From Risk Assessment to Management: Cardiovascular Complications in Pre- and Post-Kidney Transplant Recipients: A Narrative Review
Abstract
1. Introduction
2. Assessment of Cardiovascular Risk in Kidney Transplant Recipients
2.1. Epidemiology of Cardiovascular Events
2.1.1. Cardiovascular Morbidity and Mortality
2.1.2. Early Cardiovascular Events
2.2. Cardiovascular Risk Factors
2.2.1. Traditional Risk Factors
2.2.2. Non-Classical Risk Factors
2.3. Strategies for Screening for Coronary Artery Disease in Potential Kidney Transplant Recipient (PKTR)
2.3.1. Pretransplant Coronary Artery Disease Screening: Why?
2.3.2. Performance of the Main Available Tests in the CKD Population
2.3.3. Which Screening Strategy to Choose?
3. Management of Post-Transplant Cardiovascular Risk
3.1. Treatment of Traditional Risk Factors in Kidney Transplant Recipients
| Modality of Screening | Timing of Screening | Treatment | Target | Guidelines | |
| Hypertension | Home blood pressure self-monitoring and measurement at office visit. | Blood pressure measurement at every visit |
|
| AHA 2017 [113] |
| Dyslipidemia | Measurement of LDL-C, HDL-C, and triglycerides | Annually |
|
| KDIGO 2013 [114] 2019 ESC/EAS Guidelines [116] |
| Diabetes | Fasting plasma glucose, HbA1c, or oral glucose tolerance testing | Weekly during the first month, then at months 3, 6, 9, and 12, followed by annual assessments or when increasing the CNI dose. |
| HbA1c 7–7.5% | KDIGO 2009 [128] |
| Smoking | Discuss tobacco use at each visit and assess whether the patient is willing to quit or needs assistance. | At every visit |
| Smoking cessation | KDIGO 2009 [128] |
3.2. Treatment of Non-Classical Risk Factors Management in Kidney Transplant Recipients
3.3. The Role of Lifestyle Modifications
3.4. RAAS Inhibitors in Kidney Transplant Recipients
| Study (Year) | Population (N) | Design | Key Findings | Reference |
|---|---|---|---|---|
| Midtvedt et al. (2001) | 72 | RCT | Nifedipine improved GFR by 20%; lisinopril reduced proteinuria. | [147] |
| Heinze et al. (2006) | 1513 | Observational cohort | RAS inhibitors reduced graft loss risk (HR 0.65) and mortality (HR 0.61). | [148] |
| Opelz et al. (2006) | 17,209 | Observational cohort | No significant improvement in graft (HR 1.05) or patient survival (HR 1.01). | [149] |
| Knoll et al. (2016) | 213 | RCT | No significant effect on ESRD, mortality, or creatinine doubling in KTRs. | [150] |
| Cheungpasitporn et al. (2016) | 20,024 | Meta-analysis | No reduction in allograft loss or mortality; heterogeneous study designs. | [151] |
| Kovarik et al. (2019) | 48 | Cross-sectional | ACEis promoted anti-inflammatory RAS pathways but showed intrarenal escape. | [152] |
3.5. Emerging Therapies to Manage Cardiovascular Risk
3.6. Challenges of Immunosuppression from a Cardiovascular Perspective
3.6.1. T-Cells and Their Cardiovascular Impact
3.6.2. Off-Targets Adverse Effects of Maintenance Therapy: A Personalized Strategy
3.6.3. Addressing CNI Toxicity
3.6.4. mTOR Inhibitors: A Perilous Alternative
3.6.5. Belatacept: A Promising Alternative
3.6.6. Early Steroid Withdrawal: A Beneficial Strategy for Select Patients
3.7. Vascular Access Management After Transplantation: What Is the Best Option?
3.7.1. Cardiovascular Implications of AVF
3.7.2. AVF Management Post-Transplantation
| Study | Design | Patients (N) Period | Intervention | Echographic Findings | Clinical Outcomes | Ref. |
| Stoumpos et al. | Observational study | 1330 (2010–2020) | None, analysis of existing AVFs | Increased left ventricular mass, elevated cardiac output | Higher risk of new-onset heart failure post-transplant (adjusted Hazard Ratio 2.14) | [68] |
| Rao et al. | Randomized controlled trial | 64 (2013–2017) | AVF ligation | Decrease in left ventricular mass, atrial volumes, and NT-proBNP levels | Improved cardiac remodeling, no impact on eGFR | [195] |
| Hetz et al. | Randomized controlled trial | 28 (2013–2018) | Prophylactic ligation | Reduction in cardiac volumes and pulmonary systolic pressures | Prevention of high-output heart failure, decrease of NT pro BNP, no serum creatinine differences. | [196] |
| Keller et al. | Observational Prospective study | 49 (2013–2015) | None, comparative analysis | Higher LVEDD and LVESD in high-flow AVF compared with normal-flow AVF | Associated with hemodynamic abnormalities | [67] |
| Janeckova et al. | Observational study | 40 (2018–2023) | Flow reduction/ligation | Significant reduction in arterial flow and cardiac output post-ligation | 88.3% primary patency after flow reduction, lower dyspnea according NYHA grade III, lower creatininemia and improve GFR | [197] |
3.7.3. Evidence for AVF Ligation
3.7.4. Individualized AVF Management
4. Management of Post-Transplant Cardiovascular Disease
4.1. Management of Coronary Artery Diseases in Kidney Transplant Recipients
4.2. Management of Cerebrovascular Disease in Kidney Transplant Recipients
4.3. Management of Atrial Fibrillation in Kidney Transplant Recipients
4.4. Management of Peripheral Artery Disease in Kidney Transplant Recipients
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.-M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef]
- Strohmaier, S.; Wallisch, C.; Kammer, M.; Geroldinger, A.; Heinze, G.; Oberbauer, R.; Haller, M.C. Survival Benefit of First Single-Organ Deceased Donor Kidney Transplantation Compared with Long-term Dialysis Across Ages in Transplant-Eligible Patients With Kidney Failure. JAMA Netw. Open 2022, 5, e2234971. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998, 32, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Disney, A.P.S. Demography and survival of patients receiving treatment for chronic renal failure in Australia and New Zealand: Report on dialysis and renal transplantation treatment from the Australia and New Zealand dialysis and transplant registry. Am. J. Kidney Dis. 1995, 25, 165–175. [Google Scholar] [CrossRef]
- Ojo, A.O.; Hanson, J.A.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Port, F.K. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000, 57, 307–313. [Google Scholar] [CrossRef]
- Awan, A.A.; Niu, J.; Pan, J.S.; Erickson, K.F.; Mandayam, S.; Winkelmayer, W.C.; Navaneethan, S.D.; Ramanathan, V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996–2014). Am. J. Nephrol. 2018, 48, 472–481. [Google Scholar] [CrossRef]
- Ying, T.; Shi, B.; Kelly, P.J.; Pilmore, H.; Clayton, P.A.; Chadban, S.J. Death after Kidney Transplantation: An Analysis by Era and Time Post-Transplant. J. Am. Soc. Nephrol. 2020, 31, 2887–2899. [Google Scholar] [CrossRef]
- Wyld, M.L.R.; De La Mata, N.L.; Masson, P.; O’Lone, E.; Kelly, P.J.; Webster, A.C. Cardiac Mortality in Kidney Transplant Patients: A Population-based Cohort Study 1988–2013 in Australia and New Zealand. Transplantation 2021, 105, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Helve, S.; Helanterä, I.; Laine, M.; Nieminen, T.; Finne, P.; Helve, J. Trends and Specific Causes of Cardiovascular Mortality after Kidney Transplantation in Finland. Clin. J. Am. Soc. Nephrol. 2023, 19, 355–363. [Google Scholar] [CrossRef]
- Acosta, E.; Mehta, N.; Myrskylä, M.; Ebeling, M. Cardiovascular Mortality Gap Between the United States and Other High Life Expectancy Countries in 2000–2016. J. Gerontol. Ser. B 2022, 77, S148–S157. [Google Scholar] [CrossRef] [PubMed]
- Mayrdorfer, M.; Liefeldt, L.; Osmanodja, B.; Naik, M.G.; Schmidt, D.; Duettmann, W.; Hammett, C.; Schrezenmeier, E.; Friedersdorff, F.; Wu, K.; et al. A single centre in-depth analysis of death with a functioning kidney graft and reasons for overall graft failure. Nephrol. Dial. Transplant. 2022, 38, 1857–1866. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Weir, M.R.; Adey, D.B.; House, A.A.; Bostom, A.G.; Kusek, J.W. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation—Evidence from the FAVORIT study. Clin. Transplant. 2012, 26, E438–E446. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, C.A.; Rao, A.; Middleton, R.; Kalra, P.A. Post-Transplant Cardiovascular Disease in Kidney Transplant Recipients: Incidence, Risk Factors, and Outcomes in the Era of Modern Immunosuppression. J. Clin. Med. 2024, 13, 2734. [Google Scholar] [CrossRef]
- Andersson, C.; Hansen, D.; Sørensen, S.S.; McGrath, M.; McCausland, F.R.; Torp-Pedersen, C.; Schou, M.; Køber, L.; Pfeffer, M.A. Long-term cardiovascular events, graft failure, and mortality in kidney transplant recipients. Eur. J. Intern. Med. 2024, 121, 109–113. [Google Scholar] [CrossRef]
- Aakhus, S.; Dahl, K.; Widerøe, T.E. Cardiovascular disease in stable renal transplant patients in Norway: Morbidity and mortality during a 5-yr follow-up. Clin. Transplant. 2004, 18, 596–604. [Google Scholar] [CrossRef]
- Mathur, A.K.; Chang, Y.-H.; Steidley, D.E.; Heilman, R.; Khurmi, N.; Wasif, N.; Etzioni, D.; Moss, A.A. Patterns of Care and Outcomes in Cardiovascular Disease After Kidney Transplantation in the United States. Transplant. Direct 2017, 3, e126. [Google Scholar] [CrossRef]
- Lentine, K.L.; Brennan, D.C.; Schnitzler, M.A. Incidence and predictors of myocardial infarction after kidney transplantation. J. Am. Soc. Nephrol. 2005, 16, 496–506. [Google Scholar] [CrossRef]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Peng, Y.; Maclean, J.R.; Weinhandl, E.D.; Kasiske, B.L.; PORT Investigators. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2010, 10, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.R.; Fallahi, A.; Kim, M.C.; Esquitin, R.; Robbins, M.J. Coronary artery disease in a large renal transplant population: Implications for management. Am. J. Transplant. 2011, 11, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.S.; Liu, S.; Han, J.; Stedman, M.R.; Baiocchi, M.; Tan, J.C.; Chertow, G.M.; Fearon, W.F. Association of Pretransplant Coronary Heart Disease Testing with Early Kidney Transplant Outcomes. JAMA Intern. Med. 2023, 183, 134–141. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; deFilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef]
- de Mattos, A.M.; Siedlecki, A.; Gaston, R.S.; Perry, G.J.; Julian, B.A.; Kew, C.E.I.; Deierhoi, M.H.; Young, C.; Curtis, J.J.; Iskandrian, A.E. Systolic Dysfunction Portends Increased Mortality among Those Waiting for Renal Transplant. J. Am. Soc. Nephrol. 2008, 19, 1191. [Google Scholar] [CrossRef]
- Wali, R.K.; Wang, G.S.; Gottlieb, S.S.; Bellumkonda, L.; Hansalia, R.; Ramos, E.; Drachenberg, C.; Papadimitriou, J.; Brisco, M.A.; Blahut, S.; et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J. Am. Coll. Cardiol. 2005, 45, 1051–1060. [Google Scholar] [CrossRef]
- Lenihan, C.R.; Montez-Rath, M.E.; Scandling, J.D.; Turakhia, M.P.; Winkelmayer, W.C. Outcomes after kidney transplantation of patients previously diagnosed with atrial fibrillation. Am. J. Transplant. 2013, 13, 1566–1575. [Google Scholar] [CrossRef]
- Lentine, K.L.; Schnitzler, M.A.; Abbott, K.C.; Li, L.; Xiao, H.; Burroughs, T.E.; Takemoto, S.K.; Willoughby, L.M.; Gavard, J.A.; Brennan, D.C. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2006, 1, 288–296. [Google Scholar] [CrossRef]
- Lentine, K.L.; Villines, T.C.; Axelrod, D.; Kaviratne, S.; Weir, M.R.; Costa, S.P. Evaluation and Management of Pulmonary Hypertension in Kidney Transplant Candidates and Recipients: Concepts and Controversies. Transplantation 2017, 101, 166. [Google Scholar] [CrossRef]
- Alhamad, E.H.; Al-Ghonaim, M.; Alfaleh, H.F.; Cal, J.P.; Said, N. Pulmonary hypertension in end-stage renal disease and post renal transplantation patients. J. Thorac. Dis. 2014, 6, 606–616. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Lunawat, D.; Abraham, G.; Matthew, M.; Mullasari, A.; Nagarajan, P.; Reddy, Y.N.V. Progressive pulmonary hypertension: Another criterion for expeditious renal transplantation. Saudi J. Kidney Dis. Transplant. 2013, 24, 925–929. [Google Scholar] [CrossRef]
- Basile, C.; Lomonte, C.; Vernaglione, L.; Casucci, F.; Antonelli, M.; Losurdo, N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Daragó, A.; Szabó, E.; Barkó, D.; Schwegler, G.; Szabó, R.P.; Nagy, A.C.; Nemes, B. Effects of Kidney Transplantation on Valvular Heart Diseases. Transplant. Proc. 2021, 53, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.C.; Hshieh, P.; Cruess, D.; Agodoa, L.Y.C.; Welch, P.G.; Taylor, A.J.; Yuan, C.M. Hospitalized valvular heart disease in patients on renal transplant waiting list: Incidence, clinical correlates and outcomes. Clin. Nephrol. 2003, 59, 79–87. [Google Scholar] [CrossRef]
- Fox, H.; Büttner, S.; Hemmann, K.; Asbe-Vollkopf, A.; Doss, M.; Beiras-Fernandez, A.; Moritz, A.; Zeiher, A.M.; Scheuermann, E.; Geiger, H.; et al. Transcatheter aortic valve implantation improves outcome compared to open-heart surgery in kidney transplant recipients requiring aortic valve replacement. J. Cardiol. 2013, 61, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Marcassi, A.P.; Yasbek, D.C.; Pestana, J.O.M.; Fachini, F.C.; De Lira Filho, E.B.; Cassiolato, J.L.; Canziani, M.E.F. Ventricular arrhythmia in incident kidney transplant recipients: Prevalence and associated factors. Transpl. Int. 2011, 24, 67–72. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Hill, G.S.; Nochy, D.; Loupy, A. Accelerated arteriosclerosis: A form of transplant arteriopathy. Curr. Opin. Organ Transplant. 2010, 15, 11. [Google Scholar] [CrossRef]
- Freedman, B.I.; Cohen, A.H. Hypertension-attributed nephropathy: What’s in a name? Nat. Rev. Nephrol. 2016, 12, 27–36. [Google Scholar] [CrossRef]
- Loupy, A.; Vernerey, D.; Viglietti, D.; Aubert, O.; Duong Van Huyen, J.-P.; Empana, J.-P.; Bruneval, P.; Glotz, D.; Legendre, C.; Jouven, X.; et al. Determinants and Outcomes of Accelerated Arteriosclerosis. Circ. Res. 2015, 117, 470–482. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Maclean, J.R.; Snyder, J.J. Acute Myocardial Infarction and Kidney Transplantation. J. Am. Soc. Nephrol. 2006, 17, 900. [Google Scholar] [CrossRef] [PubMed]
- De Lima, J.J.G.; Gowdak, L.H.W.; David-Neto, E.; Bortolotto, L.A. Early cardiovascular events and cardiovascular death after renal transplantation: Role of pretransplant risk factors. Clin. Exp. Nephrol. 2021, 25, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.; Saeed, M.J.; Shpigel, A.; Novak, E.; Alhamad, T.; Stwalley, D.; Rich, M.W.; Brown, D.L. The association of preoperative cardiac stress testing with 30-day death and myocardial infarction among patients undergoing kidney transplantation. PLoS ONE 2019, 14, e0211161. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.; Forsyth, J.L.; Oniscu, G.C.; Robb, M.; Watson, C.; Fotheringham, J.; Roderick, P.J.; Ravanan, R.; Taylor, D.M. A propensity score–matched analysis indicates screening for asymptomatic coronary artery disease does not predict cardiac events in kidney transplant recipients. Kidney Int. 2021, 99, 431–442. [Google Scholar] [CrossRef]
- Pilmore, H.; Dent, H.; Chang, S.; McDonald, S.P.; Chadban, S.J. Reduction in cardiovascular death after kidney transplantation. Transplantation 2010, 89, 851–857. [Google Scholar] [CrossRef]
- Carpenter, M.A.; John, A.; Weir, M.R.; Smith, S.R.; Hunsicker, L.; Kasiske, B.L.; Kusek, J.W.; Bostom, A.; Ivanova, A.; Levey, A.S.; et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J. Am. Soc. Nephrol. 2014, 25, 1554–1562. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Anjum, S.; Shah, R.; Skogen, J.; Kandaswamy, C.; Danielson, B.; O’Shaughnessy, E.A.; Dahl, D.C.; Silkensen, J.R.; Sahadevan, M.; et al. Hypertension after kidney transplantation. Am. J. Kidney Dis. 2004, 43, 1071–1081. [Google Scholar] [CrossRef]
- Speer, C.; Benning, L.; Morath, C.; Zeier, M.; Frey, N.; Opelz, G.; Döhler, B.; Tran, T.H. Blood Pressure Goals and Outcomes in Kidney Transplant Recipients in an Analysis of the Collaborative Transplant Study. Kidney Int. Rep. 2024, 10, 780–790. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Gao, W. Gender differences in cardiovascular outcomes of kidney transplant recipients: A retrospective cohort study. Medicine 2024, 103, e39568. [Google Scholar] [CrossRef]
- Roodnat, J.I.; Mulder, P.G.; Zietse, R.; Rischen-Vos, J.; van Riemsdijk, I.C.; IJzermans, J.N.; Weimar, W. Cholesterol as an independent predictor of outcome after renal transplantation. Transplantation 2000, 69, 1704–1710. [Google Scholar] [CrossRef]
- Holdaas, H.; Fellström, B.; Jardine, A.G.; Holme, I.; Nyberg, G.; Fauchald, P.; Grönhagen-Riska, C.; Madsen, S.; Neumayer, H.-H.; Cole, E.; et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: A multicentre, randomised, placebo-controlled trial. Lancet Lond. Engl. 2003, 361, 2024–2031. [Google Scholar] [CrossRef]
- Holdaas, H.; Fellström, B.; Cole, E.; Nyberg, G.; Olsson, A.G.; Pedersen, T.R.; Madsen, S.; Grönhagen-Riska, C.; Neumayer, H.-H.; Maes, B.; et al. Long-term Cardiac Outcomes in Renal Transplant Recipients Receiving Fluvastatin: The ALERT Extension Study. Am. J. Transplant. 2005, 5, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes mellitus after kidney transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Cosio, F.G.; Kudva, Y.; van der Velde, M.; Larson, T.S.; Textor, S.C.; Griffin, M.D.; Stegall, M.D. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005, 67, 2415–2421. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Sampaio, M.S.; Vincenti, F.; Bunnapradist, S. Associations of Pretransplant Diabetes Mellitus, New-Onset Diabetes After Transplant, and Acute Rejection With Transplant Outcomes: An Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) Database. Am. J. Kidney Dis. 2010, 56, 1127–1139. [Google Scholar] [CrossRef]
- Caillard, S.; Eprinchard, L.; Perrin, P.; Braun, L.; Heibel, F.; Moreau, F.; Kessler, L.; Moulin, B. Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: Role of systematic screening by oral glucose tolerance test. Transplantation 2011, 91, 757–764. [Google Scholar] [CrossRef]
- Lim, W.H.; Lok, C.E.; Kim, S.J.; Knoll, G.; Shah, B.R.; Naylor, K.; Luo, B.; Vinegar, M.; Dixon, S.N.; Hawley, C.; et al. Impact of Pretransplant and New-Onset Diabetes After Transplantation on the Risk of Major Adverse Cardiovascular Events in Kidney Transplant Recipients: A Population-based Cohort Study. Transplantation 2021, 105, 2470–2481. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Klinger, D. Cigarette smoking in renal transplant recipients. J. Am. Soc. Nephrol. 2000, 11, 753–759. [Google Scholar] [CrossRef]
- Hurst, F.P.; Altieri, M.; Patel, P.P.; Jindal, T.R.; Guy, S.R.; Sidawy, A.N.; Agodoa, L.Y.; Abbott, K.C.; Jindal, R.M. Effect of smoking on kidney transplant outcomes: Analysis of the United States Renal Data System. Transplantation 2011, 92, 1101–1107. [Google Scholar] [CrossRef]
- Lentine, K.L.; Rey, L.A.R.; Bacchi, G.; Wasi, N.; Schmitz, L.; Salvalaggio, P.R.; Abbott, K.C.; Schnitzler, M.A.; Neri, L.; Brennan, D.C. Obesity and cardiac risk after kidney transplantation: Experience at one center and comprehensive literature review. Transplantation 2008, 86, 303. [Google Scholar] [CrossRef] [PubMed]
- Gwon, J.G.; Choi, J.; Jung, C.W.; Lee, C.H.; Oh, S.W.; Jo, S.-K.; Cho, W.Y.; Park, J.B.; Huh, K.H.; Ro, H.; et al. Impact of changes in waist-to-hip ratio after kidney transplantation on cardiovascular outcomes. Sci. Rep. 2021, 11, 783. [Google Scholar] [CrossRef]
- Soveri, I.; Abedini, S.; Holdaas, H.; Jardine, A.; Eriksson, N.; Fellström, B. Metabolic syndrome and cardiovascular risk in renal transplant recipients: Effects of statin treatment. Clin. Transplant. 2009, 23, 914–920. [Google Scholar] [CrossRef]
- Helanterä, I.; Salmela, K.; Kyllönen, L.; Koskinen, P.; Grönhagen-Riska, C.; Finne, P. Pretransplant Dialysis Duration and Risk of Death After Kidney Transplantation in the Current Era. Transplantation 2014, 98, 458. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, K.M.; Akaberi, S.; Isaksson, E.; Reihnér, E.; Czuba, T.; Prütz, K.-G.; Clyne, N.; Almquist, M. Cardiovascular and Cerebrovascular Events After Parathyroidectomy in Patients on Renal Replacement Therapy. World J. Surg. 2019, 43, 1981–1988. [Google Scholar] [CrossRef]
- Tapiawala, S.N.; Tinckam, K.J.; Cardella, C.J.; Schiff, J.; Cattran, D.C.; Cole, E.H.; Kim, S.J. Delayed graft function and the risk for death with a functioning graft. J. Am. Soc. Nephrol. 2010, 21, 153–161. [Google Scholar] [CrossRef]
- Keller, N.; Monnier, A.; Caillard, S.; Cognard, N.; Geny, B.; Moulin, B.; Talha, S. High-flow arteriovenous fistula and hemodynamic consequences at 1 year after kidney transplantation. Semin. Dial. 2022, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, S.; Van Rhijn, P.; Mangion, K.; Thomson, P.C.; Mark, P.B. Arteriovenous fistula for haemodialysis as a predictor of de novo heart failure in kidney transplant recipients. Clin. Kidney J. 2024, 17, sfae105. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Chakkera, H.A.; Roel, J. Explained and unexplained ischemic heart disease risk after renal transplantation. J. Am. Soc. Nephrol. 2000, 11, 1735–1743. [Google Scholar] [CrossRef]
- Fernández-Fresnedo, G.; Escallada, R.; Rodrigo, E.; De Francisco, A.L.M.; Cotorruelo, J.G.; Sanz De Castro, S.; Zubimendi, J.A.; Ruiz, J.C.; Arias, M. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation 2002, 73, 1345–1348. [Google Scholar] [CrossRef]
- Hiremath, S.; Fergusson, D.A.; Fergusson, N.; Bennett, A.; Knoll, G.A. Renin-Angiotensin System Blockade and Long-term Clinical Outcomes in Kidney Transplant Recipients: A Meta-analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2017, 69, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Carpenter, M.A.; Levey, A.S.; Ivanova, A.; Cole, E.H.; Hunsicker, L.; Kasiske, B.L.; Kim, S.J.; Kusek, J.W.; Bostom, A.G. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: The favorit trial. Am. J. Transplant. 2012, 12, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Riella, L.V.; Funakoshi, T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: A systematic review and meta-analysis. Am. J. Transplant. 2014, 14, 2317–2327. [Google Scholar] [CrossRef]
- Wen, X.; Casey, M.J.; Santos, A.H.; Hartzema, A.; Womer, K.L. Comparison of Utilization and Clinical Outcomes for Belatacept- and Tacrolimus-Based Immunosuppression in Renal Transplant Recipients. Am. J. Transplant. 2016, 16, 3202–3211. [Google Scholar] [CrossRef]
- Divard, G.; Aubert, O.; Debiais-Deschamp, C.; Raynaud, M.; Goutaudier, V.; Sablik, M.; Sayeg, C.; Legendre, C.; Obert, J.; Anglicheau, D.; et al. Long-Term Outcomes after Conversion to a Belatacept-Based Immunosuppression in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2024, 19, 628. [Google Scholar] [CrossRef]
- Bakri, R.S.; Afzali, B.; Covic, A.; Sriskantharan, R.; Bharma-Ariza, P.; Park, W.-H.; Sriharan, M.; Dalton, N.; Wierzbicki, A.S.; Crook, M.A.; et al. Cardiovascular disease in renal allograft recipients is associated with elevated sialic acid or markers of inflammation. Clin. Transplant. 2004, 18, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, C.; Foley, R.; Jeffery, J.; Negrijn, C.; Tribula, C.; Parfrey, P. Electrocardiographic Left Ventricular Hypertrophy in Renal Transplant Recipients: Prognostic Value and Impact of Blood Pressure and Anemia. J. Am. Soc. Nephrol. 2003, 14, 462. [Google Scholar] [CrossRef]
- Ibernon, M.; Moreso, F.; Ruiz-Majoral, A.; Sarrias, X.; Sarrias, M.; Grinyó, J.M.; Serón, D. Contribution of anemia and hypertension to left ventricular hypertrophy during the initial 2 years after renal transplantation. Transplant. Proc. 2011, 43, 2199–2204. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Writing Group for the CKD Prognosis Consortium; Grams, M.E.; Coresh, J.; Matsushita, K.; Ballew, S.H.; Sang, Y.; Surapaneni, A.; Alencar de Pinho, N.; Anderson, A.; Appel, L.J.; et al. Estimated Glomerular Filtration Rate, Albuminuria, and Adverse Outcomes: An Individual-Participant Data Meta-Analysis. JAMA 2023, 330, 1266–1277. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Littrell, K.; Arko, C.; Frederick, P.D.; Blaney, M. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: A collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation 2007, 116, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Sosnov, J.; Lessard, D.; Goldberg, R.J.; Yarzebski, J.; Gore, J.M. Differential symptoms of acute myocardial infarction in patients with kidney disease: A community-wide perspective. Am. J. Kidney Dis. 2006, 47, 378–384. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Cheng, X.S.; Mohanty, S.; Turner, V.; Mastrodicasa, D.; Winther, S.; Fleischmann, D.; Tan, J.C.; Fearon, W.F. Coronary Computed Tomography Angiography in Diagnosing Obstructive Coronary Artery Disease in Patients with Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2021, 11, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.; Svensson, M.; Jørgensen, H.S.; Bouchelouche, K.; Gormsen, L.C.; Pedersen, B.B.; Holm, N.R.; Bøtker, H.E.; Ivarsen, P.; Bøttcher, M. Diagnostic Performance of Coronary CT Angiography and Myocardial Perfusion Imaging in Kidney Transplantation Candidates. JACC Cardiovasc. Imaging 2015, 8, 553–562. [Google Scholar] [CrossRef]
- El-Tallawi, K.C.; Aljizeeri, A.; Nabi, F.; Al-Mallah, M.H. Myocardial Perfusion Imaging Using Positron Emission Tomography. Methodist DeBakey Cardiovasc. J. 2020, 16, 114–121. [Google Scholar] [CrossRef]
- Winther, S.; Svensson, M.; Jørgensen, H.S.; Rasmussen, L.D.; Holm, N.R.; Gormsen, L.C.; Bouchelouche, K.; Bøtker, H.E.; Ivarsen, P.; Bøttcher, M. Prognostic Value of Risk Factors, Calcium Score, Coronary CTA, Myocardial Perfusion Imaging, and Invasive Coronary Angiography in Kidney Transplantation Candidates. JACC Cardiovasc. Imaging 2018, 11, 842–854. [Google Scholar] [CrossRef]
- Dahl, J.N.; Nielsen, M.B.; Rasmussen, L.D.; Ivarsen, P.; Williams, M.C.; Svensson, M.H.S.; Birn, H.; Bøttcher, M.; Winther, S. Coronary Plaque Characteristics in Patients With Chronic Kidney Failure: Impact on Cardiovascular Events and Mortality. Circ. Cardiovasc. Imaging 2024, 17, e017066. [Google Scholar] [CrossRef]
- Cheng, X.S.; VanWagner, L.B.; Costa, S.P.; Axelrod, D.A.; Bangalore, S.; Norman, S.P.; Herzog, C.A.; Lentine, K.L.; On behalf of the American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Cardiovascular Radiology and Intervention. Emerging Evidence on Coronary Heart Disease Screening in Kidney and Liver Transplantation Candidates: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e299–e324. [Google Scholar] [CrossRef]
- Lange, S.; Mędrzycka-Dąbrowska, W.; Zorena, K.; Dąbrowski, S.; Ślęzak, D.; Malecka-Dubiela, A.; Rutkowski, P. Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review. Int. J. Environ. Res. Public. Health 2021, 18, 3000. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Gewirtz, H.; Marwick, T.H.; Kwong, R.Y.; Raggi, P.; Al-Mallah, M.H.; Herzog, C.A. Cardiac Imaging for Coronary Heart Disease Risk Stratification in Chronic Kidney Disease. JACC Cardiovasc. Imaging 2021, 14, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Dundon, B.K.; Pisaniello, A.D.; Nelson, A.J.; Maia, M.; Teo, K.S.L.; Worthley, S.G.; Coates, P.T.; Russ, G.R.; Faull, R.J.; Bannister, K.; et al. Dobutamine stress cardiac MRI for assessment of coronary artery disease prior to kidney transplantation. Am. J. Kidney Dis. 2015, 65, 808–809. [Google Scholar] [CrossRef]
- Ripley, D.P.; Kannoly, S.; Gosling, O.E.; Hossain, E.; Chawner, R.R.; Moore, J.; Shore, A.C.; Bellenger, N.G. Safety and feasibility of dobutamine stress cardiac magnetic resonance for cardiovascular assessment prior to renal transplantation. J. Cardiovasc. Med. Hagerstown Md 2014, 15, 288–294. [Google Scholar] [CrossRef]
- Golzar, Y.; Doukky, R. Stress SPECT Myocardial Perfusion Imaging in End-Stage Renal Disease. Curr. Cardiovasc. Imaging Rep. 2017, 10, 13. [Google Scholar] [CrossRef]
- Wang, L.W.; Fahim, M.A.; Hayen, A.; Mitchell, R.L.; Baines, L.; Lord, S.; Craig, J.C.; Webster, A.C. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst. Rev. 2011, 2011, CD008691. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, J.R.; Jolink, F.E.J.; Benjamens, S.; Monroy-Gonzalez, A.G.; Pol, R.A.; Slart, R.H.J.A. Diagnostic accuracy of myocardial perfusion imaging in patients evaluated for kidney transplantation: A systematic review and meta-analysis. J. Nucl. Cardiol. 2022, 29, 3405–3415. [Google Scholar] [CrossRef]
- Ives, C.W.; AlJaroudi, W.A.; Kumar, V.; Farag, A.; Rizk, D.V.; Oparil, S.; Iskandrian, A.E.; Hage, F.G. Prognostic value of myocardial perfusion imaging performed pre-renal transplantation: Post-transplantation follow-up and outcomes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1998–2008. [Google Scholar] [CrossRef]
- Helve, S.; Laine, M.; Sinisalo, J.; Helanterä, I.; Hänninen, H.; Lammintausta, O.; Lehtonen, J.; Finne, P.; Nieminen, T. Even mild reversible myocardial perfusion defects predict mortality in patients evaluated for kidney transplantation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1019–1025. [Google Scholar] [CrossRef]
- Poli, F.E.; Gulsin, G.S.; McCann, G.P.; Burton, J.O.; Graham-Brown, M.P. The assessment of coronary artery disease in patients with end-stage renal disease. Clin. Kidney J. 2019, 12, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Masson, P.; Turner, R.M.; Lord, S.W.; Baines, L.A.; Craig, J.C.; Webster, A.C. Prognostic value of cardiac tests in potential kidney transplant recipients: A systematic review. Transplantation 2015, 99, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Phawanawichian, C.; Kaolawanich, Y.; Skulratanasak, P.; Ratanasit, N. Prognostic value of dobutamine stress echocardiography for the long-term outcomes in kidney transplant candidates. Cardiovasc. Diagn. Ther. 2024, 14, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, N.T.; Prakken, N.H.J.; Gareb, B.; Benjamens, S.; Sanders, J.S.F.; Slart, R.H.J.A.; Pol, R.A. Prognostic value of single photon emission computed tomography myocardial perfusion imaging for the prediction of MACE in pre- kidney transplant recipients: A systematic review and meta-analysis. Transplant. Rev. Orlando Fla 2024, 38, 100879. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Huck, D.M.; Weber, B.; Schreiber, B.; Pandav, J.; Parks, S.; Hainer, J.; Brown, J.M.; Divakaran, S.; Blankstein, R.; Dorbala, S.; et al. Comparative Effectiveness of PET and SPECT MPI for Predicting Cardiovascular Events After Kidney Transplant. Circ. Cardiovasc. Imaging 2024, 17, e015858. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lindahl, B.; Carrero, J.J.; Evans, M.; Szummer, K.; Jernberg, T. Cardiac Troponins and Their Prognostic Importance in Patients with Suspected Acute Coronary Syndrome and Renal Dysfunction. Clin. Chem. 2017, 63, 1409–1417. [Google Scholar] [CrossRef]
- Szramowska, A.; Bielecki, M.; Grzeszczyk, M.; Łabyk, A.; Kurnicka, K.; Pruszczyk, P.; Roik, M. High-sensitivity cardiac troponin T in detecting obstructive coronary artery disease in hemodialysis patients listed for kidney transplantation. Kardiol. Pol. 2024, 82, 285–291. [Google Scholar] [CrossRef]
- Palamuthusingam, D.; Pascoe, E.M.; Hawley, C.M.; Johnson, D.W.; Fahim, M. Revised cardiac risk index in predicting cardiovascular complications in patients receiving chronic kidney replacement therapy undergoing elective general surgery. Perioper. Med. Lond. Engl. 2024, 13, 70. [Google Scholar] [CrossRef]
- Silver, S.A.; Huang, M.; Nash, M.M.; Prasad, G.V.R. Framingham risk score and novel cardiovascular risk factors underpredict major adverse cardiac events in kidney transplant recipients. Transplantation 2011, 92, 183–189. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, 708–714. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Dahl, J.N.; Jespersen, B.; Ivarsen, P.; Birn, H.; Winther, S. External Validation of Proposed American Heart Association Algorithm for Cardiovascular Screening Before Kidney Transplantation. J. Am. Heart Assoc. 2023, 12, e031150. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.B.; Webster, A.C.; Masson, P.; O’connell, P.J.; Craig, J.C. Antihypertensives for kidney transplant recipients: Systematic review and meta-analysis of randomized controlled trials. Transplantation 2009, 88, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Kohnle, M.; Pietruck, F.; Kribben, A.; Philipp, T.; Heemann, U.; Witzke, O. Ezetimibe for the Treatment of Uncontrolled Hypercholesterolemia in Patients with High-Dose Statin Therapy After Renal Transplantation. Am. J. Transplant. 2006, 6, 205–208. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Lemahieu, W.P.D.; Hermann, M.; Asberg, A.; Verbeke, K.; Holdaas, H.; Vanrenterghem, Y.; Maes, B.D. Combined therapy with atorvastatin and calcineurin inhibitors: No interactions with tacrolimus. Am. J. Transplant. 2005, 5, 2236–2243. [Google Scholar] [CrossRef]
- Migliozzi, D.R.; Asal, N.J. Clinical Controversy in Transplantation: Tacrolimus Versus Cyclosporine in Statin Drug Interactions. Ann. Pharmacother. 2020, 54, 171–177. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B.; On behalf of the American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; et al. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Hecking, M.; Haidinger, M.; Döller, D.; Werzowa, J.; Tura, A.; Zhang, J.; Tekoglu, H.; Pleiner, J.; Wrba, T.; Rasoul-Rockenschaub, S.; et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J. Am. Soc. Nephrol. 2012, 23, 739–749. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-Y.; Chang, L.-Y.; Chen, J.-Y.; Pan, H.-C.; Tseng, C.-S.; Chueh, J.S.; Wu, V.-C. The outcomes of SGLT-2 inhibitor utilization in diabetic kidney transplant recipients. Nat. Commun. 2024, 15, 10043. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kwon, S.; Jeon, Y.; Kim, Y.H.; Kwon, H.; Kim, Y.S.; Lee, H.; Kim, Y.-L.; Kim, C.-D.; Park, S.-H.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, e404. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Krisanapan, P.; Suppadungsuk, S.; Sanpawithayakul, K.; Thongprayoon, C.; Pattharanitima, P.; Tangpanithandee, S.; Mao, M.A.; Miao, J.; Cheungpasitporn, W. Safety and efficacy of glucagon-like peptide-1 receptor agonists among kidney transplant recipients: A systematic review and meta-analysis. Clin. Kidney J. 2024, 17, sfae018. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. S3), S1–S155. [CrossRef]
- Pirsch, J.D.; Henning, A.K.; First, M.R.; Fitzsimmons, W.; Gaber, A.O.; Reisfield, R.; Shihab, F.; Woodle, E.S. New-Onset Diabetes After Transplantation: Results From a Double-Blind Early Corticosteroid Withdrawal Trial. Am. J. Transplant. 2015, 15, 1982–1990. [Google Scholar] [CrossRef]
- Devresse, A.; Gohy, S.; Robert, A.; Kanaan, N. How to manage cigarette smoking in kidney transplant candidates and recipients? Clin. Kidney J. 2021, 14, 2295. [Google Scholar] [CrossRef]
- Rangaswami, J.; Mathew, R.O.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular disease in the kidney transplant recipient: Epidemiology, diagnosis and management strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef] [PubMed]
- el-Agroudy, A.E.; Wafa, E.W.; Gheith, O.E.; Shehab el-Dein, A.B.; Ghoneim, M.A. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation 2004, 77, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, B.; Bridson, J.M.; Sharma, A.; Halawa, A. From chronic kidney disease to kidney transplantation: The impact of obesity and its treatment modalities. Transplant. Rev. Orlando Fla 2016, 30, 203–211. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Opelz, G.; Döhler, B. Cardiovascular death in kidney recipients treated with renin-angiotensin system blockers. Transplantation 2014, 97, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Lubetzky, M.; Tantisattamo, E.; Molnar, M.Z.; Lentine, K.L.; Basu, A.; Parsons, R.F.; Woodside, K.J.; Pavlakis, M.; Blosser, C.D.; Singh, N.; et al. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am. J. Transplant. 2021, 21, 2937–2949. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Loupy, A.; Segev, D.L. Antibody-mediated rejection: New approaches in prevention and management. Am. J. Transplant. 2018, 18, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Okoli, G.N.; Rabbani, R.; Lam, O.L.T.; Reddy, V.K.; Askin, N.; Rampersad, C.; Trachtenberg, A.; Wiebe, C.; Nickerson, P.; et al. Effectiveness of T cell–mediated rejection therapy: A systematic review and meta-analysis. Am. J. Transplant. 2022, 22, 772–785. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, L.; Xia, B.; Zhang, X.; Liang, P.; Hu, X. Systematic review and meta-analysis of the efficacy of exercise intervention in kidney transplant recipients. World J. Urol. 2023, 41, 3449–3469. [Google Scholar] [CrossRef]
- Painter, P.L.; Hector, L.; Ray, K.; Lynes, L.; Paul, S.M.; Dodd, M.; Tomlanovich, S.L.; Ascher, N.L. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am. J. Kidney Dis. 2003, 42, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef]
- Osté, M.C.J.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.B.; de Borst, M.H.; van den Berg, E.; Soedamah-Muthu, S.S.; Kromhout, D.; Navis, G.J.; Bakker, S.J.L. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am. J. Transplant. 2018, 18, 2523–2533. [Google Scholar] [CrossRef]
- Palmer, S.C.; Maggo, J.K.; Campbell, K.L.; Craig, J.C.; Johnson, D.W.; Sutanto, B.; Ruospo, M.; Tong, A.; Strippoli, G.F. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2017, 4, CD011998. [Google Scholar] [CrossRef] [PubMed]
- Pedrollo, E.F.; Corrêa, C.; Nicoletto, B.B.; Corrêa Souza, G.; Leitão, C.B. What is Known About Dietary Interventions and Body Weight Management After Kidney Transplantation? A Scoping Review. J. Ren. Nutr. 2023, 33, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Midtvedt, K.; Hartmann, A.; Foss, A.; Fauchald, P.; Nordal, K.P.; Rootwelt, K.; Holdaas, H. Sustained improvement of renal graft function for two years in hypertensive renal transplant recipients treated with nifedipine as compared to lisinopril. Transplantation 2001, 72, 1787–1792. [Google Scholar] [CrossRef]
- Heinze, G.; Mitterbauer, C.; Regele, H.; Kramar, R.; Winkelmayer, W.C.; Curhan, G.C.; Oberbauer, R. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J. Am. Soc. Nephrol. 2006, 17, 889–899. [Google Scholar] [CrossRef]
- Opelz, G.; Zeier, M.; Laux, G.; Morath, C.; Döhler, B. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: A collaborative transplant study report. J. Am. Soc. Nephrol. 2006, 17, 3257–3262. [Google Scholar] [CrossRef]
- Knoll, G.A.; Fergusson, D.; Chassé, M.; Hebert, P.; Wells, G.; Tibbles, L.A.; Treleaven, D.; Holland, D.; White, C.; Muirhead, N.; et al. Ramipril versus placebo in kidney transplant patients with proteinuria: A multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 318–326. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sathick, I.J.J.; Erickson, S.B. The Effect of Renin-angiotensin System Inhibitors on Kidney Allograft Survival: A Systematic Review and Meta-analysis. N. Am. J. Med. Sci. 2016, 8, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, J.J.; Kaltenecker, C.C.; Kopecky, C.; Domenig, O.; Antlanger, M.; Werzowa, J.; Eskandary, F.; Kain, R.; Poglitsch, M.; Schmaldienst, S.; et al. Intrarenal Renin-Angiotensin-System Dysregulation after Kidney Transplantation. Sci. Rep. 2019, 9, 9762. [Google Scholar] [CrossRef]
- Ujjawal, A.; Schreiber, B.; Verma, A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: What is the evidence? Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221090001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-glucose cotransporter-2 inhibitor therapy in kidney transplant patients with type 2 or post-transplant diabetes: An observational multicentre study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef]
- Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists in kidney transplantation: Time to consider? Nephrol. Dial. Transplant. 2018, 33, 2080–2091. [Google Scholar] [CrossRef]
- Mahzari, M.M.; Alluhayyan, O.B.; Almutairi, M.H.; Bayounis, M.A.; Alrayani, Y.H.; Omair, A.A.; Alshahrani, A.S. Safety and efficacy of semaglutide in post kidney transplant patients with type 2 diabetes or Post-Transplant diabetes. J. Clin. Transl. Endocrinol. 2024, 36, 100343. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, E.; Bourdon, F.; Teta, D. SGLT2 inhibitors in diabetic and non-diabetic kidney transplant recipients: Current knowledge and expectations. Front. Nephrol. 2024, 4, 1332397. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Park, M. Post-Transplant Cardiovascular Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1878. [Google Scholar] [CrossRef]
- Schiffrin, E.L. T lymphocytes: A role in hypertension? Curr. Opin. Nephrol. Hypertens. 2010, 19, 181–186. [Google Scholar] [CrossRef]
- Carney, E.F. Acute kidney injury: Targeting Treg cells to protect the kidney. Nat. Rev. Nephrol. 2017, 13, 444. [Google Scholar] [CrossRef]
- Lichtenstein, K.A.; Armon, C.; Buchacz, K.; Chmiel, J.S.; Buckner, K.; Tedaldi, E.M.; Wood, K.; Holmberg, S.D.; Brooks, J.T. HIV Outpatient Study (HOPS) Investigators Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin. Infect. Dis. 2010, 51, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, V.; Price, A.; Cheru, N.T.; Venkatadri, R.; Dogan, M.; Costlow, G.; Mohammad, S.; Sharma, R. ST2+ T-Regulatory Cells in Renal Inflammation and Fibrosis after Ischemic Kidney Injury. J. Am. Soc. Nephrol. 2024, 36, 73–86. [Google Scholar] [CrossRef]
- Feng, G.; Bajpai, G.; Ma, P.; Koenig, A.; Bredemeyer, A.; Lokshina, I.; Lai, L.; Förster, I.; Leuschner, F.; Kreisel, D.; et al. CCL17 Aggravates Myocardial Injury by Suppressing Recruitment of Regulatory T Cells. Circulation 2022, 145, 765–782. [Google Scholar] [CrossRef]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Crepin, T.; Chalopin, J.-M.; Tiberghien, P.; Saas, P. Polyclonal antithymocyte globulin and cardiovascular disease in kidney transplant recipients. J. Am. Soc. Nephrol. 2014, 25, 1349–1356. [Google Scholar] [CrossRef]
- Müller, T.F.; Grebe, S.O.; Neumann, M.C.; Heymanns, J.; Radsak, K.; Sprenger, H.; Lange, H. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation 1997, 64, 1432–1437. [Google Scholar] [CrossRef]
- Sandal, S.; Bae, S.; McAdams-DeMarco, M.; Massie, A.B.; Lentine, K.L.; Cantarovich, M.; Segev, D.L. Induction immunosuppression agents as risk factors for incident cardiovascular events and mortality after kidney transplantation. Am. J. Transplant. 2019, 19, 1150–1159. [Google Scholar] [CrossRef]
- Opałka, B.; Żołnierczuk, M.; Grabowska, M. Immunosuppressive Agents-Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. J. Clin. Med. 2023, 12, 6935. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Parodi-Rullán, R.; Khuchua, Z.; Kuznetsov, A.V. Mitochondrial permeability transition in cardiac ischemia-reperfusion: Whether cyclophilin D is a viable target for cardioprotection? Cell. Mol. Life Sci. 2017, 74, 2795–2813. [Google Scholar] [CrossRef]
- Bedo, D.; Beaudrey, T.; Florens, N. Unraveling Chronic Cardiovascular and Kidney Disorder through the Butterfly Effect. Diagnostics 2024, 14, 463. [Google Scholar] [CrossRef]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torquetenovirus viremia for early prediction of graft rejection after kidney transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef]
- Hirt, S.W.; Bara, C.; Barten, M.J.; Deuse, T.; Doesch, A.O.; Kaczmarek, I.; Schulz, U.; Stypmann, J.; Haneya, A.; Lehmkuhl, H.B. Everolimus in heart transplantation: An update. J. Transplant. 2013, 2013, 683964. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.-C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343. [Google Scholar] [CrossRef]
- Ekperikpe, U.S.; Mandal, S.; Bhopatkar, A.A.; Shields, C.A.; Coley, C.A.; Chambers, C.L.; Johnson, T.D.; Cornelius, D.C.; Williams, J.M. Abatacept Decreases Renal T-cell Infiltration and Renal Inflammation and Ameliorates Progressive Renal Injury in Obese Dahl Salt-sensitive Rats Before Puberty. J. Cardiovasc. Pharmacol. 2024, 83, 635–645. [Google Scholar] [CrossRef]
- Kallikourdis, M.; Martini, E.; Carullo, P.; Sardi, C.; Roselli, G.; Greco, C.M.; Vignali, D.; Riva, F.; Ormbostad Berre, A.M.; Stølen, T.O.; et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat. Commun. 2017, 8, 14680. [Google Scholar] [CrossRef]
- Ewing, M.M.; Karper, J.C.; Abdul, S.; de Jong, R.C.M.; Peters, H.A.B.; de Vries, M.R.; Redeker, A.; Kuiper, J.; Toes, R.E.M.; Arens, R.; et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol. 2013, 168, 1965–1974. [Google Scholar] [CrossRef]
- Vincenti, F.; Blancho, G.; Durrbach, A.; Friend, P.; Grinyo, J.; Halloran, P.F.; Klempnauer, J.; Lang, P.; Larsen, C.P.; Mühlbacher, F.; et al. Five-year safety and efficacy of belatacept in renal transplantation. J. Am. Soc. Nephrol. 2010, 21, 1587–1596. [Google Scholar] [CrossRef]
- Durrbach, A.; Pestana, J.M.; Florman, S.; Del Carmen Rial, M.; Rostaing, L.; Kuypers, D.; Matas, A.; Wekerle, T.; Polinsky, M.; Meier-Kriesche, H.U.; et al. Long-Term Outcomes in Belatacept—Versus Cyclosporine-Treated Recipients of Extended Criteria Donor Kidneys: Final Results From BENEFIT-EXT, a Phase III Randomized Study. Am. J. Transplant. 2016, 16, 3192–3201. [Google Scholar] [CrossRef]
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21, 21–137. [Google Scholar] [CrossRef]
- Woodle, E.S.; First, M.R.; Pirsch, J.; Shihab, F.; Gaber, A.O.; Van Veldhuisen, P. Astellas Corticosteroid Withdrawal Study Group A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann. Surg. 2008, 248, 564–577. [Google Scholar] [CrossRef]
- Serrano, O.K.; Kandaswamy, R.; Gillingham, K.; Chinnakotla, S.; Dunn, T.B.; Finger, E.; Payne, W.; Ibrahim, H.; Kukla, A.; Spong, R.; et al. Rapid Discontinuation of Prednisone in Kidney Transplant Recipients: 15-Year Outcomes From the University of Minnesota. Transplantation 2017, 101, 2590–2598. [Google Scholar] [CrossRef]
- Bae, S.; Garonzik Wang, J.M.; Massie, A.B.; Jackson, K.R.; McAdams-DeMarco, M.A.; Brennan, D.C.; Lentine, K.L.; Coresh, J.; Segev, D.L. Early Steroid Withdrawal in Deceased-Donor Kidney Transplant Recipients with Delayed Graft Function. J. Am. Soc. Nephrol. 2020, 31, 175–185. [Google Scholar] [CrossRef]
- Bae, S.; Chen, Y.; Sandal, S.; Lentine, K.L.; Schnitzler, M.; Segev, D.L.; McAdams DeMarco, M.A. Association of early steroid withdrawal with kidney transplant outcomes in first-transplant and retransplant recipients. Nephrol. Dial. Transplant. 2024, gfae218. [Google Scholar] [CrossRef]
- Johnson, J.C.; Malik, M.; Engebretsen, T.L.; Mujtaba, M.; Lea, A.S.; Stevenson, H.L.; Kueht, M.L. Assessing Long-Term Adverse Outcomes in Older Kidney Transplant Recipients: A Propensity Score-Matched Comparison of Early Steroid Withdrawal Versus Continuous Steroid Immunosuppression Using a Large Real-World Database. Drugs Aging 2024, 41, 915–927. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef]
- Robinson, B.M.; Bieber, B.; Pisoni, R.L.; Port, F.K. Dialysis Outcomes and Practice Patterns Study (DOPPS): Its strengths, limitations, and role in informing practices and policies. Clin. J. Am. Soc. Nephrol. 2012, 7, 1897–1905. [Google Scholar] [CrossRef]
- Stoumpos, S.; Rankin, A.; Hall Barrientos, P.; Mangion, K.; McGregor, E.; Thomson, P.C.; Stevenson, K.; Welsh, P.; Kasthuri, R.; Kingsmore, D.B.; et al. Interrogating the haemodynamic effects of haemodialysis arteriovenous fistula on cardiac structure and function. Sci. Rep. 2021, 11, 18102. [Google Scholar] [CrossRef]
- Liu, Z.; Hilbelink, D.R.; Crockett, W.B.; Gerdes, A.M. Regional changes in hemodynamics and cardiac myocyte size in rats with aortocaval fistulas. 1. Developing and established hypertrophy. Circ. Res. 1991, 69, 52–58. [Google Scholar] [CrossRef]
- Aala, A.; Sharif, S.; Parikh, L.; Gordon, P.C.; Hu, S.L. High-Output Cardiac Failure and Coronary Steal With an Arteriovenous Fistula. Am. J. Kidney Dis. 2018, 71, 896–903. [Google Scholar] [CrossRef]
- Maresca, B.; Filice, F.B.; Orlando, S.; Ciavarella, G.M.; Scrivano, J.; Volpe, M.; Pirozzi, N. Early echocardiographic modifications after flow reduction by proximal radial artery ligation in patients with high-output heart failure due to high-flow forearm arteriovenous fistula. J. Vasc. Access 2020, 21, 753–759. [Google Scholar] [CrossRef]
- Wilmink, T.; Hollingworth, L.; Dasgupta, I. Access ligation in transplant patients. J. Vasc. Access 2016, 17 (Suppl. S1), S64–S68. [Google Scholar] [CrossRef]
- Vajdič Trampuž, B.; Arnol, M.; Gubenšek, J.; Ponikvar, R.; Buturović Ponikvar, J. A national cohort study on hemodialysis arteriovenous fistulas after kidney transplantation—Long-term patency, use and complications. BMC Nephrol. 2021, 22, 344. [Google Scholar] [CrossRef]
- Manca, O.; Pisano, G.L.; Carta, P.; Manca, E.M.; Piredda, G.B.; Pili, G.; Logias, F.; Lo Jacono, F.; Barracca, A. The management of hemodialysis arteriovenous fistulas in well functioning renal transplanted patients: Many doubts, few certainties. J. Vasc. Access 2005, 6, 182–186. [Google Scholar] [CrossRef]
- Rao, N.N.; Stokes, M.B.; Rajwani, A.; Ullah, S.; Williams, K.; King, D.; Macaulay, E.; Russell, C.H.; Olakkengil, S.; Carroll, R.P.; et al. Effects of Arteriovenous Fistula Ligation on Cardiac Structure and Function in Kidney Transplant Recipients. Circulation 2019, 139, 2809–2818. [Google Scholar] [CrossRef]
- Hetz, P.; Pirklbauer, M.; Müller, S.; Posch, L.; Gummerer, M.; Tiefenthaler, M. Prophylactic Ligature of AV Fistula Prevents High Output Heart Failure after Kidney Transplantation. Am. J. Nephrol. 2020, 51, 511–519. [Google Scholar] [CrossRef]
- Janeckova, J.; Bachleda, P.; Utikal, P.; Orsag, J. Management of Arteriovenous Fistula After Successful Kidney Transplantation in Long-Term Follow-Up. Transpl. Int. 2024, 37, 12841. [Google Scholar] [CrossRef]
- Yasir, M.B.; Man, R.K.; Gogikar, A.; Nanda, A.; Niharika Janga, L.S.; Sambe, H.G.; Mohamed, L. A Systematic Review Exploring the Impact of Arteriovenous Fistula Ligature on High-Output Heart Failure in Renal Transplant Recipients. Ann. Vasc. Surg. 2024, 100, 67–80. [Google Scholar] [CrossRef]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2015, 66, 1209–1220. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron, D.J.; O’Brien, S.M.; Fleg, J.L.; Kretov, E.I.; Briguori, C.; Kaul, U.; Reynolds, H.R.; Mazurek, T.; Sidhu, M.S.; et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N. Engl. J. Med. 2020, 382, 1608–1618. [Google Scholar] [CrossRef]
- Herzog, C.A.; Simegn, M.A.; Xu, Y.; Costa, S.P.; Mathew, R.O.; El-Hajjar, M.C.; Gulati, S.; Maldonado, R.A.; Daugas, E.; Madero, M.; et al. Kidney Transplant List Status and Outcomes in the ISCHEMIA-CKD Trial. J. Am. Coll. Cardiol. 2021, 78, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Ivanov, J.; Ko, D.; Fremes, S.; Rao, V.; Jolly, S.; Cantor, W.J.; Lavi, S.; Overgaard, C.B.; Ruel, M.; et al. Clinical outcomes of treatment by percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with chronic kidney disease undergoing index revascularization in Ontario. Circ. Cardiovasc. Interv. 2015, 8, e001973. [Google Scholar] [CrossRef]
- Li, X.; Xiao, F.; Zhang, S. Coronary revascularisation in patients with chronic kidney disease and end-stage renal disease: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e14506. [Google Scholar] [CrossRef] [PubMed]
- SHARP Collaborative Group Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am. Heart J. 2010, 160, 785–794.e10. [CrossRef]
- Lee, M.-S.; Batiste, C.; Onwuzurike, J.; Elkoustaf, R.; Wu, Y.-L.; Chen, W.; Kahwaji, J.; Sahota, A.; Lee, R.L. Pretransplant cardiac stress testing and transplant wait time in kidney transplantation candidates. Open Heart 2024, 11, e002738. [Google Scholar] [CrossRef] [PubMed]
- Pullen, L.C. Rethinking coronary heart disease tests in pretransplant evaluation: Cardiologists no longer screen asymptomatic patients for coronary artery disease-so why are transplant centers still doing it? Am. J. Transplant. 2023, 23, 1087–1089. [Google Scholar] [CrossRef]
- Ying, T.; Gill, J.; Webster, A.; Kim, S.J.; Morton, R.; Klarenbach, S.W.; Kelly, P.; Ramsay, T.; Knoll, G.A.; Pilmore, H.; et al. Canadian-Australasian Randomised trial of screening kidney transplant candidates for coronary artery disease-A trial protocol for the CARSK study. Am. Heart J. 2019, 214, 175–183. [Google Scholar] [CrossRef]
- Kopparam, R.V.; Grady, D.; Redberg, R.F. Coronary Heart Disease Testing Before Kidney Transplant-A Call for Revised Guidance. JAMA Intern. Med. 2023, 183, 287–288. [Google Scholar] [CrossRef]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.-M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction Through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef]
- Florens, N.; Kasam, R.K.; Rudman-Melnick, V.; Lin, S.-C.; Prasad, V.; Molkentin, J.D. Interleukin-33 Mediates Cardiomyopathy After Acute Kidney Injury by Signaling to Cardiomyocytes. Circulation 2023, 147, 746–758. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hsu, C.-Y.; Yang, J.; Tan, T.C.; Zheng, S.; Ordonez, J.D.; Liu, K.D. Acute Kidney Injury and Risk of Heart Failure and Atherosclerotic Events. Clin. J. Am. Soc. Nephrol. 2018, 13, 833–841. [Google Scholar] [CrossRef]
- Florens, N.; Aymes, E.; Gauthier, V.; Frimat, L.; Laville, M.; Bedo, D.; Beaudrey, T.; Amouyel, P.; Mansencal, N.; Lange, C.; et al. Acute kidney injury as a key predictor of cardiovascular events in chronic kidney disease patients: The CKD-REIN study. Clin. Kidney J. 2024, 17, sfae337. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Webster, A.C.; Hong, M.; Turner, R.; Lindley, R.I.; Craig, J.C. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2015, 30, 1162–1169. [Google Scholar] [CrossRef]
- Kelly, D.M.; Ademi, Z.; Doehner, W.; Lip, G.Y.H.; Mark, P.; Toyoda, K.; Wong, C.X.; Sarnak, M.; Cheung, M.; Herzog, C.A.; et al. Chronic Kidney Disease and Cerebrovascular Disease: Consensus and Guidance From a KDIGO Controversies Conference. Stroke 2021, 52, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Suissa, L.; Hak, J.-F.; Burtey, S.; Guillet, B.; Hache, G. Kidney disease and stroke: Epidemiology and potential mechanisms of susceptibility. Nephrol. Dial. Transplant. 2023, 38, 1940–1951. [Google Scholar] [CrossRef]
- Bobot, M.; Guedj, E.; Resseguier, N.; Faraut, J.; Garrigue, P.; Nail, V.; Hache, G.; Gonzalez, S.; McKay, N.; Vial, R.; et al. Increased Blood-Brain Barrier Permeability and Cognitive Impairment in Patients With ESKD. Kidney Int. Rep. 2024, 9, 2988–2995. [Google Scholar] [CrossRef]
- Aull-Watschinger, S.; Konstantin, H.; Demetriou, D.; Schillinger, M.; Habicht, A.; Hörl, W.H.; Watschinger, B. Pre-transplant predictors of cerebrovascular events after kidney transplantation. Nephrol. Dial. Transplant. 2008, 23, 1429–1435. [Google Scholar] [CrossRef]
- Huang, S.-T.; Yu, T.-M.; Chuang, Y.-W.; Chung, M.-C.; Wang, C.-Y.; Fu, P.-K.; Ke, T.-Y.; Li, C.-Y.; Lin, C.-L.; Wu, M.-J.; et al. The Risk of Stroke in Kidney Transplant Recipients with End-Stage Kidney Disease. Int. J. Environ. Res. Public. Health 2019, 16, 326. [Google Scholar] [CrossRef]
- Lentine, K.L.; Rocca Rey, L.A.; Kolli, S.; Bacchi, G.; Schnitzler, M.A.; Abbott, K.C.; Xiao, H.; Brennan, D.C. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin. J. Am. Soc. Nephrol. 2008, 3, 1090–1101. [Google Scholar] [CrossRef]
- Oliveras, A.; Roquer, J.; Puig, J.M.; Rodríguez, A.; Mir, M.; Orfila, M.A.; Masramon, J.; Lloveras, J. Stroke in renal transplant recipients: Epidemiology, predictive risk factors and outcome. Clin. Transplant. 2003, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, A.M.; Prather, J.; Olyaei, A.J.; Shibagaki, Y.; Keith, D.S.; Mori, M.; Norman, D.J.; Becker, T. Cardiovascular events following renal transplantation: Role of traditional and transplant-specific risk factors. Kidney Int. 2006, 70, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Guijarro, C.; Massy, Z.A.; Wiederkehr, M.R.; Ma, J.Z. Cardiovascular disease after renal transplantation. J. Am. Soc. Nephrol. 1996, 7, 158–165. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Lv, J.; Zheng, M.; Zhu, Y. Outcomes of acute ischemic stroke in kidney transplant recipients: An analysis of US Nationwide inpatient sample. Transl. Neurosci. 2022, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Shin, S.; Baek, C.H.; Chang, J.Y.; Kang, D.-W.; Kwon, S.U.; Kim, J.S.; Kim, B.J. Characteristics of stroke after liver and kidney transplantation. Front. Neurol. 2023, 14, 1123518. [Google Scholar] [CrossRef]
- Weng, S.-F.; Shen, Y.-C.; Wang, J.-J.; Tien, K.-J. Reduced risk of new onset stroke after kidney transplantation in Asian dialysis patients: A propensity score-matched, competing risk study in Taiwan. QJM Mon. J. Assoc. Physicians 2019, 112, 489–495. [Google Scholar] [CrossRef]
- Mathew, A.; Eliasziw, M.; Devereaux, P.J.; Merino, J.G.; Barnett, H.J.M.; Garg, A.X. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators Carotid endarterectomy benefits patients with CKD and symptomatic high-grade stenosis. J. Am. Soc. Nephrol. 2010, 21, 145–152. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; AbuRahma, A.F. A comparison of the 2022 Society for Vascular Surgery and the 2023 European Society for Vascular Surgery guidelines for the management of patients with asymptomatic and symptomatic carotid stenosis. J. Vasc. Surg. 2024, 79, 1272–1275. [Google Scholar] [CrossRef]
- Reiff, T.; Eckstein, H.-H.; Mansmann, U.; Jansen, O.; Fraedrich, G.; Mudra, H.; Böckler, D.; Böhm, M.; Debus, E.S.; Fiehler, J.; et al. Carotid endarterectomy or stenting or best medical treatment alone for moderate-to-severe asymptomatic carotid artery stenosis: 5-year results of a multicentre, randomised controlled trial. Lancet Neurol. 2022, 21, 877–888. [Google Scholar] [CrossRef]
- Adil, M.M.; Saeed, F.; Chaudhary, S.A.; Malik, A.; Qureshi, A.I. Comparative Outcomes of Carotid Artery Stent Placement and Carotid Endarterectomy in Patients with Chronic Kidney Disease and End-Stage Renal Disease. J. Stroke Cerebrovasc. Dis. 2016, 25, 1721–1727. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, J.B.; Mahnken, J.D.; Rigler, S.K.; Ellerbeck, E.F.; Mukhopadhyay, P.; Spertus, J.A.; Hou, Q.; Shireman, T.I. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int. 2012, 81, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, J.; Ye, P.; Xing, A.; Wu, Y.; Wu, S.; Luo, Y. Comparison of atrial fibrillation in CKD and non-CKD populations: A cross-sectional analysis from the Kailuan study. Int. J. Cardiol. 2019, 277, 125–129. [Google Scholar] [CrossRef]
- Song, J.; Navarro-Garcia, J.A.; Wu, J.; Saljic, A.; Abu-Taha, I.; Li, L.; Lahiri, S.K.; Keefe, J.A.; Aguilar-Sanchez, Y.; Moore, O.M.; et al. Chronic kidney disease promotes atrial fibrillation via inflammasome pathway activation. J. Clin. Investig. 2023, 133, e167517. [Google Scholar] [CrossRef]
- Pokorney, S.D.; Chertow, G.M.; Al-Khalidi, H.R.; Gallup, D.; Dignacco, P.; Mussina, K.; Bansal, N.; Gadegbeku, C.A.; Garcia, D.A.; Garonzik, S.; et al. Apixaban for Patients With Atrial Fibrillation on Hemodialysis: A Multicenter Randomized Controlled Trial. Circulation 2022, 146, 1735–1745. [Google Scholar] [CrossRef]
- Fu, E.L.; Desai, R.J.; Paik, J.M.; Kim, D.H.; Zhang, Y.; Mastrorilli, J.M.; Cervone, A.; Lin, K.J. Comparative Safety and Effectiveness of Warfarin or Rivaroxaban Versus Apixaban in Patients With Advanced CKD and Atrial Fibrillation: Nationwide US Cohort Study. Am. J. Kidney Dis. 2024, 83, 293–305.e1. [Google Scholar] [CrossRef]
- Elenjickal, E.J.; Travlos, C.K.; Marques, P.; Mavrakanas, T.A. Anticoagulation in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2024, 55, 146–164. [Google Scholar] [CrossRef]
- Albaladejo, P.; Bonhomme, F.; Blais, N.; Collet, J.-P.; Faraoni, D.; Fontana, P.; Godier, A.; Llau, J.; Longrois, D.; Marret, E.; et al. Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: Updated guidelines from the French Working Group on Perioperative Hemostasis (GIHP)—September 2015. Anaesth. Crit. Care Pain Med. 2017, 36, 73–76. [Google Scholar] [CrossRef]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.M.R.; Reis, A.M.; Tavares, F.P.; Oliveira, L.S.; Godoi, A.; Viana, P.; Riella, J. Safety and efficacy of direct oral anticoagulants in kidney transplant recipients: A systematic review and meta-analysis. Transplant. Rev. 2024, 39, 100899. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Chokesuwattanaskul, R.; Bathini, T.; Khoury, N.J.; Sharma, K.; Ungprasert, P.; Prasitlumkum, N.; Aeddula, N.R.; Watthanasuntorn, K.; Salim, S.A.; et al. Epidemiology and Prognostic Importance of Atrial Fibrillation in Kidney Transplant Recipients: A Meta-Analysis. J. Clin. Med. 2018, 7, 370. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef]
- Abu Dabrh, A.M.; Steffen, M.W.; Undavalli, C.; Asi, N.; Wang, Z.; Elamin, M.B.; Conte, M.S.; Murad, M.H. The natural history of untreated severe or critical limb ischemia. J. Vasc. Surg. 2015, 62, 1642–1651.e3. [Google Scholar] [CrossRef]
- Johansen, K.L.; Garimella, P.S.; Hicks, C.W.; Kalra, P.A.; Kelly, D.M.; Martens, S.; Matsushita, K.; Sarafidis, P.; Sood, M.M.; Herzog, C.A.; et al. Central and peripheral arterial diseases in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021, 100, 35–48. [Google Scholar] [CrossRef]
- Bourrier, M.; Ferguson, T.W.; Embil, J.M.; Rigatto, C.; Komenda, P.; Tangri, N. Peripheral Artery Disease: Its Adverse Consequences With and Without CKD. Am. J. Kidney Dis. 2020, 75, 705–712. [Google Scholar] [CrossRef]
- Tsuyuki, K.; Kohno, K.; Ebine, K.; Obara, T.; Aoki, T.; Muto, A.; Ninomiya, K.; Kumagai, K.; Yokouchi, I.; Yazaki, Y.; et al. Exercise-ankle brachial pressure index with one-minute treadmill walking in patients on maintenance hemodialysis. Ann. Vasc. Dis. 2013, 6, 52–56. [Google Scholar] [CrossRef]
- Arinze, N.V.; Gregory, A.; Francis, J.M.; Farber, A.; Chitalia, V.C. Unique aspects of peripheral artery disease in patients with chronic kidney disease. Vasc. Med. Lond. Engl. 2019, 24, 251–260. [Google Scholar] [CrossRef]
- Hernández, D.; Vázquez, T.; Armas-Padrón, A.M.; Alonso-Titos, J.; Casas, C.; Gutiérrez, E.; Jironda, C.; Cabello, M.; López, V. Peripheral Vascular Disease and Kidney Transplant Outcomes: Rethinking an Important Ongoing Complication. Transplantation 2021, 105, 1188–1202. [Google Scholar] [CrossRef]
- Snyder, J.J.; Kasiske, B.L.; Maclean, R. Peripheral arterial disease and renal transplantation. J. Am. Soc. Nephrol. 2006, 17, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Jindal, R.M.; Sumrani, N.; John, D.; Mondal, Z.; Tedla, F.; Salifu, M.O. Impact of renal posttransplantation amputation on allograft outcomes: A study of United States renal data system. Transplantation 2013, 95, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Jindal, R.M.; Elster, E.A.; Tedla, F.; John, D.; Sumrani, N.; Salifu, M.O. Effect of peripheral vascular disease on kidney allograft outcomes: A study of U.S. Renal data system. Transplantation 2013, 95, 810–815. [Google Scholar] [CrossRef]
- Patel, S.I.; Chakkera, H.A.; Wennberg, P.W.; Liedl, D.A.; Alrabadi, F.; Cha, S.S.; Hooley, D.D.; Amer, H.; Wadei, H.M.; Shamoun, F.E. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc. Med. Lond. Engl. 2017, 22, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Franquet, Q.; Terrier, N.; Pirvu, A.; Rambeaud, J.-J.; Long, J.-A.; Janbon, B.; Tetaz, R.; Malvezzi, P.; Jouve, T.; Descotes, J.-L.; et al. Aortic bypass surgery for asymptomatic patients awaiting a kidney transplant: A word of caution. Clin. Transplant. 2018, 32, e13218. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Cangro, C.B.; Hariharan, S.; Hricik, D.E.; Kerman, R.H.; Roth, D.; Rush, D.N.; Vazquez, M.A.; Weir, M.R. American Society of Transplantation The evaluation of renal transplantation candidates: Clinical practice guidelines. Am. J. Transplant. 2001, 1 (Suppl. S2), 3–95. [Google Scholar] [PubMed]
- Northcutt, A.; Zibari, G.; Tan, T.-W.; Coulter, A.H.; Zhang, W.W. Does kidney transplantation to iliac artery deteriorate ischemia in the ipsilateral lower extremity with peripheral arterial disease? Vascular 2015, 23, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.S.; Santos, T.S.; Pereira, C.A.; Duarte, D.B.; Neto, H.; Gomes, A.; Loureiro, L.; Martins, J.; Silva, F.; Martins, L.S.; et al. The influence of simultaneous pancreas-kidney transplantation on the evolution of diabetic foot lesions and peripheral arterial disease. J. Endocrinol. Investig. 2023, 46, 1459–1464. [Google Scholar] [CrossRef]
- Sucher, R.; Rademacher, S.; Jahn, N.; Brunotte, M.; Wagner, T.; Alvanos, A.; Sucher, E.; Seehofer, D.; Scheuermann, U.; Hau, H.-M. Effects of simultaneous pancreas-kidney transplantation and kidney transplantation alone on the outcome of peripheral vascular diseases. BMC Nephrol. 2019, 20, 453. [Google Scholar] [CrossRef]
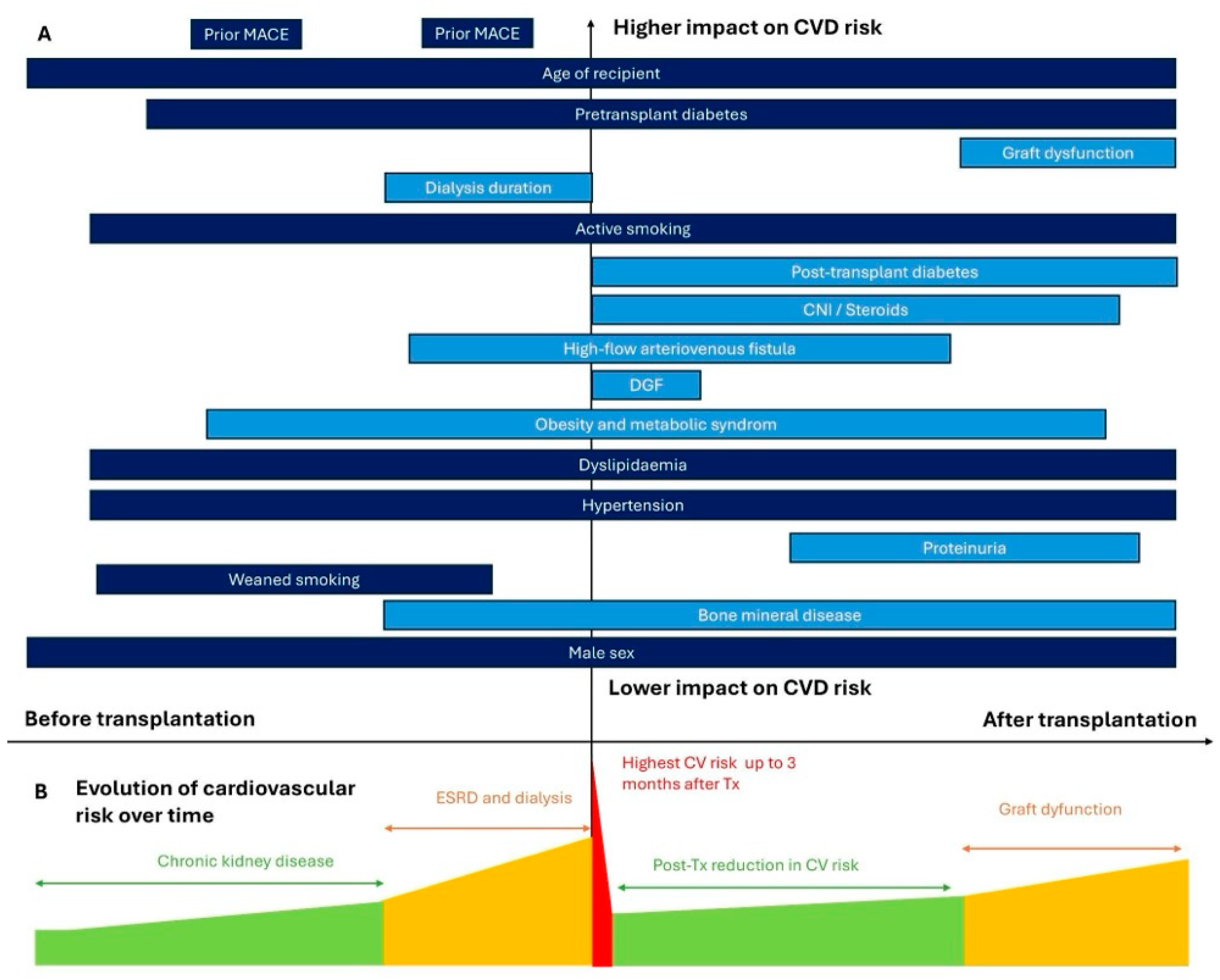
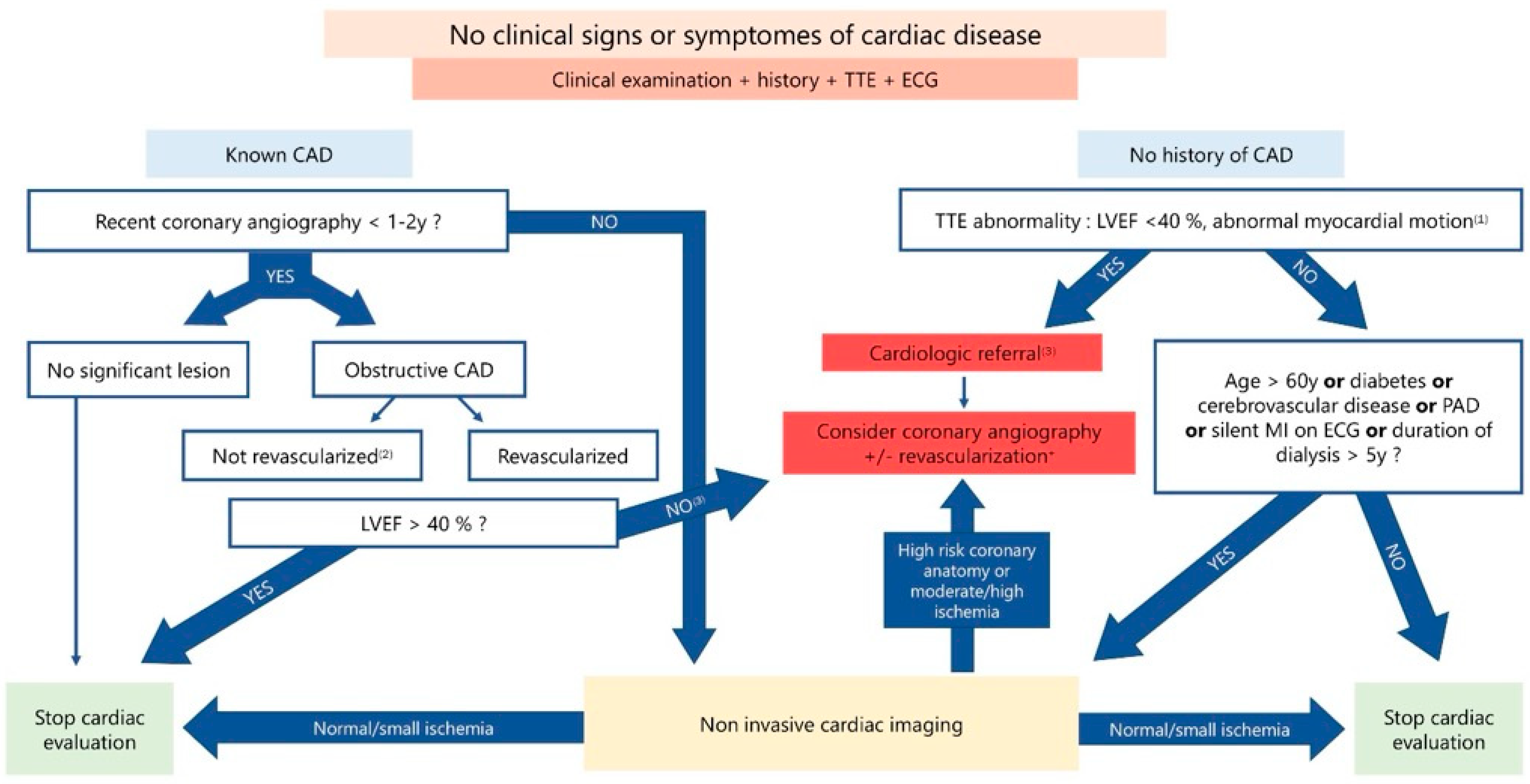
| Side Effects of Immunosuppressive Treatment | CTC | CsA | TAC | imTor | MMF | Belatacept |
|---|---|---|---|---|---|---|
| Post-transplantation diabetes melitus | ↑↑ | ↑ | ↑↑ | ↑ | ↓ | |
| Dyslipidemia | ↑ | ↑↑ | ↑ | ↑↑ | ↓ | ↓ |
| Hypertension (HTN) | ↑↑ | ↑↑ | ↑ | ↓ | ↓ | |
| Glomerular proteinuria | ↓ | ↓ | ↑↑ | |||
| Kidney fibrosis | ↑↑ | ↑↑ | ↑ | ↓ | ||
| Cardiac hypertrophy, fibrosis | ↑↑ | ↑↑ | ↑↑ | ↓ | ↓ | ↓ |
| Vascular remodeling | ↑ | ↑ | ↓ | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudrey, T.; Bedo, D.; Weschler, C.; Caillard, S.; Florens, N. From Risk Assessment to Management: Cardiovascular Complications in Pre- and Post-Kidney Transplant Recipients: A Narrative Review. Diagnostics 2025, 15, 802. https://doi.org/10.3390/diagnostics15070802
Beaudrey T, Bedo D, Weschler C, Caillard S, Florens N. From Risk Assessment to Management: Cardiovascular Complications in Pre- and Post-Kidney Transplant Recipients: A Narrative Review. Diagnostics. 2025; 15(7):802. https://doi.org/10.3390/diagnostics15070802
Chicago/Turabian StyleBeaudrey, Thomas, Dimitri Bedo, Célia Weschler, Sophie Caillard, and Nans Florens. 2025. "From Risk Assessment to Management: Cardiovascular Complications in Pre- and Post-Kidney Transplant Recipients: A Narrative Review" Diagnostics 15, no. 7: 802. https://doi.org/10.3390/diagnostics15070802
APA StyleBeaudrey, T., Bedo, D., Weschler, C., Caillard, S., & Florens, N. (2025). From Risk Assessment to Management: Cardiovascular Complications in Pre- and Post-Kidney Transplant Recipients: A Narrative Review. Diagnostics, 15(7), 802. https://doi.org/10.3390/diagnostics15070802






