The Effects of Passive Leg Raising and Maintenance Fluid Administration on Pulse Oximetry Waveform Amplitude and Peak Variability in Mechanically Ventilated Patients in Sepsis and Septic Shock
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Procedure and Measurements
2.3. Data Analysis
2.4. Statistical Analysis
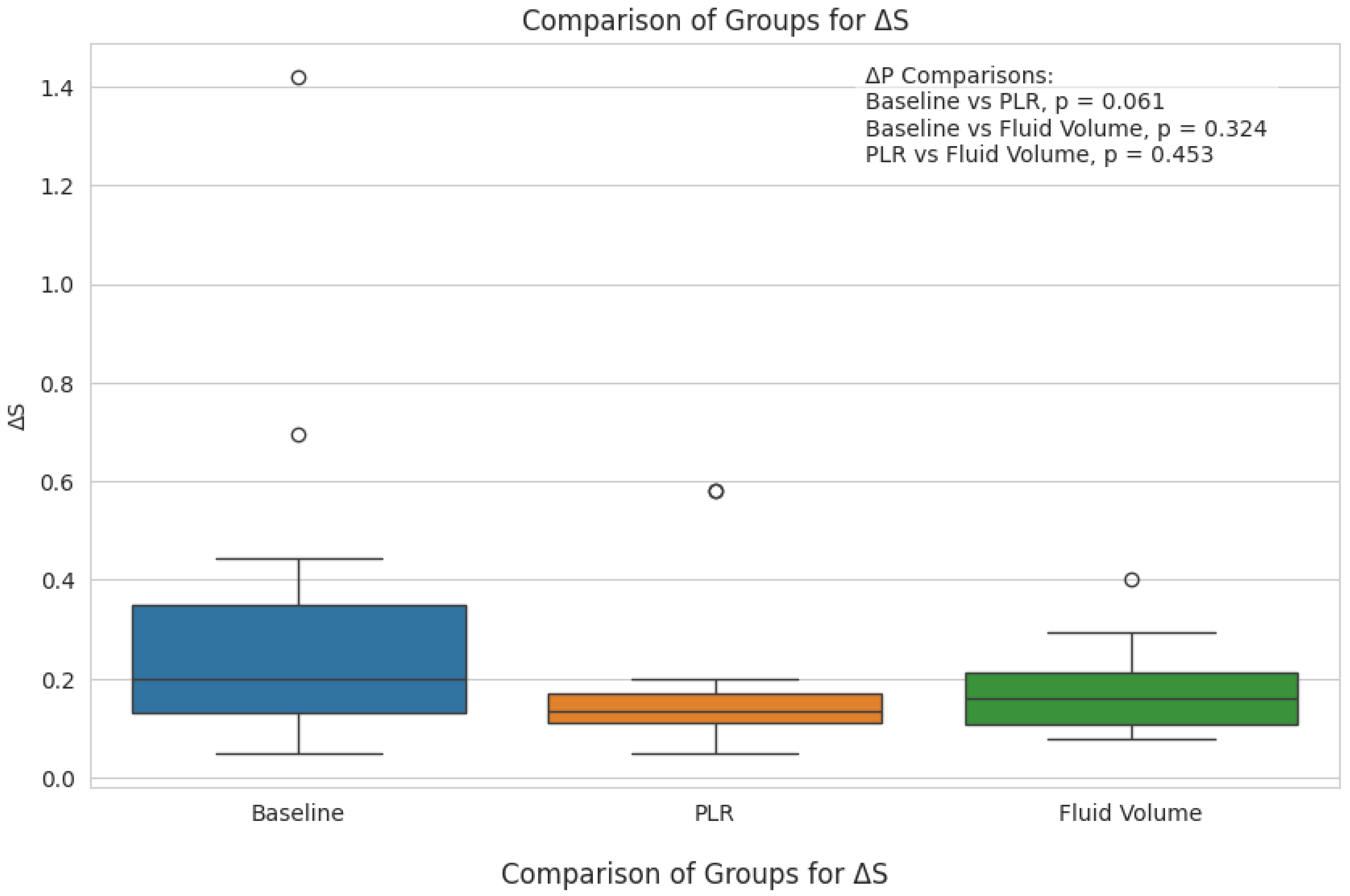
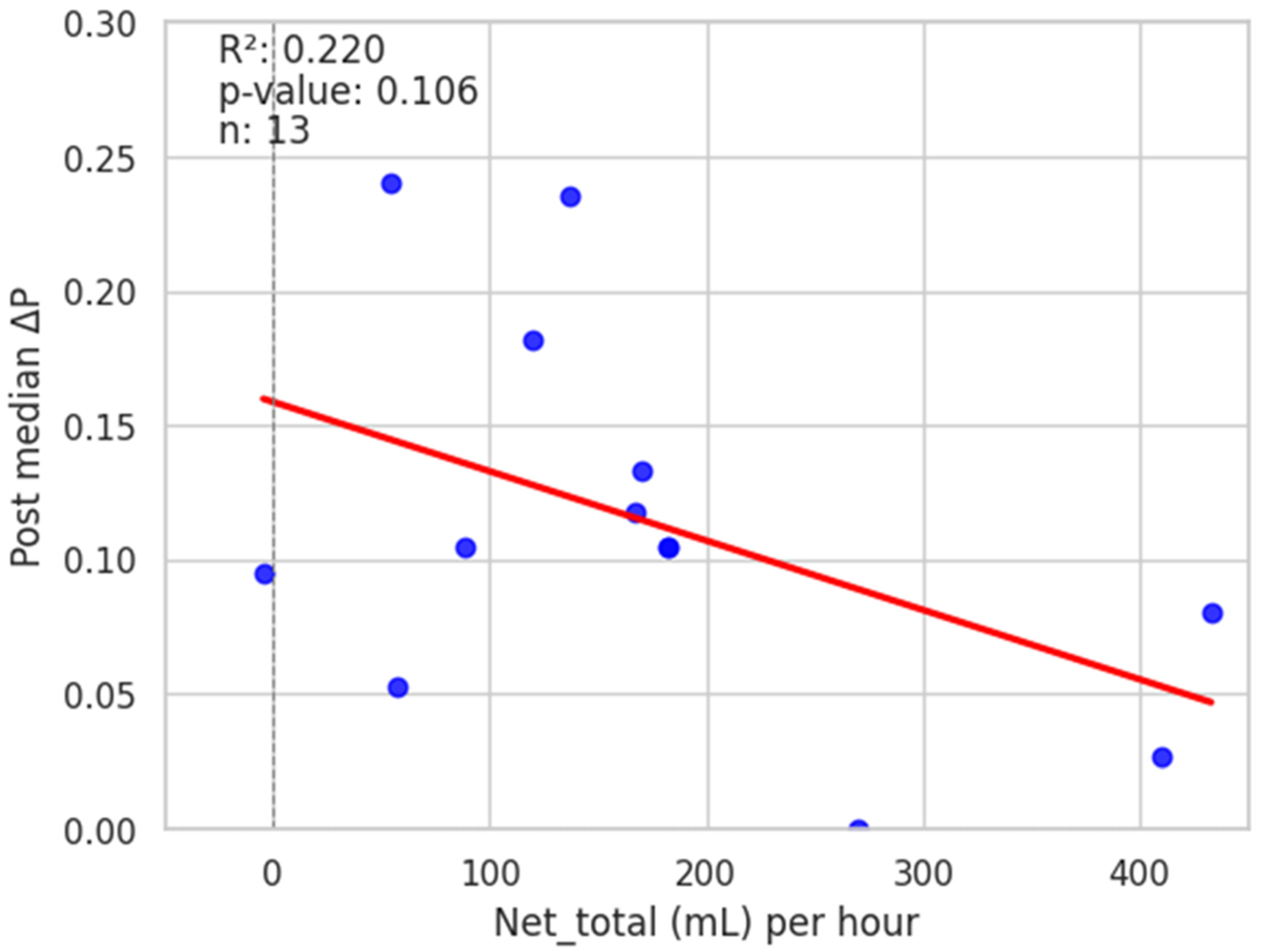
3. Results
4. Discussion
Limitations: Use of Other Measures of the Effects of Systemic Perfusion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamir, M.; Eidelman, L.; Floman, Y.; Kaplan, L.; Pizov, R. Pulse oximetry plethysmographic waveform during changes in blood volume. Br. J. Anaesth. 1999, 82, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Bendjelid, K. The pulse oximetry plethysmographic curve revisited. Curr. Opin. Crit. Care 2008, 14, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Feissel, M.; Teboul, J.L.; Merlani, P.; Badie, J.; Faller, J.P.; Bendjelid, K. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med. 2007, 33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Jabot, J.; Teboul, J.-L.; Richard, C.; Monnet, X. Passive leg raising for predicting fluid responsiveness: Importance of the postural change. Intensive Care Med. 2009, 35, 85–90. [Google Scholar] [CrossRef]
- Mallat, J.; Fischer, M.O.; Granier, M.; Vinsonneau, C.; Jonard, M.; Mahjoub, Y.; Baghdadi, F.A.; Préau, S.; Poher, F.; Rebet, O.; et al. Passive leg raising-induced changes in pulse pressure variation to assess fluid responsiveness in mechanically ventilated patients: A multicentre prospective observational study. Br. J. Anaesth. 2022, 129, 308–316. [Google Scholar] [CrossRef]
- Cannesson, M.; Attof, Y.; Rosamel, P.; Desebbe, O.; Joseph, P.; Metton, O.; Bastien, O.; Lehot, J.-J. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology 2007, 106, 1105–1111. [Google Scholar] [CrossRef]
- Delerme, S.; Renault, R.; Le Manach, Y.; Lvovschi, V.; Bendahou, M.; Riou, B.; Ray, P. Variations in pulse oximetry plethysmographic waveform amplitude induced by passive leg raising in spontaneously breathing volunteers. Am. J. Emerg. Med. 2007, 25, 637–642. [Google Scholar] [CrossRef]
- Delerme, S.; Castro, S.; Freund, Y.; Nazeyrollas, P.; Josse, M.-O.; Madonna-Py, B.; Rouff, E.; Riou, B.; Ray, P. Relation between pulse oximetry plethysmographic waveform amplitude induced by passive leg raising and cardiac index in spontaneously breathing subjects. Am. J. Emerg. Med. 2010, 28, 505–510. [Google Scholar] [CrossRef]
- Cannesson, M.; Awad, A.A.; Shelley, K. Oscillations in the plethysmographic waveform amplitude: Phenomenon hides behind artifacts. Anesthesiology 2009, 111, 206–207. [Google Scholar] [CrossRef]
- Magder, S. Clinical usefulness of respiratory variations in arterial pressure. Am. J. Respir. Crit. Care Med. 2004, 169, 151–155. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R.; Vasu, T.; Hirani, A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit. Care Med. 2009, 37, 2642–2647. [Google Scholar] [PubMed]
- Michard, F.; Boussat, S.; Chemla, D.; Anguel, N.; Mercat, A.; Lecarpentier, Y.; Richard, C.; Pinsky, M.R.; Teboul, J.-L. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am. J. Respir. Crit. Care Med. 2000, 162, 134–138. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International guidelines for nanagement of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [PubMed]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation–Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar]
- Stern, K.; Pillai, R.; Chowdhury, S.; Baydur, A. Assessment of hemodynamic changes during mechanical and spontaneous ventilation by variations in pulse oximetry waveform in critically ill patients undergoing hemodialysis: A pilot study to evaluate reliability of a noninvasive technique. J. Pulm. Respir. Med. 2018, 8, 6. [Google Scholar] [CrossRef]
- Dixon, W.J.; Massey, J.F. Introduction to Statistical Analysis, 4th ed.; McGraw-Hill Education: New York, NY, USA, 1983. [Google Scholar]
- Messina, A.; Calabrò, L.; Pugliese, L.; Lulja, A.; Sopuch, A.; Rosalba, D.; Morenghi, E.; Hernandez, G.; Monnet, X.; Cecconi, M. Fluid challenge in critically ill patients receiving haemodynamic monitoring: A systematic review and comparison of two decades. Crit. Care. 2022, 26, 186. [Google Scholar] [CrossRef]
- Acheampong, A.; Vincent, J.-L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care 2015, 19, 251. [Google Scholar]
- Hjortrup, P.B.; Haase, N.; Bundgaard, H.; Thomsen, S.L.; Winding, R.; Pettilä, V.; Aaen, A.; Lodahl, D.; Berthelsen, R.E.; Christensen, H.; et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: The CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016, 42, 1695–1705. [Google Scholar]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 155–170. [Google Scholar]
- Andrews, B.; Semler, M.W.; Muchemwa, L.; Kelly, P.; Lakhi, S.; Heimburger, D.C.; Mabula, C.; Bwalya, M.; Bernard, G.R. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: A randomized clinical trial. JAMA 2017, 318, 1233–1240. [Google Scholar] [PubMed]
- Meyhoff, T.S.; Møller, M.H.; Hjortrup, P.B.; Cronhjort, M.; Perner, A.; Wetterslev, J. Lower vs higher fluid volumes during initial management of sepsis: A systematic review with meta-analysis and trial sequential analysis. Chest 2020, 157, 1478–1496. [Google Scholar] [PubMed]
- Meyhoff, T.S.; Hjortrup, P.B.; Wetterslev, J.; Sivapalan, P.; Laake, J.H.; Cronhjort, M.; Jakob, S.M.; Cecconi, M.; Nalos, M.; Ostermann, M.; et al. Restriction of intravenous fluid in ICU patients with septic shock. N. Engl. J. Med. 2022, 386, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.C.d.F.; Barbas, C.S.V.; Queiroz, V.N.F.; Neto, A.S.; Deliberato, R.O.; Pereira, A.J.; Timenetsky, K.T.; Júnior, J.M.S.; Takaoka, F.; de Backer, D.; et al. Assessment of fluid responsiveness using pulse pressure variation, plethysmographic variability index, central venous pressure, and inferior vena cava variation in patients undergoing mechanical ventilation: A systematic review and meta-analysis. Crit. Care 2024, 28, 289. [Google Scholar] [CrossRef]
- Landsverk, S.A.; Hoiseth, L.O.; Kvandal, P.; Hisdal, J.; Skare, O.; Kirkeboen, K.A. Poor agreement between respiratory variations in pulse oximetry photoplethysmographic waveform amplitude and pulse pressure in intensive care unit patients. Anesthesiology 2008, 109, 849–855. [Google Scholar]
- Desgranges, F.P.; Desebbe, O.; Ghazouani, A.; Gilbert, K.; Keller, G.; Chiari, P.; Robin, J.; Bastien, O.; Lehot, J.J.; Cannesson, M. Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. Br. J. Anaesth. 2011, 107, 329–335. [Google Scholar]
- Muller, L.; Louart, G.; Bousquet, P.-J.; Candela, D.; Zoric, L.; de La Coussaye, J.-E.; Jaber, S.; Lefrant, J.-Y. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010, 36, 496–503. [Google Scholar] [CrossRef]
- Lima, A.P.; Beelen, P.; Bakker, J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit. Care Med. 2002, 30, 1210–1213. [Google Scholar] [CrossRef]
- Ginosar, Y.; Weiniger, C.F.; Meroz, Y.; Kurz, V.; Bdolah-Abram, T.; Babchenko, A.; Nitzan, M.; Davidson, E.M. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol. Scand. 2009, 53, 1018–1026. [Google Scholar]
- Galvin, E.M.; Niehof, S.; Medina, H.J.; Zijlstra, F.J.; van Bommel, J.; Klein, J.; Verbrugge, S.J.C. Thermographic temperature measurement compared with pinprick and cold sensation in predicting the effectiveness of regional blocks. Anesth. Analg. 2006, 102, 598–604. [Google Scholar]
- Broch, O.; Bein, B.; Gruenewald, M.; Höcker, J.; Schöttler, J.; Meybohm, P.; Steinfath, M.; Renner, J. Accuracy of the pleth variability index to predict fluid responsiveness depends on the perfusion index. Acta Anaesthesiol. Scand. 2011, 55, 686–693. [Google Scholar] [CrossRef]




| Age | 58.7 ± 18.3 |
| Sex (M/F) | 13/7 |
| BMI (kg/m2) | 26.5 ± 10 |
| Temperature (°C) | 37.1 ± 14.6 |
| Heart rate (beats/min) | 94.3 ± 27.6 |
| Blood pressure (syst/diast, mm Hg) | 104 ± 23/68 ± 12 |
| MAP (mm Hg) | 80.1 ± 14.6 |
| Respiratory rate (on ventilator, breaths/min) | 19.1 ± 4.1 |
| SpO2 (%) | 98.4 ± 1.6 |
| FiO2 | 0.59 ± 0.23 |
| Tidal volume (mL) | 455 ± 37 |
| Respiratory rate (breaths/min) | 19.3 ± 3 |
| Peak inspiratory flow (L/min) | 49.2 ± 8.4 |
| Peak airway pressure (cm H2O) | 25.2 ± 7.5 |
| Mean airway pressure (cm H2O) | 10.6 ± 2.5 |
| Positive end-expiratory pressure (cm H2O) | 5.9 ± 1.6 |
| Airway resistance (cm H2O/L/s) *** | 21.3 ± 7.1 |
| Total net volume crystalloid (mL) | 3954 ± 2734 |
| Net intravascular crystalloid administered (mL/h) | 152 ± 12 |
| Number of patients receiving vasoactive drugs at baseline: | |
| 7 |
| 2 |
| 2 |
| Individual diagnoses: | |
| Primary (source of sepsis) | Secondary |
| Upper GI bleed |
| AKI |
| Alcoholic cirrhosis |
| Uncontrolled DM |
| Encephalopathy |
| HRF |
| AKI |
| HRF |
| Testicular CA |
| Uncontrolled DM |
| Encephalopathy |
| Heroin Overdose |
| HRF |
| Multiple myeloma, |
| s/p auto-SCT | |
| Adenoca, lung |
| Vascular dementia |
| AIDS |
| Hyponatremia |
| AKI |
| Alcohol withdrawal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagihara, J.; Guo, X.; Baydur, A. The Effects of Passive Leg Raising and Maintenance Fluid Administration on Pulse Oximetry Waveform Amplitude and Peak Variability in Mechanically Ventilated Patients in Sepsis and Septic Shock. Diagnostics 2025, 15, 798. https://doi.org/10.3390/diagnostics15070798
Kagihara J, Guo X, Baydur A. The Effects of Passive Leg Raising and Maintenance Fluid Administration on Pulse Oximetry Waveform Amplitude and Peak Variability in Mechanically Ventilated Patients in Sepsis and Septic Shock. Diagnostics. 2025; 15(7):798. https://doi.org/10.3390/diagnostics15070798
Chicago/Turabian StyleKagihara, Jamie, Xinning Guo, and Ahmet Baydur. 2025. "The Effects of Passive Leg Raising and Maintenance Fluid Administration on Pulse Oximetry Waveform Amplitude and Peak Variability in Mechanically Ventilated Patients in Sepsis and Septic Shock" Diagnostics 15, no. 7: 798. https://doi.org/10.3390/diagnostics15070798
APA StyleKagihara, J., Guo, X., & Baydur, A. (2025). The Effects of Passive Leg Raising and Maintenance Fluid Administration on Pulse Oximetry Waveform Amplitude and Peak Variability in Mechanically Ventilated Patients in Sepsis and Septic Shock. Diagnostics, 15(7), 798. https://doi.org/10.3390/diagnostics15070798





