QSI and DTI of Inherited White Matter Disorders in Rat Spinal Cord: Early Detection and Comparison with Quantitative Electron Microscopy Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ex Vivo MRI Experiments
2.3. MRI ROIs Analysis and Statistics
2.4. Samples for Light and Electron Microscopy
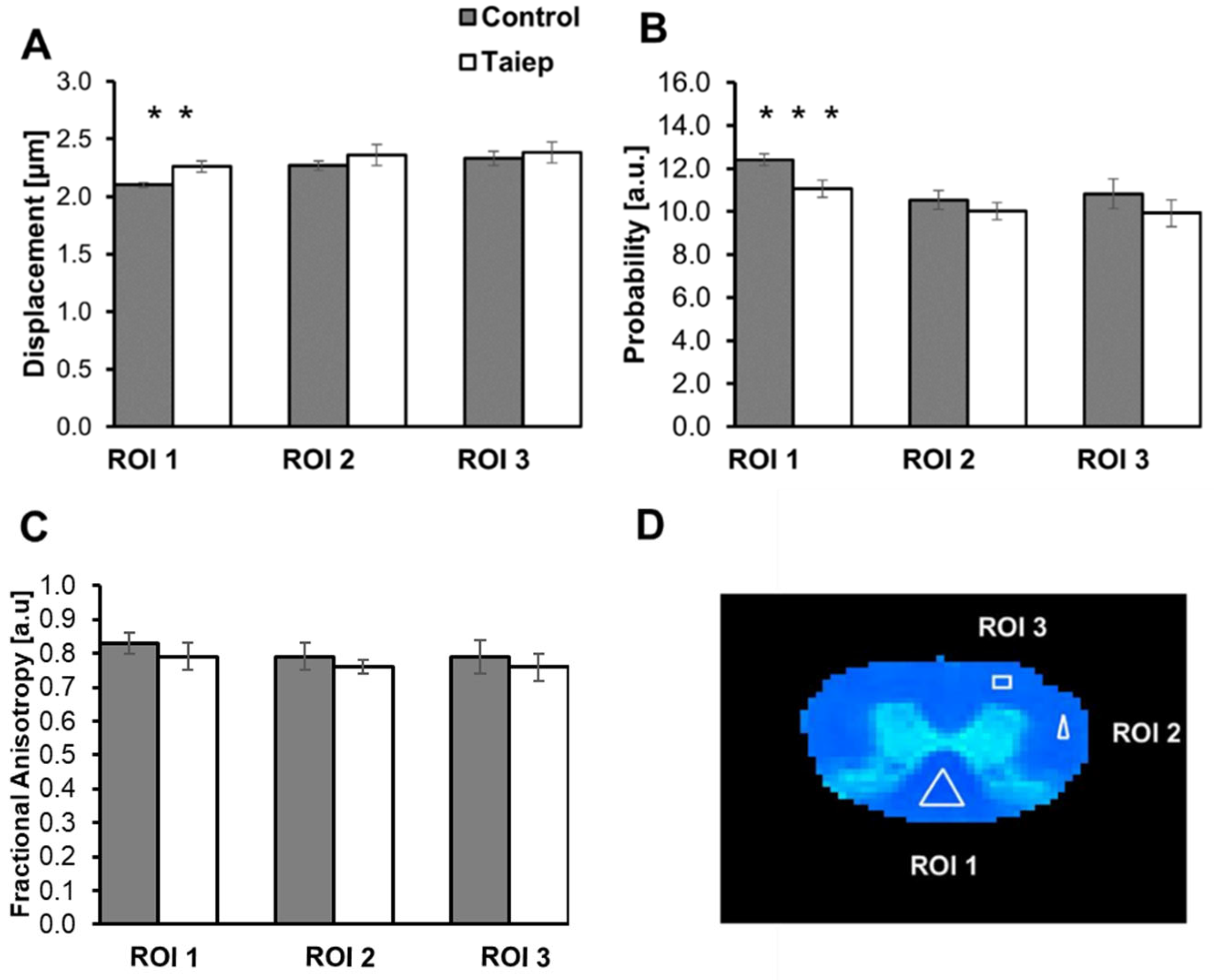
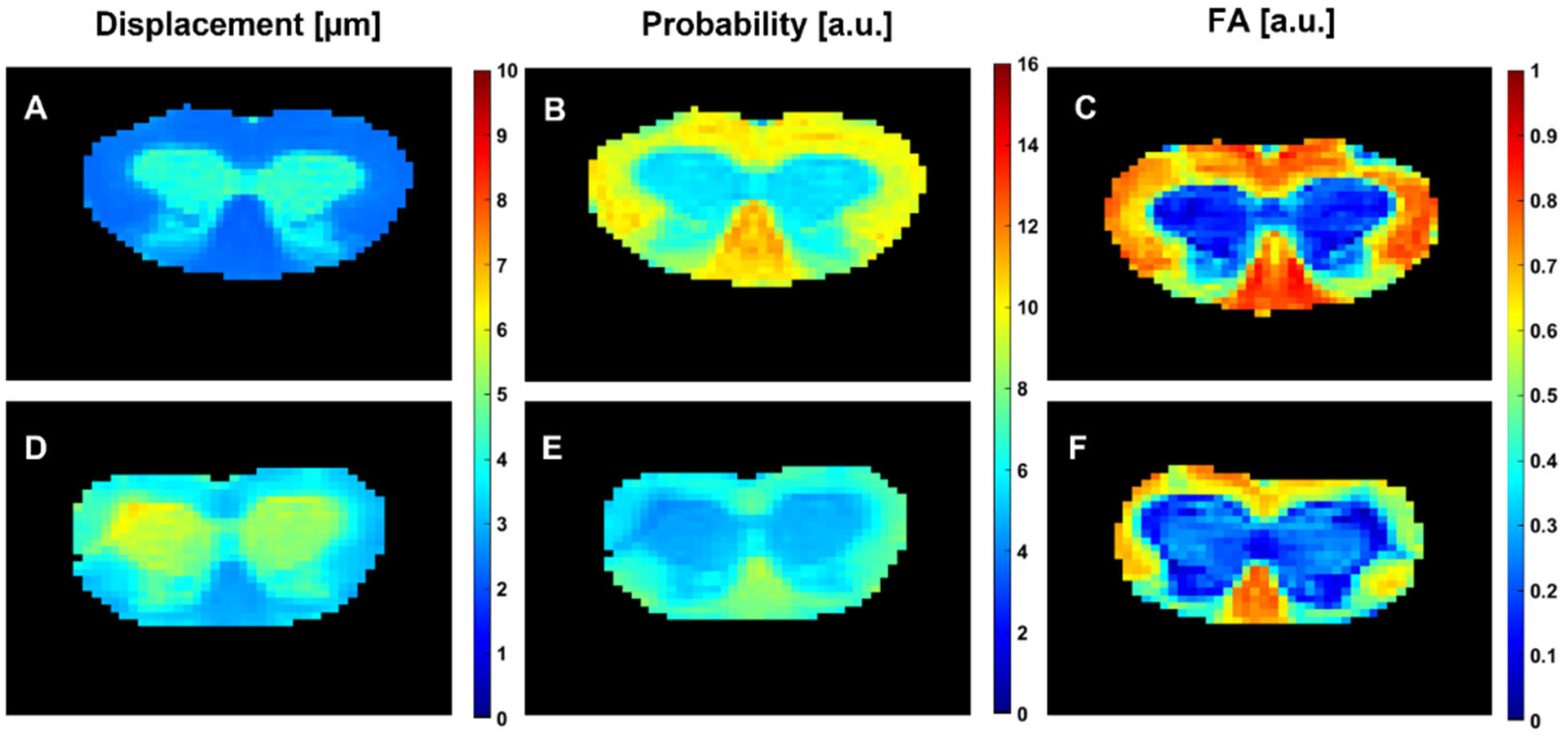
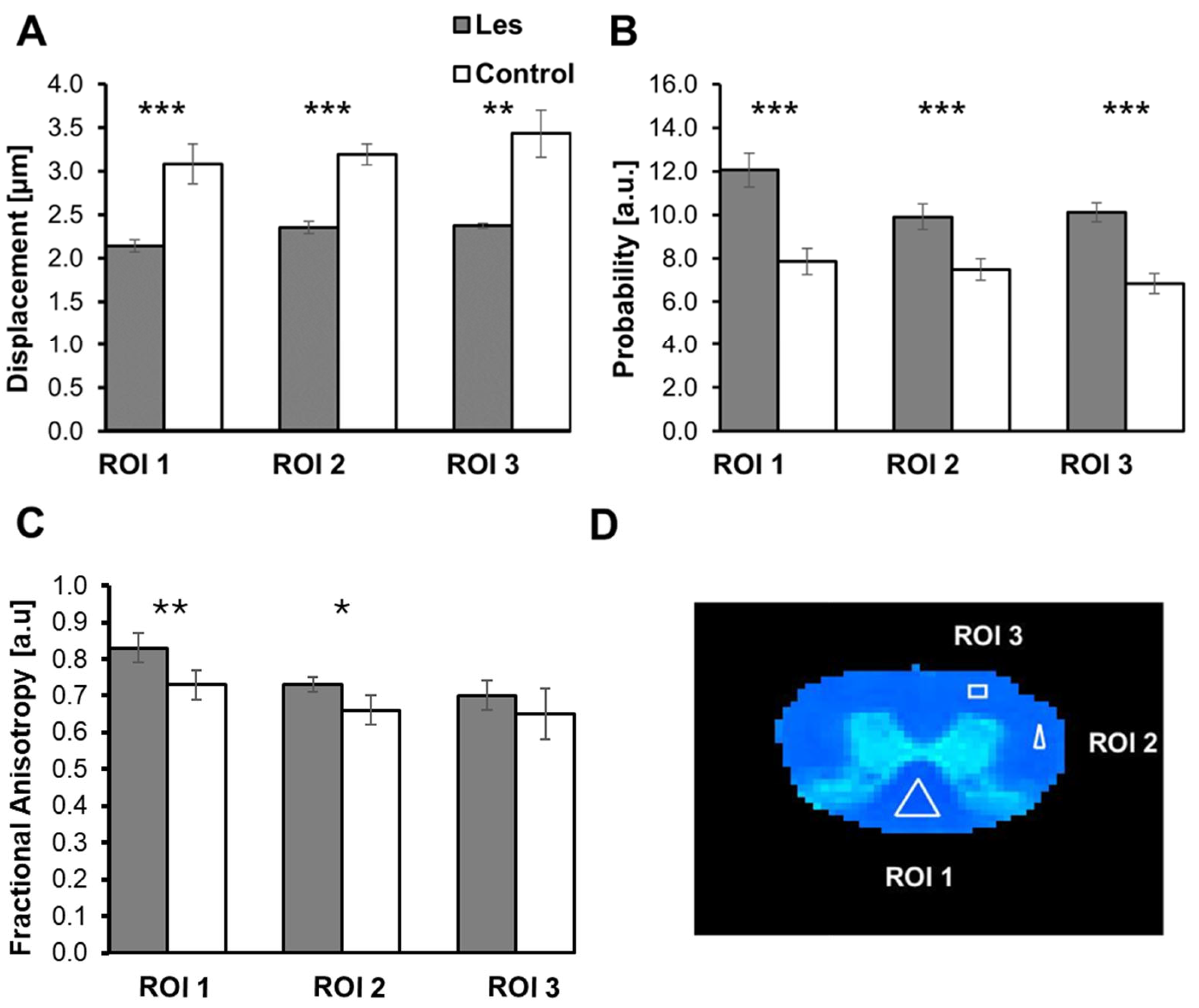
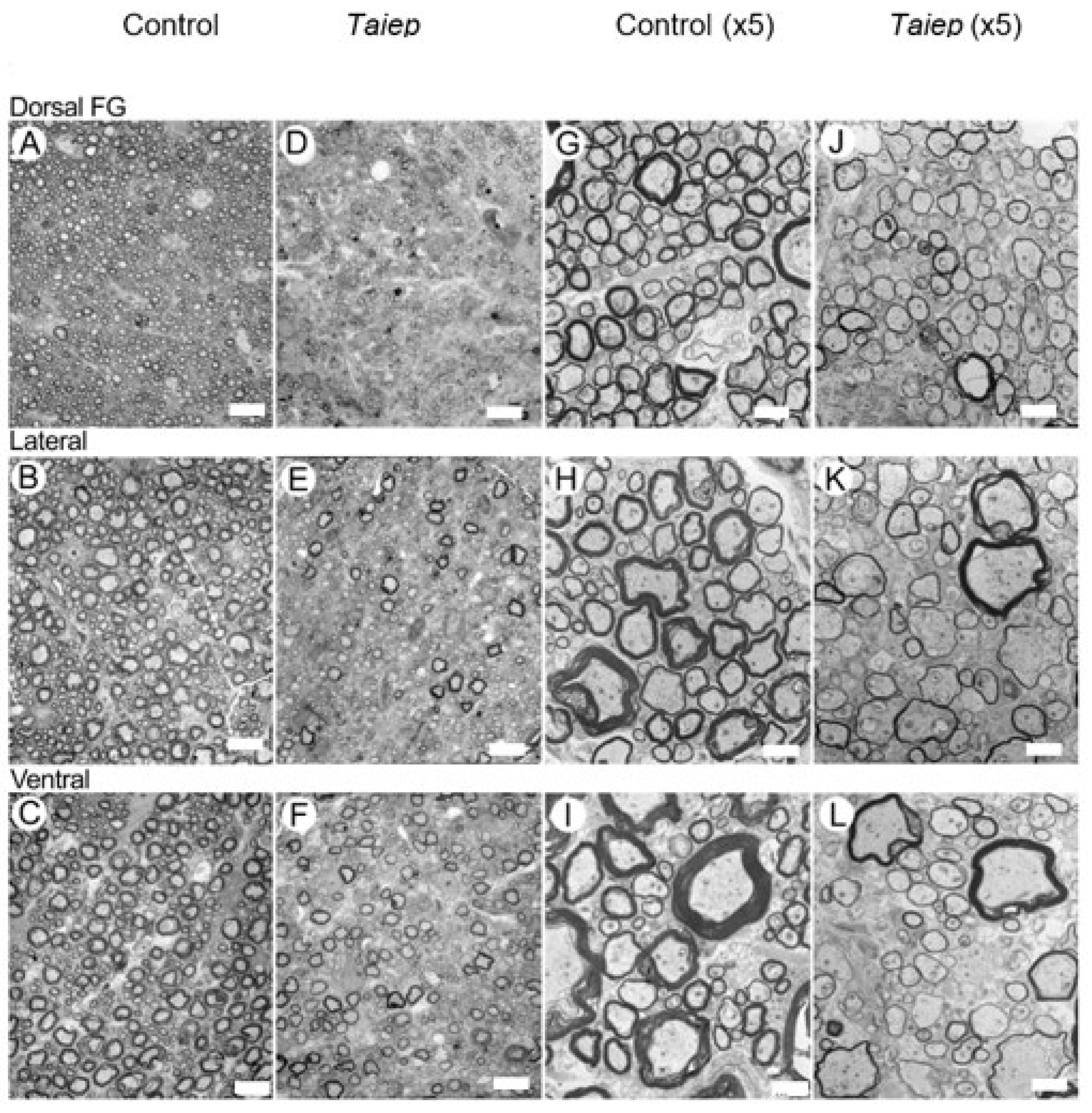
3. Results
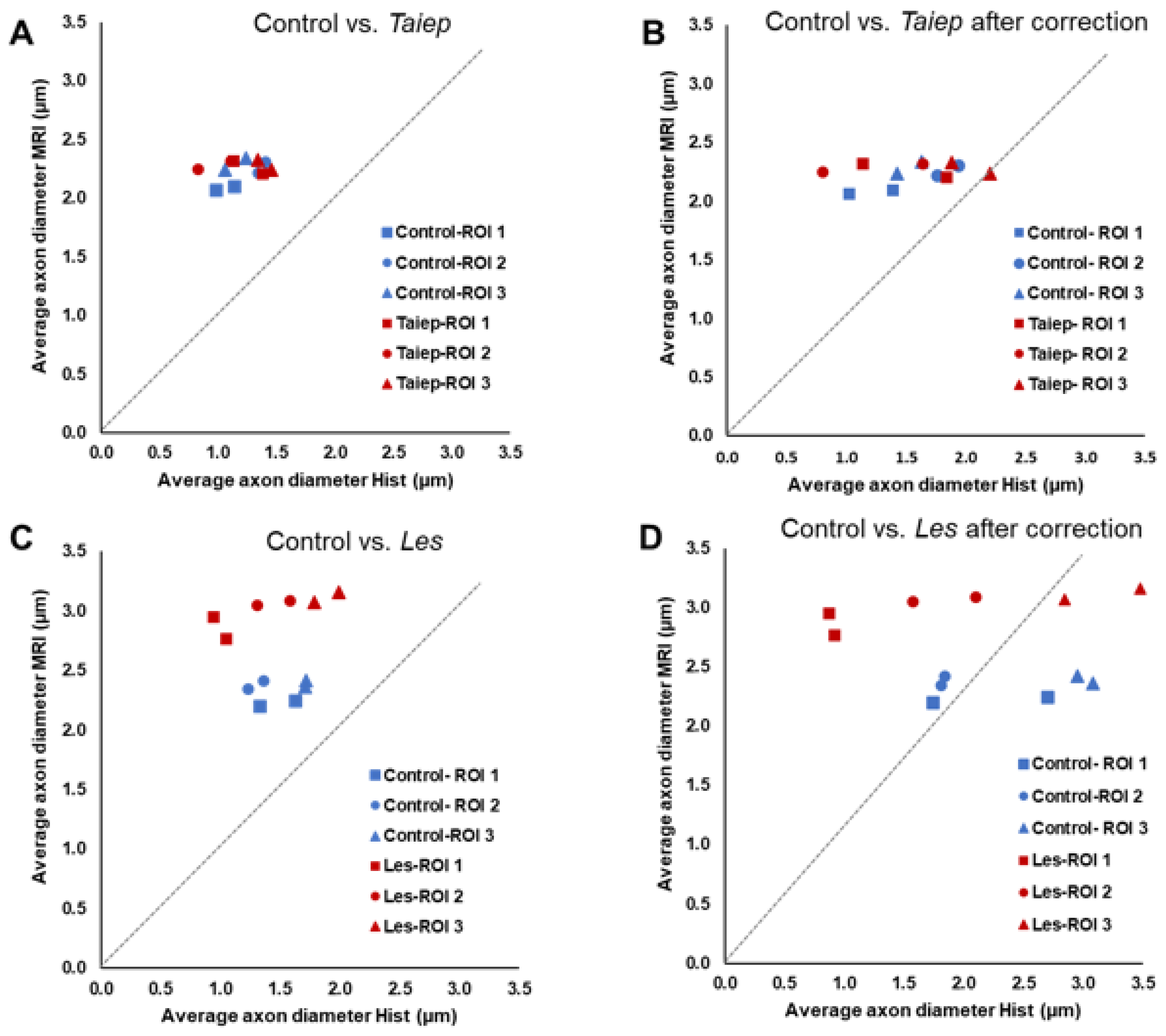
3.1. Diffusion MRI Findings in the taiep and les SCs
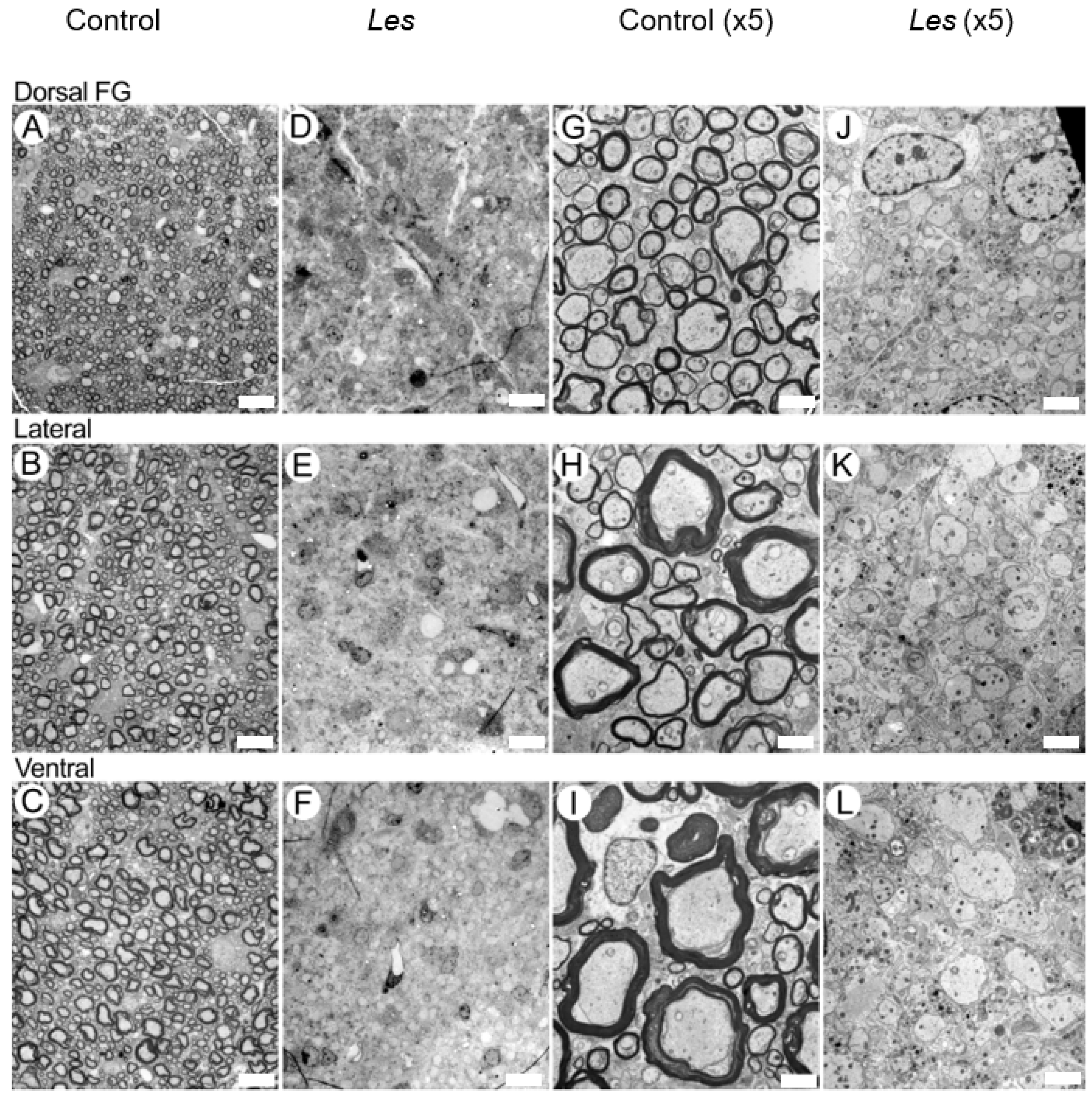
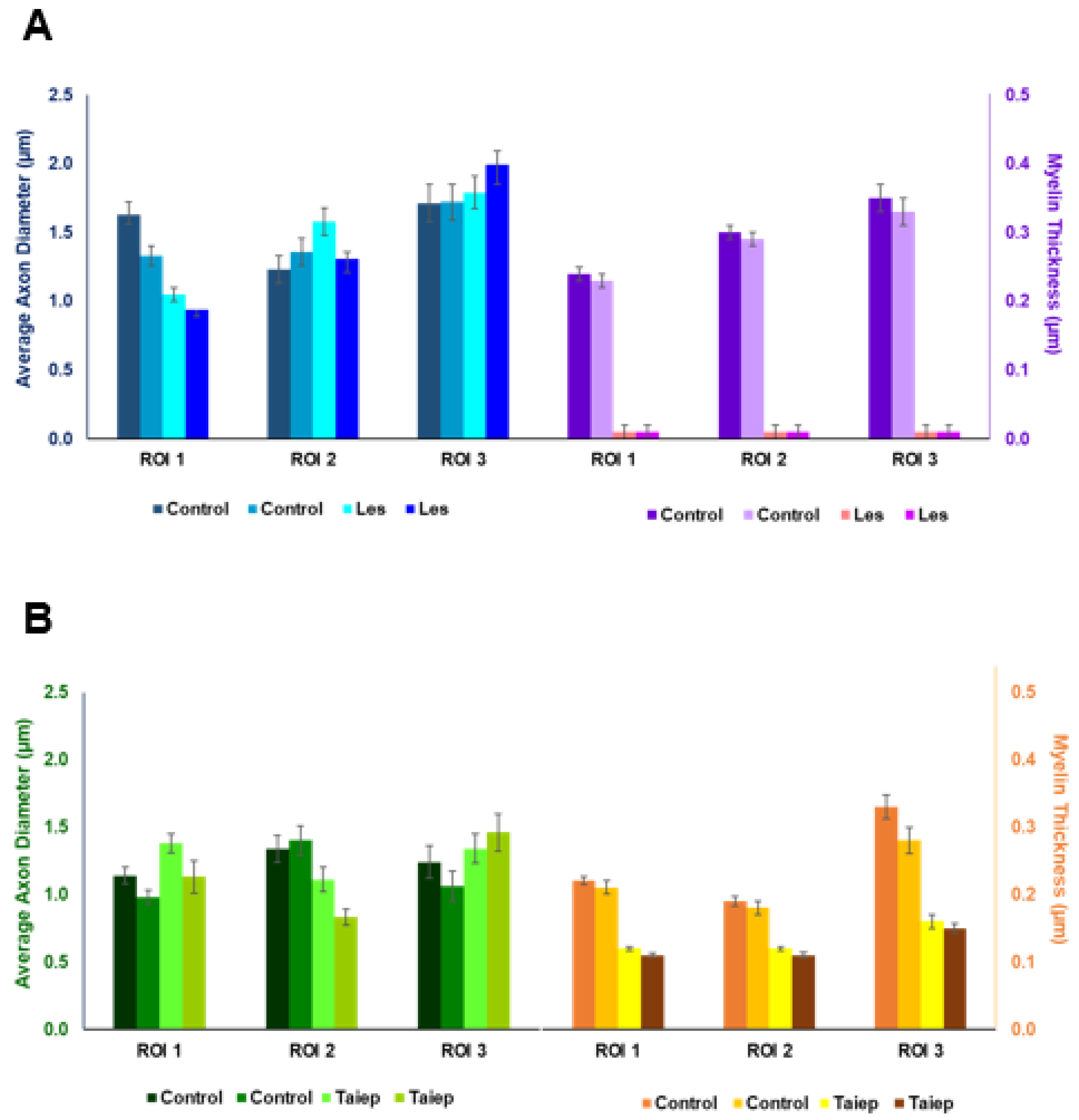
3.2. EM Findings in the Dorsal, Lateral and Ventral Columns of the taiep and les SC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Average axon diameter |
| AxD | Axial diffusivity |
| CNS | Central nervous system |
| DTI | Diffusion tensor imaging |
| EM | Electron microscopy |
| EPI | Echo planar imaging |
| FA | Fractional anisotropy |
| Les | Long–Evans Shaker |
| MBP | Myelin basic protein |
| md | Myelin-deficient |
| MD | Mean diffusivity |
| MS | Multiple sclerosis |
| PLP | Proteolipid protein |
| PZD | Probability for zero displacement |
| QSI | Q-space diffusion MRI |
| RaD | Radial diffusivity |
| RaDis | Radial displacement |
| ROI | Region of interest |
| SC | Spinal cord |
| SNR | Signal-to-noise ratio |
| WM | White matter |
Appendix A
| Displacement [µm] | Probability [a.u.] | FA [a.u] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | ROI 1 | ROI 2 | ROI 3 | ROI 1 | ROI 2 | ROI 3 | ROI 1 | ROI 2 | ROI 3 |
| Control | 2.10 ± 0.03 | 2.27 ± 0.02 | 2.28 ± 0.06 | 12.22 ± 0.47 | 10.55 ± 0.63 | 11.19 ± 0.55 | 0.85 ± 0.04 | 0.80 ± 0.05 | 0.80 ± 0.02 |
| * Control | 2.10 ± 0.02 | 2.22 ± 0.04 | 2.34 ± 0.04 | 12.41 ± 0.73 | 10.98 ± 0.68 | 11.46 ± 0.29 | 0.82 ± 0.03 | 0.80 ± 0.04 | 0.80 ± 0.03 |
| * Control | 2.07 ± 0.03 | 2.31 ± 0.04 | 2.24 ± 0.02 | 12.75 ± 0.64 | 10.66 ± 0.16 | 11.32 ± 0.25 | 0.80 ± 0.03 | 0.85 ± 0.03 | 0.86 ± 0.02 |
| Control | 2.12 ± 0.05 | 2.23 ± 0.02 | 2.36 ± 0.02 | 12.09 ± 0.43 | 10.70 ± 0.23 | 10.28 ± 0.23 | 0.82 ± 0.02 | 0.74 ± 0.02 | 0.72 ± 0.06 |
| Control | 2.12 ± 0.04 | 2.30 ± 0.01 | 2.40 ± 0.02 | 12.59 ± 0.51 | 9.82 ± 0.34 | 9.88 ± 0.36 | 0.88 ± 0.03 | 0.77 ± 0.04 | 0.76 ± 0.02 |
| Average | 2.10 ± 0.02 | 2.27 ± 0.04 | 2.33 ± 0.06 | 12.41 ± 0.27 | 10.54 ± 0.43 | 10.82 ± 0.70 | 0.83 ± 0.03 | 0.79 ± 0.04 | 0.79 ± 0.05 |
| * Taiep | 2.21 ± 0.07 | 2.32 ± 0.03 | 2.33 ± 0.03 | 11.53 ± 0.62 | 9.65 ± 0.53 | 10.13 ± 0.19 | 0.82 ± 0.07 | 0.75 ± 0.03 | 0.77 ± 0.02 |
| * Taiep | 2.32 ± 0.13 | 2.25 ± 0.02 | 2.24 ± 0.03 | 11.07 ± 0.57 | 10.30 ± 0.12 | 10.91 ± 0.11 | 0.77 ± 0.05 | 0.77 ± 0.02 | 0.73 ± 0.06 |
| Taiep | 2.22 ± 0.09 | 2.37 ± 0.08 | 2.46 ± 0.01 | 11.25 ± 0.56 | 9.58 ± 0.24 | 9.27 ± 0.04 | 0.75 ± 0.05 | 0.80 ± 0.04 | 0.78 ± 0.03 |
| Taiep | 2.32 ± 0.01 | 2.50 ± 0.07 | 2.46 ± 0.01 | 10.47 ± 0.19 | 10.44 ± 0.29 | 9.70 ± 0.37 | 0.74 ± 0.06 | 0.73 ± 0.01 | 0.71 ± 0.04 |
| Taiep | 2.25 ± 0.06 | 2.36 ± 0.04 | 2.39 ± 0.02 | 10.95 ± 0.50 | 10.14 ± 0.13 | 9.65 ± 0.02 | 0.85 ± 0.02 | 0.76 ± 0.02 | 0.83 ± 0.02 |
| Average | 2.26 ± 0.05 | 2.36 ± 0.09 | 2.38 ± 0.09 | 11.06 ± 0.39 | 10.02 ± 0.39 | 9.93 ± 0.63 | 0.79 ± 0.04 | 0.76 ± 0.02 | 0.76 ± 0.04 |
| p-value | 0.0015 | 0.0827 | 0.3457 | 0.0004 | 0.0811 | 0.0665 | 0.10002 | 0.20807 | 0.46531 |
| Displacement [µm] | Probability [a.u.] | FA [a.u] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | ROI 1 | ROI 2 | ROI 3 | ROI 1 | ROI 2 | ROI 3 | ROI 1 | ROI 2 | ROI 3 |
| Control | 2.11 ± 0.04 | 2.24 ± 0.01 | 2.36 ± 0.02 | 12.33 ± 0.21 | 10.70 ± 0.36 | 9.95 ± 0.08 | 0.82 ± 0.02 | 0.76 ± 0.05 | 0.65 ± 0.04 |
| * Control | 2.25 ± 0.03 | 2.35 ± 0.02 | 2.36 ± 0.01 | 10.96 ± 0.37 | 9.63 ± 0.21 | 9.78 ± 0.23 | 0.77 ± 0.02 | 0.70 ± 0.03 | 0.72 ± 0.06 |
| * Control | 2.20 ± 0.04 | 2.42 ± 0.07 | 2.42 ± 0.02 | 11.33 ± 0.37 | 8.93 ± 0.26 | 9.58 ± 0.11 | 0.82 ± 0.05 | 0.75 ± 0.02 | 0.75 ± 0.03 |
| Control | 2.06 ± 0.06 | 2.44 ± 0.05 | 2.33 ± 0.09 | 12.82 ± 0.65 | 10.16 ± 0.52 | 10.71 ± 0.52 | 0.90 ± 0.04 | 0.71 ± 0.04 | 0.74 ± 0.05 |
| Control | 2.09 ± 0.03 | 2.32 ± 0.00 | 2.40 ± 0.04 | 12.86 ± 0.42 | 10.07 ± 0.17 | 10.54 ± 0.32 | 0.84 ± 0.01 | 0.73 ± 0.01 | 0.66 ± 0.02 |
| Average | 2.14 ± 0.07 | 2.35 ± 0.07 | 2.37 ± 0.03 | 12.06 ± 0.78 | 9.90 ± 0.59 | 10.11 ± 0.44 | 0.83 ± 0.04 | 0.73 ± 0.02 | 0.70 ± 0.04 |
| Les | 2.87 ± 0.06 | 3.16 ± 0.07 | 3.65 ± 0.09 | 8.30 ± 0.28 | 8.30 ± 0.27 | 6.45 ± 0.25 | 0.72 ± 0.03 | 0.60 ± 0.03 | 0.60 ± 0.04 |
| Les | 3.43 ± 0.08 | 3.24 ± 0.03 | 3.57 ± 0.09 | 6.82 ± 0.18 | 7.23 ± 0.21 | 6.64 ± 0.09 | 0.67 ± 0.04 | 0.62 ± 0.02 | 0.67 ± 0.03 |
| * Les | 2.77 ± 0.08 | 3.09 ± 0.03 | 3.07 ± 0.06 | 8.52 ± 0.28 | 7.25 ± 0.26 | 7.49 ± 0.22 | 0.72 ± 0.04 | 0.72 ± 0.01 | 0.74 ± 0.04 |
| * Les | 2.95 ± 0.04 | 3.05 ± 0.06 | 3.16 ± 0.05 | 7.96 ± 0.10 | 7.72 ± 0.15 | 7.24 ± 0.15 | 0.73 ± 0.03 | 0.69 ± 0.02 | 0.70 ± 0.02 |
| Les | 3.05 ± 0.04 | 3.39 ± 0.20 | 3.72 ± 0.08 | 7.63 ± 0.22 | 6.90 ± 0.24 | 6.30 ± 0.12 | 0.80 ± 0.01 | 0.67 ± 0.03 | 0.54 ± 0.01 |
| Average | 3.01 ± 0.23 | 3.19 ± 0.12 | 3.43 ± 0.27 | 7.85 ± 0.59 | 7.48 ± 0.49 | 6.82 ± 0.46 | 0.73 ± 0.04 | 0.66 ± 0.04 | 0.65 ± 0.07 |
| p-values | 0.00075 | 0.00001 | 0.00138 | 0.00006 | 0.00023 | 0.00001 | 0.00869 | 0.03070 | 0.23894 |
| Sample | ROI | AAD | AAD After Correction | Myelin Thickness |
|---|---|---|---|---|
| Control | ROI 1 | 1.14 ± 0.06 | 1.39 ± 0.09 | 0.22 ± 0.006 |
| ROI 2 | 1.34 ± 0.10 | 1.76 ± 0.10 | 0.19 ± 0.007 | |
| ROI 3 | 1.24 ± 0.12 | 1.63 ± 0.15 | 0.33 ± 0.018 | |
| Control | ROI 1 | 0.98 ± 0.05 | 1.02 ± 0.06 | 0.21 ± 0.010 |
| ROI 2 | 1.40 ± 0.11 | 1.94 ± 0.14 | 0.18 ± 0.010 | |
| ROI 3 | 1.06 ± 0.11 | 1.43 ± 0.15 | 0.28 ± 0.020 | |
| Taiep | ROI 1 | 1.38 ± 0.07 | 1.84 ± 0.09 | 0.12 ± 0.003 |
| ROI 2 | 1.11 ± 0.09 | 1.64 ± 0.16 | 0.12 ± 0.003 | |
| ROI 3 | 1.34 ± 0.11 | 1.88 ± 0.15 | 0.16 ± 0.010 | |
| Taiep | ROI 1 | 1.13 ± 0.12 | 1.14 ± 0.07 | 0.11 ± 0.003 |
| ROI 2 | 0.83 ± 0.06 | 0.80 ± 0.08 | 0.11 ± 0.004 | |
| ROI 3 | 1.46 ± 0.14 | 2.20 ± 0.17 | 0.15 ± 0.007 | |
| Control | ROI 1 | 1.63 ± 0.09 | 2.70 ± 0.15 | 0.24 ± 0.010 |
| ROI 2 | 1.23 ± 0.10 | 1.81 ± 0.14 | 0.30 ± 0.013 | |
| ROI 3 | 1.71 ± 0.14 | 3.08 ± 0.22 | 0.35 ± 0.018 | |
| Control | ROI 1 | 1.33 ± 0.07 | 1.74 ± 0.11 | 0.23 ± 0.007 |
| ROI 2 | 1.36 ± 0.10 | 1.84 ± 0.13 | 0.29 ± 0.012 | |
| ROI 3 | 1.72 ± 0.13 | 2.95 ± 0.21 | 0.33 ± 0.017 | |
| Les | ROI 1 | 1.05 ± 0.05 | 0.92 ± 0.05 | 0.00 ± 0.000 |
| ROI 2 | 1.58 ± 0.10 | 2.10 ± 0.16 | 0.00 ± 0.000 | |
| ROI 3 | 1.79 ± 0.12 | 2.85 ± 0.19 | 0.00 ± 0.000 | |
| Les | ROI 1 | 0.94 ± 0.05 | 0.87 ± 0.06 | 0.00 ± 0.000 |
| ROI 2 | 1.31 ± 0.10 | 1.57 ± 0.13 | 0.00 ± 0.000 | |
| ROI 3 | 1.99 ± 0.14 | 3.48 ± 0.22 | 0.00 ± 0.000 |
| ROI | Protocol (ms) | WT/Les-28-Day-Old | Les-28-Day-Old | WT/Taiep-28-Day-Old | Taiep-28-Day-Old | WT/Taiep-3-Month-Old |
|---|---|---|---|---|---|---|
| ROI 1 | 90/2/30 | 2.11 ± 0.02 | 2.54 ± 0.05 | 2.15 ± 0.04 | 2.17 ± 0.03 | 2.08 ± 0.02 |
| 90/2/50 | 2.20 ± 0.04 | 3.04 ± 0.08 | 2.31 ± 0.05 | 2.19 ± 0.04 | 2.05 ± 0.03 | |
| 90/2/75 | 2.31 ± 0.05 | 3.50 ± 0.22 | 2.26 ± 0.06 | 2.39 ± 0.06 | 2.07 ± 0.02 | |
| ROI 2 | 90/2/30 | 2.16 ± 0.01 | 2.64 ± 0.11 | 2.23 ± 0.03 | 2.18 ± 0.01 | 2.12 ± 0.01 |
| 90/2/50 | 2.29 ± 0.02 | 3.21 ± 0.14 | 2.26 ± 0.06 | 2.16 ± 0.01 | 2.12 ± 0.02 | |
| 90/2/75 | 2.39 ± 0.06 | 3.58 ± 0.12 | 2.22 ± 0.01 | 2.42 ± 0.07 | 2.15 ± 0.03 | |
| ROI 3 | 90/2/30 | 2.23 ± 0.03 | 2.76 ± 0.05 | 2.26 ± 0.03 | 2.20 ± 0.01 | 2.16 ± 0.01 |
| 90/2/50 | 2.31 ± 0.03 | 3.46 ± 0.08 | 2.31 ± 0.06 | 2.20 ± 0.03 | 2.16 ± 0.01 | |
| 90/2/75 | 2.40 ± 0.04 | 4.12 ± 0.09 | 2.58 ± 0.02 | 2.38 ± 0.04 | 2.24 ± 0.03 |
References
- Bjartmar, C.; Yin, X.H.; Trapp, B.D. Axonal pathology in myelin disorders. J. Neurocytol. 1999, 28, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, M.S.; Schiffmann, R.; Mochel, F.; Wolf, N.I. Diagnostic, prognosis, and treatment of leukodystrophies. Lancet Neurol. 2019, 18, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.I.; Ffrench-Constant, C.; van der Knaap, M.S. Hypomyelinating leukodystrophies unravelling myelin biology. Nat. Rev. Neurol. 2021, 17, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Radcliff, A.B. Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp. Neurol. 2016, 283, 452–475. [Google Scholar] [CrossRef]
- Duncan, I.D.; Kondo, Y.; Zhang, S. The myelin mutants as models to study myelin repair in the leukodystrophies. Neurotherapeutics 2011, 8, 607–624. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; O′Connor, L.T.; Goetz, B.D.; Delaney, K.H. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J. Neurocytol. 1998, 27, 581–591. [Google Scholar] [CrossRef]
- O′Connor, L.T.; Goetz, B.D.; Kwiecien, J.M.; Delaney, K.H.; Fletch, A.L.; Duncan, I.D. Insertion of a retrotransposon in Mbp disrupts mRNA splicing and myelination in a new mutant rat. J. Neurosci. 1999, 19, 3404–3413. [Google Scholar] [CrossRef]
- Smith, C.M.; Mayer, J.A.; Duncan, I.D. Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant. J. Neurosci. 2013, 33, 8088–8100. [Google Scholar] [CrossRef]
- Holmgren, B.; Urba-Holmgren, R.; Riboni, L.; Vega-SaenzdeMiera, E.C. Sprague Dawley rat mutant with tremor, ataxia, tonic immobility episodes, epilepsy, and paralysis. Lab. Anim. Sci. 1989, 39, 226–228. [Google Scholar]
- Duncan, I.D.; Lunn, K.F.; Holmgren, B.; Urba-Holmgren, R.; Brignolo-Holmes, L. The taiep rat: A myelin mutant with an associated oligodendrocyte microtubular defect. J. Neurocytol. 1992, 21, 870–884. [Google Scholar] [CrossRef]
- Anch, A.M.; Powell, E.; Bloom, C.; Dyche, J.; Faulkner, K.; Richter, R.R. Locomotor analysis of the taiep rat. J. Gen. Psychol. 2000, 127, 412–425. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, M.S.; Leegwater, P.A.J.; Könst, A.A.; Visser, A.; Naidu, S.; Oudejans, C.B.; Schutgens, R.B.; Pronk, J.C. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann. Neurol. 2002, 51, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Bugiani, M.; Radcliff, A.B.; Moran, J.J.; Lopez-Anido, C.; Duong, P.; August, B.K.; Wolf, N.I.; van der Knaap, M.S.; Svaren, J. A mutation in the Tubb4a gene leads to microtubule accumulation with hypomyelination and demyelination. Ann. Neurol. 2017, 81, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K. (Ed.) Diffusion MRI: Theory, Methods and Applications; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Behrens, T.E.J.; Johansen-Berg, H. (Eds.) Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy; Academic Press: London, UK, 2009. [Google Scholar]
- Moseley, M.E.; Cohen, Y.; Kucharczyk, J.; Mintorovitch, J.; Asgari, H.S.; Wendland, M.F.; Tsuruda, J.; Norman, D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 1990, 176, 439–445. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Cohen, Y.; Anaby, D.; Morozov, D. Diffusion MRI of the spinal cord: From structural studies to pathology. NMR Biomed. 2017, 30, e3592. [Google Scholar] [CrossRef]
- Hori, M.; Fukunaga, I.; Masutani, Y.; Taoka, T.; Kamagata, K.; Suzuki, Y.; Aoki, S. Visualizing non-Gaussian diffusion: Clinical application of q-space imaging and diffusional kurtosis imaging of the brain and spine. Magn. Reson. Med. Sci. 2012, 11, 221–233. [Google Scholar] [CrossRef]
- Hori, M.; Maekawa, T.; Kamiya, K.; Hagiwara, A.; Goto, M.; Takemura, M.Y.; Fujita, S.; Andica, C.; Kamagata, K.; Cohen-Adad, J.; et al. Advanced diffusion MR imaging for multiple sclerosis in the brain and spinal cord. Magn. Reson. Med. Sci. 2022, 21, 58–70. [Google Scholar] [CrossRef]
- Assaf, Y.; Cohen, Y. Non mono-exponential attenuation of the water and N-acetyl-aspartate signals due to diffusion in brain tissue. J. Magn. Reson. 1998, 131, 69–85. [Google Scholar] [CrossRef]
- Pfeuffer, J.; Provencher, S.W.; Gruetter, R. Water diffusion in rat brain in vivo as detected at very large b values is multi-compartmental. MAGMA 1998, 8, 98–108. [Google Scholar] [CrossRef]
- Niendorf, T.; Dijkhuizen, R.M.; Norris, D.G.; Campagne, M.v.L.; Nicolay, K. Biexponential diffusion attenuation in various states of brain tissue: Implications to diffusion-weighted imaging. Magn. Reson. Med. 1996, 36, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Cohen, Y. Assignment of the water slow diffusing component in CNS using q-space diffusion MRS: Implications to fiber tract imaging. Magn. Reson. Med. 2000, 43, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Mayk, A.; Cohen, Y. Displacement imaging of spinal cord using q-space diffusion-weighted MRI. Magn. Reson. Med. 2000, 44, 713–722. [Google Scholar] [CrossRef]
- Assaf, Y.; Ben-Bashat, D.; Chapman, J.; Peled, S.; Biton, I.; Kafri, M.; Segev, Y.; Hendler, T.; Korczyn, A.; Graif, M.; et al. High b-value q-space analyzed diffusion weighted MRI: Application to multiple sclerosis. Magn. Reson. Med. 2002, 47, 115–126. [Google Scholar] [CrossRef]
- Cohen, Y.; Assaf, Y. High b-value q-space analysis diffusion-weighted MRS and MRI in neuronal tissues—A technical review. NMR Biomed. 2002, 15, 516–542. [Google Scholar] [CrossRef]
- Biton, I.E.; Mayk, A.; Kidron, D.; Assaf, Y.; Cohen, Y. Improved detectability of experimental allergic encephalomyelitis in excised swine spinal cords by high b-value q-space DWI. Exp. Neurol. 2005, 195, 437–446. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Lu, H.; Jensen, J.H.; Ramani, A.; Helpern, J.A. Three-dimensional characterization of non-Gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006, 19, 236–247. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis imaging. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef]
- Assaf, Y.; Basser, P.J. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage 2005, 25, 48–58. [Google Scholar] [CrossRef]
- Assaf, Y.; Blumenfeld-Katzir, T.; Yovel, Y.; Basser, P.J. AxCaliber: A method for measuring axon distribution from diffusion MRI. Magn. Reson. Med. 2008, 59, 1347–1354. [Google Scholar] [CrossRef]
- Alexander, D.C.; Hubbard, P.L.; Hall, M.G.; Moore, E.A.; Ptito, M.; Parker, G.J.; Dyrby, T.B. Orientaionally invariant indices of axon diameter and density from diffusion MRI. Neuroimage 2010, 52, 1374–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Palombo, M.; Ianus, A.; Guerreri, M.; Nunes, D.; Alexander, D.C.; Shemesh, N.; Zhang, H. SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. Neuroimage 2020, 215, 116835. [Google Scholar] [CrossRef]
- Shemesh, N.; Ozerslan, E.; Komlosh, M.E.; Basser, P.J.; Cohen, Y. From single-pulsed field gradient to double-pulsed field gradient NMR: Gleaning new microstructural information and developing new forms of contrast in MRI. NMR Biomed. 2010, 23, 757–780. [Google Scholar] [CrossRef]
- Shemesh, N.; Jespersen, S.N.; Alexander, D.C.; Cohen, Y.; Drobnjak, I.; Dyrby, T.B.; Finsterbusch, J.; Koch, M.A.; Kuder, T.; Laun, F.; et al. Conventions and nomenclature for double diffusion encoding NMR and MRI. Magn. Reson. Med. 2016, 75, 82–87. [Google Scholar] [CrossRef]
- Novikov, D.S.; Fieremans, E.; Jespersen, S.N.; Kiselev, V.G. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. NMR Biomed. 2019, 32, e3998. [Google Scholar] [CrossRef]
- Novikov, D.S. The present and future of microstructure MRI: From paradigm shift to normal science. J. Neurosci. Methods 2021, 351, 108947. [Google Scholar] [CrossRef]
- Does, M.D.; Parsons, E.C.; Gore, J.C. Oscillating gradient measurements of water diffusion in normal and globally ischemic rat brain. Magn. Reson. Med. 2003, 49, 206–215. [Google Scholar] [CrossRef]
- Baron, C.A.; Beaulieu, C. Oscillating gradient spin echo (OGSE) diffusion tensor imaging of the human brain. Magn. Reson. Med. 2014, 72, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Ono, J.; Harada, K.; Takahashi, M.; Maeda, M.; Ikenaka, K.; Sakurai, K.; Sakai, N.; Kagawa, T.; Fritz-Zieroth, B.; Nagai, T.; et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995, 671, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gulani, V.; Webb, A.G.; Duncan, I.D.; Lauterbur, P.C. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn. Reson. Med. 2001, 45, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-K.; Sun, S.-W.; Ramsbottom, M.J.; Changc, C.; Russellf, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef]
- Tyszka, J.M.; Readhead, C.; Bearer, E.L.; Paulter, R.G.; Jacobs, R.E. Statistical diffusion tensor histology reveals regional dysmyelination effects in the Shiverer mouse mutant. Neuroimage 2006, 21, 1058–1065. [Google Scholar] [CrossRef]
- Hofling, A.A.; Kim, J.H.; Fantz, C.R.; Sands, M.S.; Song, S.K. Diffusion tensor imaging detects axonal injury and demyelination in the spinal cord and cranial nerve of a murine model of globoid leukodystrophy. NMR Biomed. 2009, 22, 1100–1106. [Google Scholar] [CrossRef]
- DeBoy, C.A.; Zhang, J.; Dike, S.; Shats, I.; Jones, M.; Reich, D.S.; Mori, S.; Nguyen, T.; Rothstein, B.; Miller, R.H.; et al. High-resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cords. Brain 2009, 130, 2199–2210. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Field, A.S.; Duncan, I.D.; Samsonov, A.A.; Kondo, Y.; Tudorascu, D.; Alexander, A.L. High b-value and diffusion tensor imaging in a canine model of dysmyelination and brain maturation. Neuroimage 2011, 58, 829–837. [Google Scholar] [CrossRef]
- Jeong, K.E.; Lee, S.Y.; Yeom, S.; Carlson, N.; Shah, L.M.; Rose, J.; Jeong, E. Ultrahigh-b diffusion imaging for quantitative evaluation of myelination in Shiverer mouse spinal cord. Magn. Reson. Med. 2022, 87, 179–192. [Google Scholar] [CrossRef]
- Biton, I.E.; Duncan, I.D.; Cohen, Y. High b-Value q-Space diffusion MRI in myelin-deficient rat spinal cords. Magn. Reson. Imaging 2006, 24, 161–166. [Google Scholar] [CrossRef]
- Biton, I.E.; Duncan, I.D.; Cohen, Y. q-Space diffusion of myelin-deficient spinal cords. Magn. Reson. Med. 2007, 58, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shir, A.; Duncan, I.D.; Cohen, Y. QSI and DTI of excised brains of the myelin-deficient rat. Neuroimage 2009, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Anaby, D.; Duncan, I.D.; Smith, C.M.; Cohen, Y. q-Space diffusion MRI (QSI) of the disease progression in the spinal cords of the Long Evans shaker: Diffusion time and apparent anisotropy. NMR Biomed. 2013, 26, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Wehrli, F.W.; Fan, Y.; Hwang, S.N.; Schwartz, E.D.; Nissanov, J.; Hackney, D.B. Assessment of axonal fiber tract architecture in excised rat spinal cord by localized NMR q-space imaging: Simulations and experimental studies. Magn. Reson. Med. 2004, 52, 733–740. [Google Scholar] [CrossRef]
- Farrell, J.A.; Smith, S.A.; Gordon-Lipkin, E.M.; Reich, D.S.; Calabresi, P.A.; van Zijl, P.C. High b-value q-space diffusion-weighted MRI of the human cervical spinal cord in vivo: Feasibility and application to multiple sclerosis. Magn. Reson. Med. 2008, 59, 1079–1089. [Google Scholar] [CrossRef]
- Farrell, J.A.D.; Zhang, J.; Jones, M.V.; DeBoy, C.A.; Hoffman, P.N.; Landman, B.A.; Smith, S.A.; Reich, D.S.; Calabresi, P.A.; van Zijl, P.C.M. q-Space and conventional diffusion imaging of axon and myelin damage in the rat spinal cord after axotomy. Magn. Reson. Med. 2010, 63, 1323–1335. [Google Scholar] [CrossRef]
- David, G.; Mohammadi, S.; Martin, A.R.; Cohen-Adad, J.; Weiskopf, N.; Thompson, A.; Freund, P. Traumatic and non-traumatic spinal cord injury: Pathological insights from neuroimaging. Nat. Rev. Neurol. 2019, 15, 718–731. [Google Scholar] [CrossRef]
- Cory, D.G.; Garroway, A.N. Measurement of translational displacement probabilities by NMR: An indicator for compartmentation. Mag. Reson. Med. 1990, 14, 435–444. [Google Scholar] [CrossRef]
- Leemans, A.; Jeurissen, B.; Sijbers, J. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. Intl. Soc. Mag. Reson. Med. 2009, 17, 3537. [Google Scholar]
- Lee, J.; Hyun, J.; Lee, J.; Choi, E.; Shin, H.; Min, K.; Nam, Y.; Kim, H.J.; Oh, S. So you want to image myelin using MRI: An overview and practical guide for myelin water imaging. J. Magn. Reson. Imaging 2021, 53, 360–373. [Google Scholar] [CrossRef]
- Piredda, G.-F.; Hilbert, T.; Thiran, J.-P.; Kober, T. Probing myelin content of the human brain with MRI: A review. Magn. Reson. Med. 2021, 85, 627–652. [Google Scholar] [CrossRef] [PubMed]
- Lazarei, A.; Lipp, I. Can, MRI measure myelin? Systematic review, qualitative assessment, and meta-analysis of studies validating microstructural imaging with myelin histology. Neuroimage 2021, 230, 117744. [Google Scholar] [CrossRef]
- Saliani, A.; Perraud, B.; Duval, T.; Stikov, N.; Rossignol, S.; Cohen-Adad, J. Axon and myelin morphology in animal and human spinal cord. Front. Neuroanat. 2017, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Saliani, A.; Zaimi, A.; Nami, H.; Duval, T.; Stikov, N.; Cohen-Adad, J. Construction of a rat spinal cord atlas of axon morphometry. Neuroimage 2019, 202, 116156. [Google Scholar] [CrossRef]
- Nilsson, M.; Lasic, S.; Drobnjak, I.; Topgaard, D.; Westin, C.F. Resolution limit of cylinder diameter estimation by diffusion MRI: The impact of gradient waveform and orientation dispersion. NMR Biomed. 2017, 30, e3711. [Google Scholar] [CrossRef]
- Anaby, D.; Morozov, D.; Seroussi, I.; Hametner, S.; Sochen, N.; Cohen, Y. Single- and double-diffusion encoding MRI for studying ex vivo apparent axon diameter distribution in spinal cord white matter. NMR Biomed. 2019, 32, e4170. [Google Scholar] [CrossRef]
- Ong, H.H.; Wright, A.C.; Wehrli, S.L.; Souza, A.; Schwartz, E.D.; Hwang, S.N.; Wehrli, F.W. Indirect measurement of regional axon diameter in excised mouse spinal cord with q-space imaging: Simulation and experimental studies. Neuroimage 2008, 40, 1619–1632. [Google Scholar] [CrossRef]
- Benjamini, D.; Komlosh, M.E.; Holtzclaw, L.A.; Nevo, U.; Basser, P.J. White matter microstructure from nonparametric axon diameter distribution mapping. Neuroimage 2016, 135, 333–344. [Google Scholar] [CrossRef]
- Komlosh, M.; Özarslan, E.; Lizak, M.; Horkayne-Szakaly, I.; Freidlin, R.; Horkay, F.; Basser, P. Mapping average axon diameters in porcine spinal cord white matter and rat corpus callosum using d-PFG MRI. Neuroimage 2013, 78, 210–216. [Google Scholar] [CrossRef]
- Barazany, D.; Basser, P.J.; Assaf, Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain 2009, 132, 1210–1220. [Google Scholar] [CrossRef]
- Veraart, J.; Nunes, D.; Rudrapatna, U.; Fieremans, E.; Jones, D.K.; Novikov, D.S.; Shemesh, N. Noninvasive quantification of axon radii using diffusion MRI. eLife 2020, 9, e48855. [Google Scholar] [CrossRef]
- Ong, H.H.; Wehrli, F.W. Quantifying axon diameter and intra-cellular volume fraction in excised mouse spinal cord with q-space imaging. Neuroimage 2010, 51, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Gast, H.; Horowitz, A.; Krupnik, R.; Barazany, D.; Lifshits, S.; Ben-Amitay, S.; Assaf, Y. A method for in vivo mapping of axonal diameter distributions in the human brain using diffusion-based axonal spectrum imaging (AxSI). Neuroinformatics 2023, 32, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Harkins, K.D.; Beaulieu, C.; Xu, J.; Gore, J.C.; Does, M.D. A simple estimate of axons size with diffusion MRI. Neuroimage 2021, 227, 117619. [Google Scholar] [CrossRef]
- Veraart, J.; Raven, E.P.; Edwards, L.J.; Weiskopf, N.; Jones, D.K. The variability of MR axon radii estimates in the human white matter. Hum. Brain Mapp. 2021, 42, 2201–2213. [Google Scholar] [CrossRef]
- Huang, S.Y.; Nummenmaa, A.; Witzel, T.; Duval, T.; Cohen-Adad, J.; Wald, L.L.; McNab, J.A. The impact of gradient strength on in vivo diffusion MRI estimates of axon diameter. Neuroimage 2015, 220, 1777–1788. [Google Scholar] [CrossRef]
- Yamada, I.; Hikishima, K.; Miyasaka, N.; Tokairin, Y.; Ito, E.; Kawano, T.; Kobayashi, D.; Eishi, Y.; Okano, H. Esophageal carcinoma: Evaluation with q-space diffusion-weighted MR imaging ex vivo. Magn. Reson. Med. 2015, 73, 2262–2273. [Google Scholar] [CrossRef]
- Fujiyoshi, K.; Hikishima, K.; Nakahara, J.; Tsuji, O.; Hata, J.; Konomi, T.; Nagai, T.; Shibata, S.; Kaneko, S.; Iwanami, A.; et al. Application of q-space diffusion MRI for visualization of white matter. J. Neurosci. 2016, 36, 2796–2808. [Google Scholar] [CrossRef]
- Sakai, K.; Yamada, K.; Akazawa, K.; Tazoe, J.; Yasuike, M.; Nagano, H.; Ikeno, H.; Nakagawa, T. Can we shorten the q-space to make it clinically feasible. Jpn. J. Radiol. 2017, 35, 16–24. [Google Scholar] [CrossRef]
- Motegi, H.; Kufukihara, K.; Kitagawa, S.; Sekiguchi, K.; Hata, J.; Fujiwara, H.; Jinzaki, M.; Okano, H.; Nakamura, M.; Iguchi, Y.; et al. Non-lesional white matter changes depicted by q-space diffusional MRI correlate with clinical disabilities in multiple sclerosis. J. Neurol. Sci. 2024, 456, 122851. [Google Scholar] [CrossRef]
- McNab, J.A.; Edlow, B.L.; Witzel, T.; Huang, S.Y.; Bhat, H.; Heberlein, K.; Feiweier, T.; Liu, K.; Keil, B.; Cohen-Adad, J.; et al. The human connectome project and beyond: Initial applications of 300mT/m gradients. Neuroimage 2013, 80, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Witzel, T.; Keil, B.; Scholz, A.; Davids, M.; Dietz, P.; Rummert, E.; Ramb, R.; Kirsch, J.E.; Yendiki, A.; et al. Connectome 2.0: Developing the next generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome. Neuroimage 2021, 243, 118530. [Google Scholar] [CrossRef]
- Grussu, F.; Schneider, T.; Zhang, H.; Alexander, D.C.; Wheeler-Kingshott, C.A.M. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord In Vivo. Neuroimage 2015, 111, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Bejamini, D.; Basser, P.J. Magnetic resonance microdynamic imaging reveal distinct tissue microenvironments. Neuroimage 2017, 163, 183–196. [Google Scholar] [CrossRef]
- Jespersen, S.N.; Oelson, J.L.; Hansen, B.; Shemesh, N. Diffusion time dependence of microstructural parameters in fixed spinal cord. Neuroimage 2018, 182, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Oelson, J.L.; Ostergaard, L.; Shemesh, N.; Jespersen, S.N. Beyond the diffusion standard model in fixed rat spinal cord with combined linear and planar encoding. Neuroimage 2021, 231, 117849. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, M.T.; August, B.K.; Radecki, D.Z.; Reilly, M.; Komro, A.; Svaren, J.; Anaby, D.; Duncan, I.D.; Cohen, Y. QSI and DTI of Inherited White Matter Disorders in Rat Spinal Cord: Early Detection and Comparison with Quantitative Electron Microscopy Findings. Diagnostics 2025, 15, 837. https://doi.org/10.3390/diagnostics15070837
Resende MT, August BK, Radecki DZ, Reilly M, Komro A, Svaren J, Anaby D, Duncan ID, Cohen Y. QSI and DTI of Inherited White Matter Disorders in Rat Spinal Cord: Early Detection and Comparison with Quantitative Electron Microscopy Findings. Diagnostics. 2025; 15(7):837. https://doi.org/10.3390/diagnostics15070837
Chicago/Turabian StyleResende, Maysa Teixeira, Benjamin K. August, Daniel Z. Radecki, Madelyn Reilly, Abigail Komro, John Svaren, Debbie Anaby, Ian D. Duncan, and Yoram Cohen. 2025. "QSI and DTI of Inherited White Matter Disorders in Rat Spinal Cord: Early Detection and Comparison with Quantitative Electron Microscopy Findings" Diagnostics 15, no. 7: 837. https://doi.org/10.3390/diagnostics15070837
APA StyleResende, M. T., August, B. K., Radecki, D. Z., Reilly, M., Komro, A., Svaren, J., Anaby, D., Duncan, I. D., & Cohen, Y. (2025). QSI and DTI of Inherited White Matter Disorders in Rat Spinal Cord: Early Detection and Comparison with Quantitative Electron Microscopy Findings. Diagnostics, 15(7), 837. https://doi.org/10.3390/diagnostics15070837






