huang, R.; Zhu, H.; Liu, Q.; Mitra, S.; Wang, Y. Long short-term memory recurrent neural network for pharmacokinetic-pharmacodynamic modeling. Int. J. Clin. Pharmacol. Ther. 2021, 59, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Mahmoud, Q.H. A Systematic Review of Synthetic Data Generation Techniques Using Generative AI. Electronics 2024, 13, 3509. [Google Scholar] [CrossRef]
- Al-Garadi, M.A.; Yang, Y.-C.; Sarker, A. The Role of Natural Language Processing during the COVID-19 Pandemic: Health Applications, Opportunities, and Challenges. Healthcare 2022, 10, 2270. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Z.; Huang, S.; Zhang, N.; Wang, Y.; Hong, S.; Chan, J.S.K.; Chen, K.Y.; Xia, Y.; Zhang, Y.; et al. Machine Learning in Cardio-Oncology: New Insights from an Emerging Discipline. Rev. Cardiovasc. Med. 2023, 24, 296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Itchhaporia, D. Artificial intelligence in cardiology. Trends Cardiovasc. Med. 2020, 32, 34–41. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-induced Cardiotoxicity. Maedica 2013, 8, 59–67. [Google Scholar] [PubMed] [PubMed Central]
- Jacob, S.; Pathak, A.; Franck, D.; Latorzeff, I.; Jimenez, G.; Fondard, O.; Lapeyre, M.; Colombier, D.; Bruguiere, E.; Lairez, O.; et al. Early Detection and Prediction of Cardiotoxicity after Radiation Therapy for Breast Cancer: The BACCARAT Prospective Cohort Study. Radiat. Oncol. 2016, 11, 54. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; López-Candales, A. Potential Usefulness of Diastolic Parameters Measured by Strain Imaging Echocardiography in the Early Prediction of Chemotherapy-Induced Cardiotoxicity. Med. Hypotheses 2017, 101, 30–32. [Google Scholar] [CrossRef]
- Bustová, I. Riziko kardiotoxicity pri kombinované lécbē zárením a chemoterapií u pokrocilého karcionomu prsu III klinického stádia [Risk of cardiotoxicity of combination treatment radiotherapy and chemotherapy of locally advanced breast carcinoma stage III]. Klin. Onkol. 2009, 22, 17–21. (In Czech) [Google Scholar] [PubMed]
- Zhou, Y.; Hou, Y.; Hussain, M.; Brown, S.A.; Budd, T.; Tang, W.H.W.; Abraham, J.; Xu, B.; Shah, C.; Moudgil, R.; et al. Machine Learning-Based Risk Assessment for Cancer Therapy-Related Cardiac Dysfunction in 4300 Longitudinal Oncology Patients. J. Am. Heart Assoc. 2020, 9, e019628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, W.T.; Liu, C.F.; Feng, Y.H.; Liao, C.T.; Wang, J.J.; Chen, Z.C.; Lee, H.C.; Shih, J.Y. An artificial intelligence approach for predicting cardiotoxicity in breast cancer patients receiving anthracycline. Arch. Toxicol. 2022, 96, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Sangha, V.; Dhingra, L.S.; Aminorroaya, A.; Coppi, A.; Krumholz, H.M.; Baldassarre, L.A.; Khera, R. Artificial Intelligence-Enhanced Risk Stratification of Cancer Therapeutics-Related Cardiac Dysfunction Using Electrocardiographic Images. Circ. Cardiovasc. Qual. Outcomes 2025, 18, e011504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yagi, R.; Goto, S.; Himeno, Y.; Katsumata, Y.; Hashimoto, M.; MacRae, C.A.; Deo, R.C. Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline electrocardiograms. Nat. Commun. 2024, 15, 2536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.; Sohn, T.J.; Ng, B.P.; Park, C. Predicting unplanned readmission due to cardiovascular disease in hospitalized patients with cancer: A machine learning approach. Sci. Rep. 2023, 13, 13491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Droubi, S.S.; Jahangir, E.; Kochendorfer, K.M.; Krive, M.; Laufer-Perl, M.; Gilon, D.; Okwuosa, T.M.; Gans, C.P.; Arnold, J.H.; Bhaskar, S.T.; et al. Artificial intelligence modelling to assess the risk of cardiovascular disease in oncology patients. Eur. Heart J.-Digit. Health 2023, 4, 302–315. [Google Scholar] [CrossRef]
- Madan, N.; Lucas, J.; Akhter, N.; Collier, P.; Cheng, F.; Guha, A.; Zhang, L.; Sharma, A.; Hamid, A.; Ndiokho, I.; et al. Artificial intelligence and imaging: Opportunities in cardio-oncology. Am. Heart J. Plus. 2022, 15, 100126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Ouyang, D.; Baykaner, T.; Jamal, F.; Cheng, P.; Rhee, J.W. Artificial intelligence applications in cardio-oncology: Leveraging high dimensional cardiovascular data. Front. Cardiovasc. Med. 2022, 9, 941148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.-M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef]
- Chang, H.-M.; Okwuosa, T.M.; Scarabelli, T.; Moudgil, R.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Bacchiani, G.; Beggiato, M.; Colombo, A.; Cipolla, C.M. Strategies to Prevent and Treat Cardiovascular Risk in Cancer Patients. Semin. Oncol. 2013, 40, 186–198. [Google Scholar] [CrossRef]
- Yeh, E.T.H.; Bickford, C.L. Cardiovascular Complications of Cancer Therapy. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef]
- Tan, T.C.; Scherrer-Crosbie, M. Cardiac Complications of Chemotherapy: Role of Imaging. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 296. [Google Scholar] [CrossRef]
- Yagi, R.; Goto, S.; MacRae, C.A.; Deo, R.C. Expanded adaptation of an artificial intelligence model for predicting chemotherapy-induced cardiotoxicity using baseline electrocardiograms. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544-2577. [Google Scholar] [CrossRef]
- Brown, S.; Sparapani, R.; Osinski, K.; Zhang, J.; Blessing, J.; Cheng, F.; Hamid, A.; Berman, G.; Lee, K.; BagheriMohamadiPour, M.; et al. Establishing an interdisciplinary research team for cardio-oncology artificial intelligence informatics precision and health equity. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 13, 100094. [Google Scholar] [CrossRef]
- Gao, T.; Ren, H.; He, S.; Liang, D.; Xu, Y.; Chen, K.; Wang, Y.; Zhu, Y.; Dong, H.; Xu, Z.; et al. Development of an interpretable machine learning-based intelligent system of exercise prescription for cardio-oncology preventive care: A study protocol. Front. Cardiovasc. Med. 2023, 9, 1091885. [Google Scholar] [CrossRef]
- Halasz, G.; Mazzacuva, P.; Mistrulli, R. Artificial intelligence–assisted electrocardiography: A new and easily accessible approach for diagnosing cancer therapy–related cardiac dysfunction. Eur. J. Prev. Cardiol. 2023, 31, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cao, W.; Fu, S.; Yao, B.; Yang, Z.; Yin, C.; Mishra, V.; Addison, D.; Zhang, P.; Wang, D. CardioAI: A multimodal AI-based system to support symptom monitoring and risk detection of Cancer Treatment-Induced Cardiotoxicity. arXiv 2024. [Google Scholar] [CrossRef]
- Papadopoulou, S.; Dionysopoulos, D.; Mentesidou, V.; Loga, K.; Michalopoulou, S.; Koukoutzeli, C.; Efthimiadis, K.; Kantartzi, V.; Timotheadou, E.; Styliadis, I.; et al. Artificial intelligence-assisted evaluation of cardiac function by oncology staff in chemotherapy patients. Eur. Heart J.-Digit. Health 2024, 5, 278–287. [Google Scholar] [CrossRef]
- Bovelli, D.; Plataniotis, G.; Roila, F. Cardiotoxicity of Chemotherapeutic Agents and Radiotherapy-Related Heart Disease: ESMO Clinical Practice Guidelines. Ann. Oncol. 2010, 21, v277–v282. [Google Scholar] [CrossRef] [PubMed]
- Geisberg, C.; Pentassuglia, L.; Sawyer, D.B. Cardiac Side Effects of Anticancer Treatments: New Mechanistic Insights. Curr. Heart Fail. Rep. 2012, 9, 211–218. [Google Scholar] [CrossRef]
- Zhou, S.; Blaes, A.; Shenoy, C.; Sun, J.; Zhang, R. Risk prediction of heart diseases in patients with breast cancer: A deep learning approach with longitudinal electronic health records data. iScience 2024, 27, 110329. [Google Scholar] [CrossRef]
- Baldassarre, L.A.; Yang, E.H.; Cheng, R.K.; DeCara, J.M.; Dent, S.; Liu, J.E.; Rudski, L.G.; Strom, J.B.; Thavendiranathan, P.; Barac, A.; et al. Cardiovascular Care of the Oncology Patient During COVID-19: An Expert Consensus Document From the ACC Cardio-Oncology and Imaging Councils. JNCI J. Natl. Cancer Inst. 2021, 113, 513–522. [Google Scholar] [CrossRef]
- Aleman, B.M.P.; Moser, E.C.; Nuver, J.; Suter, T.M.; Maraldo, M.V.; Specht, L.; Vrieling, C.; Darby, S.C. Cardiovascular Disease after Cancer Therapy. Eur. J. Cancer Suppl. 2014, 12, 18–28. [Google Scholar] [CrossRef]
- Ruddy, K.J.; Patel, S.R.; Higgins, A.S.; Armenian, S.H.; Herrmann, J. Cardiovascular Health during and after Cancer Therapy. Cancers 2020, 12, 3737. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Hamid, A.; Akbilgic, O.; Brown, S. Trends in cardiology and oncology artificial intelligence publications. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100162. [Google Scholar] [CrossRef]
- Safavi, A.H.; Louie, A.V.; Elzibak, A.H.; Warner, A.; Donovan, E.K.; Detsky, J.S. Management of Patients with Cardiovascular Implantable Electronic Devices Undergoing Radiation Therapy: A National Survey of Canadian Multidisciplinary Radiation Oncology Professionals. Adv. Radiat. Oncol. 2023, 8, 101184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chia, P.L.; Foo, D. Overview of implantable cardioverter defibrillator and cardiac resynchronisation therapy in heart failure management. Singap. Med. J. 2016, 57, 354–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, A.; Bennett, S. Cardiac implantable electronic devices: An overview for primary care. Br. J. Gen. Pract. 2022, 72, 402–404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fonseca, M.; Cheng, E.; Do, D.; Haldar, S.; Kutty, S.; Yang, E.H.; Ghosh, A.K.; Guha, A. Bradyarrhythmias in Cardio-Oncology. South. Asian J. Cancer 2021, 10, 195–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riesenhuber, M.; Spannbauer, A.; Gwechenberger, M.; Pezawas, T.; Schukro, C.; Stix, G.; Schneider, M.; Goliasch, G.; Anvari, A.; Wrba, T.; et al. Pacemaker lead-associated tricuspid regurgitation in patients with or without pre-existing right ventricular dilatation. Clin. Res. Cardiol. 2021, 110, 884–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parkes, J.; Bryant, J.; Milne, R. Implantable cardioverter-defibrillators in arrhythmias: A rapid and systematic review of effectiveness. Heart 2002, 87, 438–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirzaei, M.; Rowshanfarzad, P.; Gill, S.; Ebert, M.A.; Dass, J. Risk of cardiac implantable device malfunction in cancer patients receiving proton therapy: An overview. Front. Oncol. 2023, 13, 1181450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, Y.M.; Lee, H.C.; Mulpuru, S.K.; Deshmukh, A.J.; Friedman, P.A.; Asirvatham, S.J.; Bradley, D.J.; Madhavan, M.; Abou Ezzeddine, O.F.; Wen, S.; et al. Cardiac resynchronization therapy for patients with mild to moderately reduced ejection fraction and left bundle branch block. Heart Rhythm. 2024, 21, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.S.; Jung, M.H.; Chang, S.; Kim, M.; Youn, J.C.; Chung, W.B.; Jung, H.O. Genetic predisposition in chemotherapy-induced cardiomyopathy in a 65-year-old female with metastatic breast cancer. ESC Heart Fail. 2024, 11, 2410–2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, R.; Nair, A. The Utility of CardioMEMS, a Wireless Hemodynamic Monitoring System in Reducing Heart Failure Related Hospital Readmissions. J. Nurse Pract. 2021, 17, 267–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Small, H.Y.; Montezano, A.C.; Rios, F.J.; Savoia, C.; Touyz, R.M. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: Understanding and managing a new syndrome. Can. J. Cardiol. 2014, 30, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Malagon, L.; Martínez, J.; Romero, J.; Munarriz, A.; Basterra, N. Cancer and implantable cardiac defibrillator. Causality, confusion or chance? Med. Clin. 2021, 157, 459–463. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Agarwal, J.P.; Pandey, K.C. Cancer patients with cardiac pacemakers needing radiation treatment: A systematic review. J. Cancer Res. Ther. 2013, 9, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Dieffenderfer, J.; Goodell, H.; Mills, S.; McKnight, M.; Yao, S.; Lin, F.; Beppler, E.; Bent, B.; Lee, B.; Misra, V.; et al. Low-Power Wearable Systems for Continuous Monitoring of Environment and Health for Chronic Respiratory Disease. IEEE J. Biomed. Health Inform. 2016, 20, 1251–1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, W.; Wang, F.; Fan, A.; Zhao, W.; Yao, W.; Yang, P. Extended Application of Inertial Measurement Units in Biomechanics: From Activity Recognition to Force Estimation. Sensors 2023, 23, 4229. [Google Scholar] [CrossRef]
- Brambilla, C.; Pirovano, I.; Mira, R.M.; Rizzo, G.; Scano, A.; Mastropietro, A. Combined Use of EMG and EEG Techniques for Neuromotor Assessment in Rehabilitative Applications: A Systematic Review. Sensors 2021, 21, 7014. [Google Scholar] [CrossRef]
- Villarejo, M.V.; Zapirain, B.G.; Zorrilla, A.M. A stress sensor based on Galvanic Skin Response (GSR) controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Gamal, A.; Ahmed, A.I.; Said, L.A.; Elbaz, A.; Herencsar, N.; Soltan, A. Internet of Things: A Comprehensive Overview on Protocols, Architectures, Technologies, Simulation Tools, and Future Directions. Energies 2023, 16, 3465. [Google Scholar] [CrossRef]
- Hughes, A.; Shandhi, M.M.H.; Master, H.; Dunn, J.; Brittain, E. Wearable Devices in Cardiovascular Medicine. Circ. Res. 2023, 132, 652–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrigendum to: How to use digital devices to detect and manage arrhythmias: An EHRA practical guide. EP Eur. 2023, 25, 486. [CrossRef]
- Faro, J.M.; Yue, K.; Singh, A.; Soni, A.; Ding, E.Y.; Shi, Q.; McManus, D.D. Wearable device use and technology preferences in cancer survivors with or at risk for atrial fibrillation. Cardiovasc. Digit. Health J. 2022, 3, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.; Okwuosa, T.; Teske, A.J.; Guha, A.; Collier, P.; Moudgil, R.; Sarkar, A.; Brown, S.A. Cardio oncology: Digital innovations, precision medicine and health equity. Front. Cardiovasc. Med. 2022, 9, 951551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef] [PubMed]

| Category | Year of Study | Author | ML Method | Data Source | Application | Performance Metrics |
|---|---|---|---|---|---|---|
| Predictive algorithms for cardiovascular risk estimation | 2023 | Han S et al. [59] | XGBoost, AdaBoost, Decision Tree | Nationwide Readmissions Database (358,629 hospitalized cancer patients) | Predicting unplanned readmission due to cardiovascular disease in hospitalized cancer patients | XGBoost had best predictive performance for unplanned cardiovascular readmissions |
| 2023 | Al-Droubi SS et al. [60] | Random Forest (RF), ANN | 20,023 oncology patient records from Vanderbilt University Medical Center | AI modeling for cardiovascular disease risk assessment in oncology patients | Accuracy: >90% for ANN, superior to RF | |
| 2022 | Chang WT et al. [56] | RF, Logistic Regression, LightGBM, KNN, MLP | Prospective study on breast cancer patients receiving anthracycline therapy (2014–2018) | Prediction of cardiotoxicity in breast cancer patients using ML models trained on clinical and imaging data | Best performing model: MLP (AUROC = 0.66, Sensitivity: 0.86, Specificity: 0.53) | |
| 2020 | Zhou Y et al. [55] | RF, SVM, Gradient Boosting | Electronic medical records of 4309 cancer patients (Cleveland Clinic, 1997–2018) | Risk assessment of cancer therapy-related cardiac dysfunction using clinical and echocardiographic data | AUROC: 0.821 (CAD), 0.787 (AF), 0.882 (HF), 0.807 (MI), 0.802 (de novo CTRCD) | |
| Integration of clinical, imaging, genetic, and biomarker data | 2025 | Oikonomou EK et al. [57] | DL (CNN applied to ECG images) | Retrospective study of 1550 patients undergoing anthracycline or trastuzumab treatment (2013–2023, Yale New Haven Health System) | AI-enhanced ECG-based risk stratification for early detection of cardiac dysfunction post-chemotherapy | Hazard ratio for high-risk patients: 3.35 for CTRCD, 13.52 for LVEF <40% |
| 2024 | Yagi R et al. [58] | AI-CTRCD Model (Transfer Learning on AI-EF Model, ECG-based) | Multicenter study on 1011 anthracycline-treated patients (Brigham & Women’s Hospital, Massachusetts General Hospital, Keio University Hospital) | AI-powered ECG analysis for predicting chemotherapy-induced cardiotoxicity before treatment initiation | Time-dependent AUC for 2-year prediction: 0.78 with AI-CTRCD vs. 0.74 without (p = 0.005) | |
| 2023 | Zheng Y et al. [48] | RF, ANN, Convolutional Neural Networks | Large-scale cardio-oncology patient registry integrating clinical and imaging data | ML-driven insights into cardio-oncology risk factors and treatment outcomes | RF and ANN demonstrated strong performance in patient risk stratification | |
| 2022 | Madan N et al. [61] | AI-Augmented Cardiac Imaging (Echocardiography, MRI, PET) | Advanced cardiac imaging databases, including echocardiography, MRI, and PET | AI-integrated imaging for early detection and prevention of cardiotoxicity in cancer patients | AI-augmented imaging models improved early detection of cardiac dysfunction | |
| 2022 | Chen H et al. [62] | DL (Multimodal Imaging) | Cardiovascular imaging and biomarker datasets (Stanford, Cedars Sinai, City of Hope) | AI-driven analysis of high-dimensional cardiovascular data for risk stratification | AI-enhanced imaging analysis improved diagnostic accuracy significantly |
| Category | Year of Study | Author | ML Method | Data Source | Application | Performance Metrics |
|---|---|---|---|---|---|---|
| Early detection of cardiotoxicity | 2024 | Jacobs JEJ et al. [18] | DL on ECG | Single-center cohort of breast cancer patients receiving anthracyclines (N = 989) | AI ECG tool to detect abnormal left ventricular ejection fraction post-anthracycline therapy | AUC = 0.93 for EF < 50%, AUC = 0.94 for EF ≤ 35% |
| 2024 | Halasz G et al. [71] | Convolutional Neural Networks (CNN) applied to ECG | Single-center study on AI-based ECG detection of abnormal LVEF post-anthracycline therapy | Screening for newly abnormal LVEF using AI-assisted ECG analysis | DL-based AI-ECG achieved high sensitivity and specificity in detecting LVEF abnormalities | |
| 2022 | Martinez DS et al. [16] | AI-enhanced ECG risk stratification | Review of AI opportunities in cardio-oncology with focus on ECG-based monitoring | Opportunities in cardio-oncology using AI-enhanced ECG assessment | N/A (Review article summarizing AI in ECG for cardio-oncology) | |
| Real-time data analysis for clinical decision support | 2023 | Brown SA et al. [69] | Patient similarity algorithms, ML for shared decision-making | Clinical decision aid tool developed for >4000 cancer survivors, integrating real-world clinical data | Shared decision-making and real-time ML-based clinical decision support | Clinical trial to evaluate improvement in cardio-oncology care using AI decision aid |
| 2023 | Gao T et al. [70] | Interpretable ML for exercise prescription | Exercise prescription model based on ML and real-time monitoring in cardio-oncology | AI-driven exercise prescription system for preventive cardio-oncology care | Expected improvement in cardiovascular outcomes with AI-based exercise prescription | |
| 2023 | Wu S et al. [72] | Multimodal AI integrating wearables and voice assistants | Wearables + AI-driven risk detection of cardiotoxicity in multimodal clinical decision systems | AI-integrated multimodal symptom monitoring and risk detection | Clinician-validated AI system for continuous monitoring and early symptom detection | |
| 2023 | Papadopoulou SL et al. [73] | AI-assisted echocardiographic evaluation by oncology staff | AI-enabled handheld ultrasound device tested in 115 chemotherapy patients | AI-supported evaluation of cardiac function by oncology staff in chemotherapy patients | Automated LVEF calculation by oncology staff achieved sensitivity of 94–95%, specificity of 87–94% |
| Category | Year of Study | Author | ML Method | Data Source | Application | Performance Metrics |
|---|---|---|---|---|---|---|
| Automated differential diagnosis | 2024 | Oikonomou EK et al. [57] | AI-enhanced ECG imaging for cardiac dysfunction risk stratification | Multicenter dataset of 1550 patients undergoing anthracycline or trastuzumab therapy | AI-enhanced ECG-based risk stratification of cancer therapy-related cardiac dysfunction | AI-ECG identified high-risk patients with 3.4× to 13.5× increased risk for cardiac dysfunction |
| 2024 | Papadopoulou SL et al. [73] | AI-assisted evaluation of cardiac function using handheld ultrasound devices | 115 chemotherapy patients evaluated for AI-enabled echocardiographic assessment | Automated LVEF assessment by oncology staff | Sensitivity: 94–95%, Specificity: 87–94% for LVEF detection using AI-enabled HUD | |
| Treatment optimization by algorithms based on clinical data | 2024 | Zhou S et al. [76] | DL applied to longitudinal EHR data for heart disease risk prediction | Longitudinal electronic health records from breast cancer patients | Prediction of heart disease risk in breast cancer patients using DL models | AUC of 0.88 for predicting cardiovascular disease risk in breast cancer patients |
| 2022 | Kappel C et al. [29] | AI-driven telemedicine platforms for equity in cardio-oncology care | Telemedicine applications for cardio-oncology care in rural communities | Improving access and equity in cardio-oncology using AI-driven telehealth solutions | Reported increase in access to cardio-oncology care in remote communities |
| Category | Year of Study | Author | ML Method | Data Source | Application | Performance Metrics |
|---|---|---|---|---|---|---|
| Treatment optimization by algorithms based on clinical data | 2024 | Nechita LC et al. [33] | ML-enhanced ECG for long-term cardiotoxicity detection | ECG-based screening for subclinical cardiotoxicity in cancer survivors | Enhancing ECG interpretation for long-term detection of cardiotoxicity | Higher detection sensitivity of ECG-based AI screening for late-onset cardiotoxicity |
| 2023 | Echefu G et al. [32] | AI applications in cardio-oncology for racial disparity analysis | Review of AI-driven healthcare disparities in cardio-oncology | Assessing AI’s role in reducing racial disparities in cardio-oncology | Recommendations for AI model bias mitigation and equitable risk assessment | |
| 2023 | Suero-Abreu GA et al. [80] | AI publication trends in cardiology and oncology research | Bibliometric analysis of AI trends in cardiology and oncology | Identifying research gaps and AI integration trends in cardiology-oncology | Growth analysis of AI research in cardio-oncology over the past decade |
| Study | Key Findings | Device Type | AI Integration | Clinical Impact |
|---|---|---|---|---|
| Kappel et al. (2023) [29] | Explored AI-enhanced telemedicine for remote cardiac monitoring. | Various implantable devices | AI-enabled remote monitoring | Improved access to cardio-oncology care in rural areas. |
| Gao et al. (2022) [70] | Developed AI-driven models for CRT optimization in cancer survivors. | CRT | AI-assisted pacing adjustments | Improved treatment response and reduced heart failure exacerbations. |
| Riesenhuber et al. (2021) [85] | Examined pacemaker outcomes in cancer patients. Found increased mortality in post-diagnosis implantations. | Pacemaker | No AI | Highlighted need for early cardiac intervention. |
| Roy et al. (2021) [92] | Investigated cancer incidence in ICD recipients. Found no conclusive causal link. | ICD | No AI | Suggested potential interactions between device materials and cancer development. |
| Lee SY et al. (2021) [89] | Assessed CRT efficacy in chemotherapy-induced cardiomyopathy. | CRT | No AI | Found CRT beneficial in restoring cardiac function in cancer patients. |
| Munshi et al. (2013) [93] | Reviewed radiotherapy risks for cancer patients with pacemakers. | Pacemaker | No AI | Emphasized safety measures needed for radiation exposure. |
| Study | Clinical Application | Device Type | Key Benefits | AI Integration |
|---|---|---|---|---|
| Hughes et al. (2023) [99] | Hypertension and Blood Pressure Monitoring | Omron HeartGuide, Withings BPM Core | Home-based blood pressure tracking, AI-driven trend analysis | Predictive analytics for hypertension risk, automated alerts |
| EHRA Guide, 2023 [100] | AI Integration and Advanced Functionalities | AI-driven ECG Analysis, DL Arrhythmia Detection | Automated arrhythmia classification, early warning systems | Personalized treatment recommendations, predictive analytics |
| Faro et al. (2022) [101] | Arrhythmia Detection and Management | KardiaMobile (AliveCor), Apple Watch ECG | Continuous arrhythmia monitoring, early AF detection | AI-powered AF detection, ML-based ECG interpretation |
| Sadler et al. (2022) [102] | Remote Patient Monitoring and Telemedicine | Fitbit, Garmin Smartwatches, BioSticker | Real-time cardiac data transmission, reduced clinic visits | Data aggregation for clinical decision support, AI-driven alerts |
| Bayoumy et al., Nat Rev Cardiol. (2021) [24] | Cardiotoxicity Surveillance | Zio XT Patch, Hexoskin Smart Shirt | Prolonged cardiac monitoring, early HF detection | AI-enhanced risk stratification for heart failure prediction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechita, L.C.; Tutunaru, D.; Nechita, A.; Voipan, A.E.; Voipan, D.; Tupu, A.E.; Musat, C.L. AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring. Diagnostics 2025, 15, 787. https://doi.org/10.3390/diagnostics15060787
Nechita LC, Tutunaru D, Nechita A, Voipan AE, Voipan D, Tupu AE, Musat CL. AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring. Diagnostics. 2025; 15(6):787. https://doi.org/10.3390/diagnostics15060787
Chicago/Turabian StyleNechita, Luiza Camelia, Dana Tutunaru, Aurel Nechita, Andreea Elena Voipan, Daniel Voipan, Ancuta Elena Tupu, and Carmina Liana Musat. 2025. "AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring" Diagnostics 15, no. 6: 787. https://doi.org/10.3390/diagnostics15060787
APA StyleNechita, L. C., Tutunaru, D., Nechita, A., Voipan, A. E., Voipan, D., Tupu, A. E., & Musat, C. L. (2025). AI and Smart Devices in Cardio-Oncology: Advancements in Cardiotoxicity Prediction and Cardiovascular Monitoring. Diagnostics, 15(6), 787. https://doi.org/10.3390/diagnostics15060787






