Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions
Abstract
1. Introduction
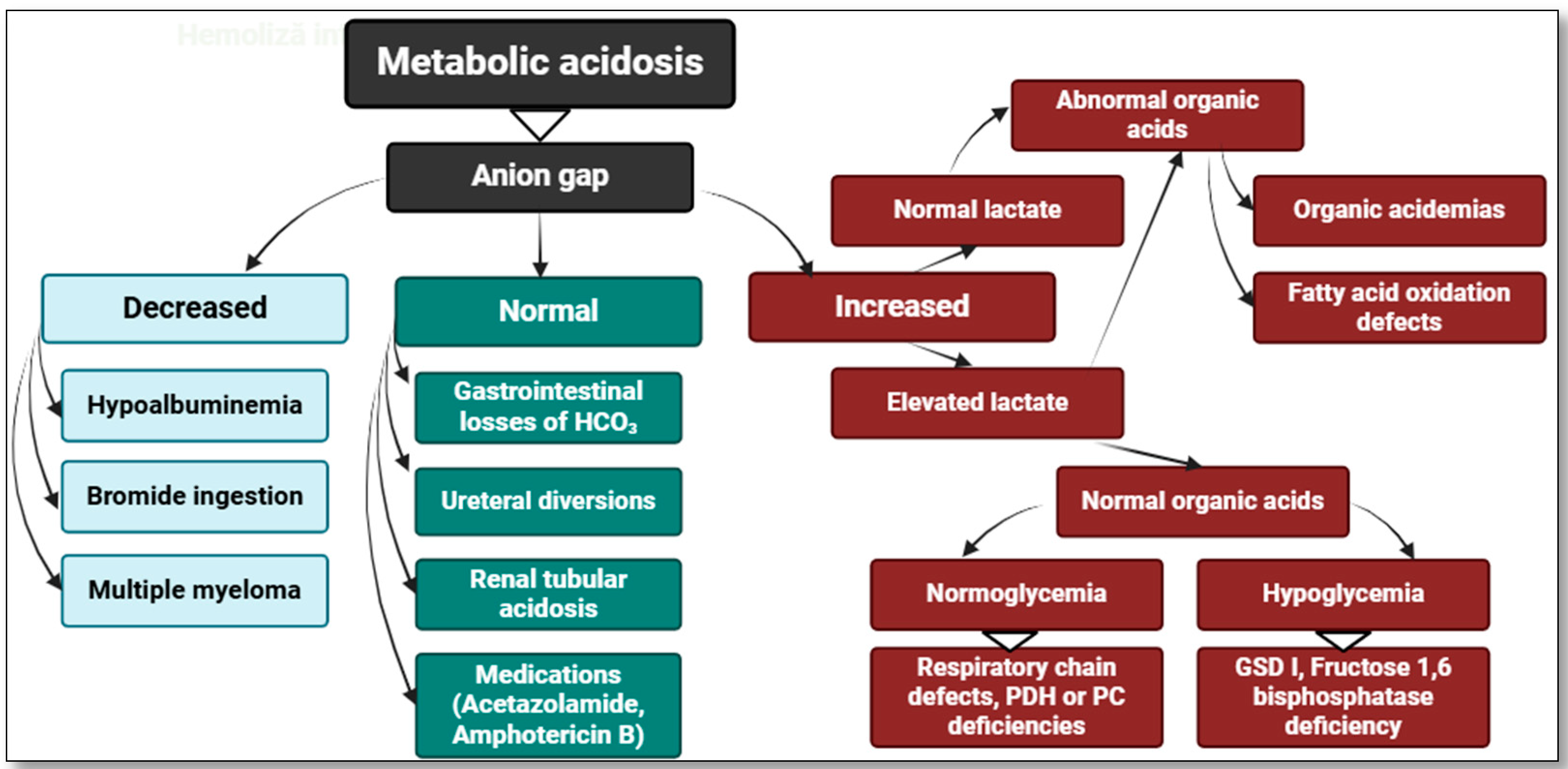
2. Hyperlactatemia—Definitions and Classifications
3. Lactate—Friend or Foe? Classic and Current Theories
4. The Role of Lactate in Pediatric Pathology
5. The Assessment of Lactate Levels—Particularities in Children
6. Future Directions of Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, K.P.; Kapetanstrataki, M.; Wilkins, B.; Slater, A.J.; Ward, V.; Parslow, R.C. Lactate, Base Excess, and the Pediatric Index of Mortality: Exploratory Study of an International, Multicenter Dataset. Pediatr. Crit. Care Med. 2022, 23, e268–e276. [Google Scholar] [CrossRef] [PubMed]
- Kompanje, E.J.; Jansen, T.C.; van der Hoven, B.; Bakker, J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med. 2007, 33, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Huckabee, W.E. Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients. Am. J. Med. 1961, 30, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 2023, 83, 3904–3920.e7. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 2022, 7, 305, Erratum in: Signal Transduct Target Ther. 2022, 7, 372. https://doi.org/10.1038/s41392-022-01206-5. [Google Scholar] [CrossRef]
- Lee, D.C.; Sohn, H.A.; Park, Z.Y.; Oh, S.; Kang, Y.K.; Lee, K.M.; Kang, M.; Jang, Y.J.; Yang, S.J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Q.; Zheng, J.; Yang, Y.; Zhang, X.; Ma, A.; Qin, Y.; Qin, Z.; Zheng, X. The function and mechanism of lactate and lactylation in tumor metabolism and microenvironment. Genes. Dis. 2022, 10, 2029–2037. [Google Scholar] [CrossRef]
- Matsushita, F.Y.; Krebs, V.L.J.; De Carvalho, W.B. Association between Serum Lactate and Morbidity and Mortality in Neonates: A Systematic Review and Meta-Analysis. Children 2023, 10, 1796. [Google Scholar] [CrossRef] [PubMed]
- Belu, A.; Trandafir, L.M.; Țarcă, E.; Cojocaru, E.; Frăsinariu, O.; Stârcea, M.; Moscalu, M.; Tiutiuca, R.C.; Luca, A.C.; Galaction, A. Variations in Biochemical Values under Stress in Children with SARS-CoV-2 Infection. Diagnostics 2022, 12, 1213. [Google Scholar] [CrossRef]
- Kushimoto, S.; Akaishi, S.; Sato, T.; Nomura, R.; Fujita, M.; Kudo, D.; Kawazoe, Y.; Yoshida, Y.; Miyagawa, N. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med. Surg. 2016, 3, 293–297. [Google Scholar] [CrossRef]
- Choudhary, R.; Sitaraman, S.; Choudhary, A. Lactate clearance as the predictor of outcome in pediatric septic shock. J. Emerg. Trauma Shock 2017, 10, 55–59. [Google Scholar] [CrossRef]
- Jat, K.R.; Jhamb, U.; Gupta, V.K. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J. Crit. Care Med. 2011, 15, 102–107. [Google Scholar] [CrossRef]
- Emhoff, C.W.; Messonnier, L.A. Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review. Nutrients 2023, 15, 3213. [Google Scholar] [CrossRef] [PubMed]
- Soghier, L.M.; Fratantoni, K.; Reyes, C. (Eds.) Reference Range Values for Pediatric Care, 2nd ed.; American Academy of Pediatrics: Washington, DC, USA, 2019. [Google Scholar]
- Kruse, O.; Grunnet, N.; Barfod, C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: A systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 74. [Google Scholar] [CrossRef]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Radej, J.; Horak, J.; Karvunidis, T.; Valesova, L.; Kriz, M.; Matejovic, M. Lactate: The Fallacy of Oversimplification. Biomedicines 2023, 11, 3192. [Google Scholar] [CrossRef]
- Lee, T.Y. Lactate: A multifunctional signaling molecule. Yeungnam Univ. J. Med. 2021, 38, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Ganesh, K.; Sharma, R.N.; Varghese, J.; Pillai, M.G. A profile of metabolic acidosis in patients with sepsis in an Intensive Care Unit setting. Int. J. Crit. Illn. Inj. Sci. 2016, 6, 178–181. [Google Scholar] [CrossRef]
- Cohen, R.D.; Woods, H.F. Lactic acidosis revisited. Diabetes 1983, 32, 181–191. [Google Scholar] [CrossRef]
- Woods, H.F.; Cohen, R.D. Clinical and Biochemical Aspects of Lactic Acidosis; Blackwell Scientific: Oxford, UK, 1976; pp. 1–20. [Google Scholar]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, Y.; Zhao, X.; Yu, J. From metabolic byproduct to immune modulator: The role of lactate in tumor immune escape. Front. Immunol. 2024, 15, 1492050. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Meyer, T.; Faude, O.; Scharhag, J.; Urhausen, A.; Kindermann, W. Is lactic acidosis a cause of exercise induced hyperventilation at the respiratory compensation point? Br. J. Sports Med. 2004, 38, 622–625. [Google Scholar] [CrossRef]
- Roy, R.M.; Allawzi, A.; Burns, N.; Sul, C.; Rubio, V.; Graham, J.; Stenmark, K.; Nozik, E.S.; Tuder, R.M.; Vohwinkel, C.U. Lactate produced by alveolar type II cells suppresses inflammatory alveolar macrophages in acute lung injury. FASEB J. 2023, 37, e23316. [Google Scholar] [CrossRef]
- Topjian, A.A.; Clark, A.E.; Casper, T.C.; Berger, J.T.; Schleien, C.L.; Dean, J.M.; Moler, F.W.; Pediatric Emergency Care Applied Research Network. Early lactate elevations following resuscitation from pediatric cardiac arrest are associated with increased mortality. Pediatr. Crit. Care Med. 2013, 14, e380–e387. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Gopalasingam, N.; Pedersen, M.G.B.; Nyvad, J.T.; Rittig, N.; Søndergaard, E.; Wiggers, H.; Møller, N.; Nielsen, R. Cardiovascular effects of lactate in healthy adults. Crit. Care 2025, 29, 30. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Vavřička, J.; Brož, P.; Follprecht, D.; Novák, J.; Kroužecký, A. Modern Perspective of Lactate Metabolism. Physiol. Res. 2024, 73, 499–514. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Lee, D.; Xiong, W.C. Lactate Metabolism, Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13398. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Liu, C.; Ehle, C.; Iyer-Bierhoff, A.; Liu, B.; Heinzel, T.; Xing, S. Histone Deacetylase Inhibitor (SAHA) Reduces Mortality in an Endotoxemia Mouse Model by Suppressing Glycolysis. Int. J. Mol. Sci. 2023, 24, 12448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, Y.; Zou, J.; Gao, S.; Wu, D.; Li, X.; Hu, N.; Zhao, J.; Huang, W.; Chen, H. Lactate Mediates the Bone Anabolic Effect of High-Intensity Interval Training by Inducing Osteoblast Differentiation. J. Bone Jt. Surg. Am. 2023, 105, 369–379. [Google Scholar] [CrossRef]
- Cosgriff, N.; Moore, E.E.; Sauaia, A.; Kenny-Moynihan, M.; Burch, J.M.; Galloway, B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: Hypothermia and acidoses revisited. J. Trauma 1997, 42, 857–861, discussion 861–862. [Google Scholar]
- Henry, S.; Scalea, T.M. Resuscitation in the new millennium. Surg. Clin. N. Am. 1999, 79, 1259–1267. [Google Scholar]
- Duceac, L.D.; Tarca, E.; Ciuhodaru, M.I.; Tantu, M.M.; Goroftei, R.E.B.; Banu, E.A.; Damir, D.; Glod, M.; Luca, A.C. Study on the Mechanism of Antibiotic Resistance. Rev. Chim. 2019, 70, 199–201. [Google Scholar]
- Martin, M.J.; FitzSullivan, E.; Salim, A.; Brown, C.V.; Demetriades, D.; Long, W. Discordance between lactate and base deficit in the surgical intensive care unit: Which one do you trust? Am. J. Surg. 2006, 191, 625–630. [Google Scholar]
- Husain, F.A.; Martin, M.J.; Mullenix, P.S.; Steele, S.R.; Elliott, D.C. Serum lactate and base deficit as predictors of mortality and morbidity. Am. J. Surg. 2003, 185, 485–491. [Google Scholar] [CrossRef]
- Ramanathan, R.; Parrish, D.W.; Hartwich, J.E.; Haynes, J.H. Utility of admission serum lactate in pediatric trauma. J. Pediatr. Surg. 2015, 50, 598–603. [Google Scholar] [CrossRef]
- Colak, M.; Arda Kilinc, M.; Güven, R.; Onur Kutlu, N. Procalcitonin and blood lactate level as predictive biomarkers in pediatric multiple trauma patients’ pediatric intensive care outcomes: A retrospective observational study. Medicine 2023, 102, e36289. [Google Scholar] [CrossRef] [PubMed]
- Englert, J.A.; Rogers, A.J. Metabolism, Metabolomics, and Nutritional Support of Patients with Sepsis. Clin. Chest Med. 2016, 37, 321–331. [Google Scholar] [CrossRef]
- Hatherill, M.; McIntyre, A.G.; Wattie, M.; Murdoch, I.A. Early hyperlactataemia in critically ill children. Intensive Care Med. 2000, 26, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Ha, E.J.; Jhang, W.K.; Park, S.J. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013, 39, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Gorgis, N.; Asselin, J.M.; Fontana, C.; Heidersbach, R.S.; Flori, H.R.; Ward, S.L. Evaluation of the Association of Early Elevated Lactate with Outcomes in Children with Severe Sepsis or Septic Shock. Pediatr. Emerg. Care 2019, 35, 661–665. [Google Scholar] [CrossRef]
- Abdelaziz, T.A.; Karam, N.A.; Ismail, W.I.; Askary, N.M.A.; Baz, E.G. Lactate dynamics in paediatric patients with severe sepsis: Insights from a prospective cohort study. BMC Pediatr. 2024, 24, 345. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Elhadidi, A.S.; El-Nagar, M.A.; Hassouna, H.M. Can Lactate Clearance Predict Mortality in Critically Ill Children? J. Pediatr. Intensive Care 2021, 12, 112–117. [Google Scholar] [CrossRef]
- Lee, J.Y.; Byun, Y.H.; Park, J.S.; Lee, J.S.; Ryu, J.M.; Choi, S.J. Lactic acid level as an outcome predictor in pediatric patients with intussusception in the emergency department. BMC Pediatr. 2020, 20, 184. [Google Scholar] [CrossRef]
- Duceac, L.D.; Marcu, C.; Ichim, D.L.; Ciomaga, I.M.; Tarca, E.; Iordache, A.C.; Ciuhodaru, M.I.; Florescu, L.; Tutunaru, D.; Luca, A.C.; et al. Antibiotic Molecules Involved in Increasing Microbial Resistance. Rev. Chim. 2019, 70, 2622–2626. [Google Scholar]
- Tamas, V.; Ishimine, P. Comparison of Lactic Acid Levels in Children with Suspected and Confirmed Intussusception. J. Emerg. Med. 2017, 53, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S.; Farias, J.S.; Villarreal, E.G.; Flores, S. Serum Lactate and Mortality during Pediatric Admissions: Is 2 Really the Magic Number? J. Pediatr. Intensive Care 2022, 11, 83–90. [Google Scholar] [CrossRef]
- Carragher, F.; Champion, M. Inherited metabolic disease. In Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A.P., Ayling, R.M., Eds.; Churchill Livingstone: London, UK, 2014; pp. 461–483. [Google Scholar] [CrossRef]
- Nasir, H.; Afzal, M.F.; Hamid, M.H.; Laeeq, A. Diagnostic accuracy of cerebrospinal fluid lactate in confirmed cases of acute bacterial meningitis in children. Pak. J. Med. Sci. 2020, 36, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Tuuli, M.G.; Stout, M.J.; Macones, G.A.; Cahill, A.G. Umbilical Cord Venous Lactate for Predicting Arterial Lactic Acidemia and Neonatal Morbidity at Term. Obstet. Gynecol. 2016, 127, 674–680. [Google Scholar] [CrossRef]
- Dabas, A.; Agrawal, S.; Tyagi, V.; Sharma, S.; Rastogi, V.; Jhamb, U.; Dabla, P.K. Point of Care Testing of Serum Electrolytes and Lactate in Sick Children. EJIFCC 2021, 32, 158–166. [Google Scholar]
- Scheer, B.; Perel, A.; Pfeiffer, U.J. Clinical review: Complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit. Care 2002, 6, 199–204. [Google Scholar] [CrossRef]
- Nygaard, U.; Dungu, K.H.S.; von Linstow, M.L.; Lundstrøm, K.; Zhang, H.; Vissing, N.H. Lactate as a Screening Tool for Critical Illness in a Pediatric Emergency Department. Pediatr. Emerg. Care 2023, 39, 735–738. [Google Scholar] [CrossRef]
- Samaraweera, S.A.; Gibbons, B.; Gour, A.; Sedgwick, P. Arterial versus venous lactate: A measure of sepsis in children. Eur. J. Pediatr. 2017, 176, 1055–1060. [Google Scholar] [CrossRef]
- Murdoch, I.A.; Turner, C.; Dalton, R.N. Arterial or mixed venous lactate measurement in critically ill children. Is there a difference? Acta Paediatr. (1992) 1994, 83, 412–413. [Google Scholar] [CrossRef]
- Fernández Sarmiento, J.; Araque, P.; Yepes, M.; Mulett, H.; Tovar, X.; Rodriguez, F. Correlation between Arterial Lactate and Central Venous Lactate in Children with Sepsis. Crit. Care Res. Pract. 2016, 2016, 7839739. [Google Scholar] [CrossRef] [PubMed]
- Phumeetham, S.; Kaowchaweerattanachart, N.; Law, S.; Chanthong, P.; Pratumvinit, B. Close correlation between arterial and central venous lactate concentrations of children in shock: A cross-sectional study. Clin. Chim. Acta 2017, 472, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, A.; Herghelegiu, C.G.; Voinea, S.; Dimitriu, M.C.T.; Ples, L.; Bohiltea, R.E.; Braila, A.D.; Nastase, L.; Bacalbasa, N.; Chivu, L.I.; et al. Umbilical cord lactate compared with pH as predictors of intrapartum asphyxia. Exp. Ther. Med. 2021, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef]
- Ogik, V.; Muyingo, M.; Musooko, M.; Nankunda, J. Umbilical artery lactate levels and associated maternal and newborn characteristics at Mulago National Referral Hospital: A cross-sectional observational study. BMJ Open 2021, 11, e043827. [Google Scholar] [CrossRef]
- Badmus, O.M.; Adenaya, O.R.; Aderinwale, O.A.; Ewuoso, B.O.; Awolaja, B.S.; Ade-Onojobi, A.O. Umbilical arterial blood lactate as predictor of early neonatal outcome and evaluation of intrapartum asphyxia. J. Taibah Univ. Med. Sci. 2024, 19, 911–918. [Google Scholar] [CrossRef]
- Allanson, E.R.; Waqar, T.; White, C.; Tunçalp, Ö.; Dickinson, J.E. Umbilical lactate as a measure of acidosis and predictor of neonatal risk: A systematic review. BJOG 2017, 124, 584–594. [Google Scholar] [CrossRef]
- Sabat, J.; Gould, S.; Gillego, E.; Hariprashad, A.; Wiest, C.; Almonte, S.; Lucido, D.J.; Gave, A.; Leitman, I.M.; Eiref, S.D. The use of finger-stick blood to assess lactate in critically ill surgical patients. Ann. Med. Surg. 2016, 10, 41–48. [Google Scholar] [CrossRef]
- Walther, L.H.; Zegers, F.; Nybo, M.; Mogensen, C.B.; Christensen, E.F.; Lassen, A.T.; Mikkelsen, S. Accuracy of a point-of-care blood lactate measurement device in a prehospital setting. J. Clin. Monit. Comput. 2022, 36, 1679–1687. [Google Scholar] [CrossRef]
- Fauchère, J.C.; Bauschatz, A.S.; Arlettaz, R.; Zimmermann-Bär, U.; Bucher, H.U. Agreement between capillary and arterial lactate in the newborn. Acta Paediatr. 2002, 91, 78–81. [Google Scholar] [CrossRef]
- Brody, E.I.; Genuini, M.; Auvin, S.; Lodé, N.; Brunet, S.R. Prehospital capillary lactate in children differentiates epileptic seizure from febrile seizure, syncope, and psychogenic nonepileptic seizure. Epilepsy Behav. 2022, 127, 108551. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Soleman, J.; Kozyrev, D.A.; Jabang, J.N.; Stein, M.; Grisaru-Soen, G.; Benvenisti, H.; Sadot, E.; Friedman, S.; Ayalon, I.; et al. The value of cerebrospinal fluid lactate levels in diagnosing CSF infections in pediatric neurosurgical patients. Child’s Nerv. Syst. 2019, 35, 1147–1153. [Google Scholar] [CrossRef]
- Bedetti, L.; Lugli, L.; Marrozzini, L.; Baraldi, A.; Leone, F.; Baroni, L.; Lucaccioni, L.; Rossi, C.; Roversi, M.F.; D’Amico, R.; et al. Safety and Success of Lumbar Puncture in Young Infants: A Prospective Observational Study. Front. Pediatr. 2021, 9, 692652. [Google Scholar] [CrossRef]
- Huy, N.T.; Thao, N.T.; Diep, D.T.; Kikuchi, M.; Zamora, J.; Hirayama, K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: A systemic review and meta-analysis. Crit. Care 2010, 14, R240. [Google Scholar] [CrossRef]
- Nazir, M.; Wani, W.A.; Malik, M.A.; Mir, M.R.; Ashraf, Y.; Kawoosa, K.; Ali, S.W. Cerebrospinal fluid lactate: A differential biomarker for bacterial and viral meningitis in children. J. Pediatr. 2018, 94, 88–92. [Google Scholar] [CrossRef]
- Yamada, K.; Toribe, Y.; Yanagihara, K.; Mano, T.; Akagi, M.; Suzuki, Y. Diagnostic accuracy of blood and CSF lactate in identifying children with mitochondrial diseases affecting the central nervous system. Brain Dev. 2012, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Magner, M.; Szentiványi, K.; Svandová, I.; Ješina, P.; Tesařová, M.; Honzík, T.; Zeman, J. Elevated CSF-lactate is a reliable marker of mitochondrial disorders in children even after brief seizures. Eur. J. Paediatr. Neurol. 2011, 15, 101–108. [Google Scholar] [CrossRef]
- Benoist, J.F.; Alberti, C.; Leclercq, S.; Rigal, O.; Jean-Louis, R.; Ogier de Baulny, H.; Porquet, D.; Biou, D. Cerebrospinal fluid lactate and pyruvate concentrations and their ratio in children: Age-related reference intervals. Clin. Chem. 2003, 49, 487–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boterberg, S.; Vantroys, E.; De Paepe, B.; Van Coster, R.; Roeyers, H. Urine lactate concentration as a non-invasive screener for metabolic abnormalities: Findings in children with autism spectrum disorder and regression. PLoS ONE 2022, 17, e0274310. [Google Scholar] [CrossRef]
- Sughimoto, K.; Levman, J.; Baig, F.; Berger, D.; Oshima, Y.; Kurosawa, H.; Aoki, K.; Seino, Y.; Ueda, T.; Liu, H.; et al. Machine learning predicts blood lactate levels in children after cardiac surgery in paediatric ICU. Cardiol. Young 2023, 33, 388–395. [Google Scholar] [CrossRef]
- Filipeanu, D.; Luca, F.A.; Maha, L.-G.; Țigănaș, C.G.; Țarcă, V. The nexus between digital skills’ dynamics and employment in the pandemic context. East. J. Eur. Stud. 2024, 14, 245–264. [Google Scholar]
- Tan, V.P.; Miyamoto, S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 2015, 11, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L. Oxamate, but Not Selective Targeting of LDH-A, Inhibits Medulloblastoma Cell Glycolysis, Growth and Motility. Brain Sci. 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, S.; Yao, Y.; Zhang, G. Role of Human Monocarboxylate Transporter 1 (hMCT1) and 4 (hMCT4) in Tumor Cells and the Tumor Microenvironment. Cancer Manag. Res. 2023, 15, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, F.; Tang, Y.; Li, L.; Li, L. Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs. Molecules 2024, 29, 5656. [Google Scholar] [CrossRef]
| Lactate | ||
|---|---|---|
| Function | Classic Conceptions | Current Conceptions |
| Metabolism | Byproduct of anaerobic metabolism linked to muscle fatigue, especially during high-intensity activity [25] | Energy substrate, as well as main gluconeogenic precursor [26] |
| Immunity | Not traditionally seen as having a significant impact on immune responses. | Suppression of T cell activity in tumor microenvironment [27], CD8+ T cell-dependent tumor growth inhibition in mice bearing MC38 tumors following subcutaneous administration of sodium lactate [28] |
| Respiratory | Exercise-induced lactic acidosis stimulates ventilation and triggers hyperventilation at RCP (Respiratory Compensation Point) [29]. | Lactate produced by alveolar type II cells suppresses inflammatory alveolar macrophages and mitigates acute lung injury by shifting macrophage cytokine expression toward an anti-inflammatory phenotype [30] |
| Cardiovascular | Elevated lactate levels within the first 12 h following successful resuscitation from cardiac arrest, reflecting hypoxia and associated anaerobic metabolism, were linked to higher mortality in children [31]. | Half-molar lactate infusions may serve as a potential treatment for fluid resuscitation, demonstrating enhanced cardiac function, as indicated by increases in cardiac output, stroke volume, and left ventricular ejection fraction in healthy individuals (when compared to sodium-matched hypertonic sodium chloride) [32]. |
| Muscular | Serum lactate was identified as a potential biomarker for muscle fatigue following intense exercise [33]. | Lactate serves as an energy source for muscles, enhancing exercise capacity and endurance, while also supporting muscle recovery and adaptation following exercise [34]. |
| Neurologic | Lactate production in the brain was thought to result from insufficient oxygen supply, impaired oxidative metabolism, or a mismatch between glycolysis and oxidative processes [35]. | Lactate is now recognized as an essential fuel for the brain, playing a pivotal role in supporting cognitive function and neuronal activity, particularly in processes such as learning and memory, cerebral blood flow, neurogenesis, cerebral microangiogenesis, energy metabolism, and neuroprotection [36,37]. |
| Bone health | No established connection to bone health. | Lactate may mediate the bone anabolic effects during high-intensity interval training by inducing osteoblast differentiation, offering a potential cost-effective therapeutic strategy for bone augmentation [38]. |
| Study, Publication Year, Region | Design | Period | Population Sample | Disease | Department | Main Findings |
|---|---|---|---|---|---|---|
| Murdoch et al., 1994, London [63] | Comparative study | - | 7 children, 2.3–10.8 years old, mean age 6.5 years old | Septic shock (n = 3), ARDS * (n = 2) and severe pulmonary hypertension (n = 2) | PICU ** | The mean difference between arterial and mixed venous lactate was 0.02 mmol/L, with limits of agreement from −0.20 to 0.24. The differences were clinically insignificant (p = 0.36). |
| Fernández Sarmiento et al., 2016, Colombia [64] | Retrospective study | 3 years and 4 months (January 2009–May 2012) | 42 children, 1 month–17 years and 11 months old, mean age 2.3 years old | Sepsis and/or septic shock | PICU | A strong correlation between arterial and central venous lactate levels was found (Spearman’s rho = 0.897, p < 0.001). No significant differences were found between arterial and central venous lactate regarding age, weight, or diagnosis. |
| Samaraweera et al., 2017, London [62] | Retrospective study | 3 years and 4 months (June 2012–October 2015) | 60 children ≤17 years old, mean age 4.4 (SD = ±4.4) years old | Sepsis | PICU | A venous lactate concentration ≤2 mmol/L may serve as a surrogate for arterial lactate during the initial management of pediatric sepsis. If the venous lactate level exceeds 2 mmol/L, confirmation with an arterial sample is required. |
| Phumeetham et al., 2017, Bangkok, Thailand [65] | Prospective study | 1 year (October 2013–October 2014) | 48 children, 1 month–18 years old, median age 4.5 years old | Shock (54.1% septic, 12.5% neurogenic, 16.7% hypovolemic, 16.7% cardiogenic) | PICU | Venous and arterial lactate showed strong correlation (r = 0.962, p < 0.0001). The mean difference was 0.20 mmol/L (95% CI: 0.08–0.32), with limits of agreement from −0.74 to 1.13 mmol/L. Venous lactate is a reliable alternative to arterial sampling in shock. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belu, A.; Filip, N.; Trandafir, L.M.; Spoială, E.L.; Țarcă, E.; Zamosteanu, D.; Ghiga, G.; Bernic, J.; Jehac, A.; Cojocaru, E. Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions. Diagnostics 2025, 15, 816. https://doi.org/10.3390/diagnostics15070816
Belu A, Filip N, Trandafir LM, Spoială EL, Țarcă E, Zamosteanu D, Ghiga G, Bernic J, Jehac A, Cojocaru E. Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions. Diagnostics. 2025; 15(7):816. https://doi.org/10.3390/diagnostics15070816
Chicago/Turabian StyleBelu, Alina, Nina Filip, Laura Mihaela Trandafir, Elena Lia Spoială, Elena Țarcă, Diana Zamosteanu, Gabriela Ghiga, Jana Bernic, Alina Jehac, and Elena Cojocaru. 2025. "Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions" Diagnostics 15, no. 7: 816. https://doi.org/10.3390/diagnostics15070816
APA StyleBelu, A., Filip, N., Trandafir, L. M., Spoială, E. L., Țarcă, E., Zamosteanu, D., Ghiga, G., Bernic, J., Jehac, A., & Cojocaru, E. (2025). Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions. Diagnostics, 15(7), 816. https://doi.org/10.3390/diagnostics15070816








