Vascular Endothelial Growth Factor Variants (936C/T, 634C/G, 2578A/C) and Their Genotype–Haplotype Association with Recurrent Implantation Failure in Infertile Women: A Single-Center Analytical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Recruitment
2.1.1. Hormone Level Determination
2.1.2. Genetic Methods
2.2. DNA Isolation
2.3. PCR Amplification
2.4. Restriction Fragment Length Polymorphism (RFLP)
Statistical Analysis
3. Results
3.1. Association Between the VEGF–936C/T, VEGF–634C/G, and VEGF–2578C/A Gene Polymorphisms and Recurrent Implantation Failure
3.2. Association of Haplotypes of VEGF Gene Polymorphisms with the Odds of RIF
3.3. Comparisons of Hormones Values by Genotypes of VEGF Gene Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2023, 13, 1061766. [Google Scholar]
- Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; Macklon, N. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [PubMed]
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. RbE 2018, 16, 121. [Google Scholar]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Zeadna, A.; Son, W.Y.; Moon, J.H.; Dahan, M.H. A comparison of biochemical pregnancy rates between women who underwent IVF and fertile controls who conceived spontaneously. Hum. Reprod. 2015, 30, 783–788. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. 2015 Assisted Reproductive Technology National Summary Report; US Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Moragianni, V.A.; Jones, S.M.; Ryley, D.A. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil. Steril. 2012, 98, 102–108. [Google Scholar]
- Orvieto, R.; Meltcer, S.; Nahum, R.; Rabinson, J.; Anteby, E.Y.; Ashkenazi, J. The influence of body mass index on in vitro fertilization outcome. Int. J. Gynaecol. Obstet. 2009, 104, 53–55. [Google Scholar]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6 (Suppl. S2), S125–S140. [Google Scholar] [CrossRef]
- Waylen, A.L.; Metwally, M.; Jones, G.L.; Wilkinson, A.J.; Ledger, W.L. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: A meta-analysis. Hum. Reprod. Update 2009, 15, 31–44. [Google Scholar] [PubMed]
- Künzle, R.; Mueller, M.D.; Hänggi, W.; Birkhäuser, M.H.; Drescher, H.; Bersinger, N.A. Semen quality of male smokers and nonsmokers in infertile couples. Fertil. Steril. 2003, 79, 287–291. [Google Scholar] [PubMed]
- Li, D.; Zheng, L.; Zhao, D.; Xu, Y.; Wang, Y. The role of immune cells in recurrent spontaneous abortion. Reprod. Sci. 2021, 28, 3303–3315. [Google Scholar] [CrossRef]
- Mrozikiewicz, A.E.; Ozarowski, M.; Jedrzejczak, P. Biomolecular markers of recurrent implantation failure—A review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1747. [Google Scholar]
- Cakiroglu, Y.; Tiras, B. Determining diagnostic criteria and cause of recurrent implantation failure. Curr. Opin. Obstet. Gynecol. 2020, 32, 198–204. [Google Scholar]
- Sheikhansari, G.; Pourmoghadam, Z.; Danaii, S.; Mehdizadeh, A.; Yousefi, M. Etiology and management of recurrent implantation failure: A focus on intra-uterine PBMC-therapy for RIF. J. Reprod. Immunol. 2020, 139, 103121. [Google Scholar]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar]
- Sugino, N.; Kashida, S.; Karube-Harada, A.; Takiguchi, S.; Kato, H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction 2002, 123, 379–387. [Google Scholar] [CrossRef]
- Rowe, A.J.; Wulff, C.; Fraser, H.M. Localization of mRNA for vascular endothelial growth factor (VEGF), angiopoietins and their receptors during the peri-implantation period and early pregnancy in marmosets (Callithrix jacchus). Reproduction 2003, 126, 227–238. [Google Scholar] [CrossRef]
- Kapiteijn, K.; Koolwijk, P.; van der Weiden, R.M.F.; van Nieuw Amerongen, G.; Plaisier, M.; van Hinsbergh, V.W.M.; Helmerhorst, F.M. Human embryo-conditioned medium stimulates in-vitro endometrial angiogenesis. Fertil. Steril. 2006, 85, 1232–1239. [Google Scholar] [PubMed]
- Shibuya, M. VEGF-VEGFR System as a Target for Suppressing Inflammation and other Diseases. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 135–144. [Google Scholar] [PubMed]
- Edgell, T.A.; Evans, J.; Lazzaro, L.; Boyes, K.; Sridhar, M.; Catt, S.; Rombauts, L.J.F.; Vollenhoven, B.J.; Salamonsen, L.A. Assessment of potential biomarkers of pre-receptive and receptive endometrium in uterine fluid and a functional evaluation of the potential role of CSF3 in fertility. Cytokine 2018, 111, 222–229. [Google Scholar] [PubMed]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583. [Google Scholar]
- Alitalo, K.; Carmeliet, P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002, 1, 219–227. [Google Scholar]
- Kruessel, J.S.; Behr, B.; Milki, A.A.; Hirchenhain, J.; Wen, Y.; Bielfeld, P.; Polan, M.L. Vascular endothelial growth factor (VEGF) mRNA splice variants are differentially expressed in human blastocysts. Mol. Hum. Reprod. 2001, 7, 57–63. [Google Scholar]
- Goodman, C.; Jeyendran, R.S.; Coulam, C.B. Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod. BioMedicine Online 2008, 16, 720–723. [Google Scholar]
- Bansal, R.; Ford, B.; Bhaskaran, S.; Thum, M.; Bansal, A. Elevated Levels of Serum Vascular Endothelial Growth Factor-A Are Not Related to NK Cell Parameters in Recurrent IVF Failure. J. Reprod. Infertil. 2017, 18, 280–287. [Google Scholar]
- Chen, X.; Man, G.C.W.; Liu, Y.; Wu, F.; Huang, J.; Li, T.C.; Wang, C.C. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am. J. Reprod. Immunol. 2017, 78, e12693. [Google Scholar]
- Gao, Y.; Wang, P.L. Increased CD56+ NK cells and enhanced Th1 responses in human unexplained recurrent spontaneous abortion. Genet. Mol. Res. 2015, 14, 18103–18109. [Google Scholar]
- Santi, A.; Felser, R.S.; Mueller, M.D.; Wunder, D.M.; McKinnon, B.; Bersinger, N.A. Increased endometrial placenta growth factor (PLGF) gene expression in women with successful implantation. Fertil. Steril. 2011, 96, 663–668. [Google Scholar] [PubMed]
- Cottrell, H.N.; Wu, J.; Rimawi, B.H.; Duran, J.M.; Spencer, J.B.; Sidell, N.; Rajakumar, A. Human endometrial stromal cell plasticity: Reversible sFlt1 expression negatively coincides with decidualization. Hypertens. Pregnancy 2017, 36, 204–211. [Google Scholar]
- Zeng, H.; Hu, L.; Xie, H.; Ma, W.; Quan, S. Polymorphisms of vascular endothelial growth factor and recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 304, 297–307. [Google Scholar]
- Jung, Y.W.; Ahn, E.H.; Kim, J.O.; An, H.J.; Cho, S.H.; Kim, Y.R. Association of genetic polymorphisms in VEGF -460, -7 and -583 and hematocrit level with the development of idiopathic recurrent pregnancy loss and a meta-analysis. J. Gene Med. 2018, 20, e3048. [Google Scholar]
- Boudjenah, R.; Molina-Gomes, D.; Wainer, R.; de Ma-zancourt, P.; Selva, J.; Vialard, F. The vascular endo-thelial growth factor (VEGF) +405 G/C polymor-phism and its relationship with recurrent implanta-tion failure in women in an IVF programme with ICSI. J. Assist. Reprod. Genet. 2012, 29, 1415–1420. [Google Scholar]
- Gupta, P.; Deo, S.; Jaiswar, S.P.; Sankhwar, P.L. Case control study to compare serum vascular endo-thelial growth factor (VEGF) level in women with recurrent pregnancy loss (RPL) compared to women with term pregnancy. J. Obstet. Gynaecol. India. 2019, 69 (Suppl. S2), 95–102. [Google Scholar] [PubMed]
- Zhao, J.; Li, D.; Tang, H.; Tang, L. Association of vascular endothelial growth factor polymorphisms with polycystic ovarian syndrome risk: A meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 18. [Google Scholar]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of Anti-VEGF/VEGFR Agents on Animal Models of Endometriosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar]
- Turienzo, A.; Lledó, B.; Ortiz, J.A.; Morales, R.; Sanz, J.; Llácer, J.; Bernabeu, R. Prevalence of candidate single nucleotide polymorphisms on p53, IL-11, IL-10, VEGF and APOE in patients with repeated implantation failure (RIF) and pregnancy loss (RPL). Hum. Fertil. 2020, 23, 117–122. [Google Scholar]
- Kim, O.J.; Hong, S.H.; Oh, S.H.; Kim, T.G.; Min, K.T.; Oh, D.; Kim, N.K. Association between VEGF polymorphisms and homocysteine levels in patients with ischemic stroke and silent brain infarction. Stroke 2011, 42, 2393–2402. [Google Scholar]
- Watson, C.J.; Webb, N.J.; Bottomley, M.J.; Brenchley, P.E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: Correlation with variation in VEGF protein production. Cytokine 2000, 12, 1232–1235. [Google Scholar] [PubMed]
- Wongpiyabovorn, J.; Hirankarn, N.; Ruchusatsawat, K.; Yooyongsatit, S.; Benjachat, T.; Avihingsanon, Y. The association of single nucleotide polymorphism within vascular endothelial growth factor gene with systemic lupus erythematosus and lupus nephritis. Int. J. Immunogenet. 2011, 38, 63–67. [Google Scholar]
- Hansen, T.F.; Spindler, K.G.; Lorentzen, K.A.; Olsen, D.A.; Andersen, R.F.; Lindebjerg, J.; Brandslund, I.; Jakobsen, A. The importance of −460 C/T and +405 G/C single nucleotide polymorphisms to the function of vascular endothelial growth factor A in colorectal cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 751–758. [Google Scholar]
- Awata, T.; Inoue, K.; Kurihara, S.; Ohkubo, T.; Watanabe, M.; Inukai, K.; Inoue, I.; Katayama, S. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 2002, 51, 1635–1639. [Google Scholar] [PubMed]
- Li, Y.Z.; Wang, L.J.; Li, X.; Li, S.L.; Wang, J.L.; Wu, Z.H.; Gong, L.; Zhang, X.D. Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: An updated systematic review and meta-analysis of 14 case-control studies. Genet. Mol. Res. 2013, 12, 1035–1044. [Google Scholar] [PubMed]
- Papazoglou, D.; Galazios, G.; Koukourakis, M.I.; Kontomanolis, E.N.; Maltezos, E. Association of 634G/C and 936C/T. Polymorphisms of the vascular endothelial Growth factor with spontaneous preterm Delivery. Acta Obs. Gynecol. Scand. 2004, 83, 461–465. [Google Scholar]
- Liu, Q.; Li, Y.; Zhao, J.; Sun, D.L.; Duan, Y.N.; Wang, N.; Zhou, R.M.; Kang, S. Association of polymorphisms 21154G/A and 22578C/A in the vascular endothelial growth factor gene with decreased risk of endometriosis in Chinese women. Hum. Reprod. 2009, 24, 2660–2666. [Google Scholar]
- Moreno, V.; Gonzalez, J.; Pelegri, D. SNPassoc: SNPs-Based Whole Genome Association Studies. R Package Version 2.1-0. 2022. Available online: https://CRAN.R-project.org/package=SNPassoc (accessed on 15 March 2025).
- Daniel, S.; Sinnwell, J.P. haplo.stats: Statistical Analysis of Haplotypes with Traits and Covariates When Linkage Phase is Ambiguous. R Package Version 1.9.5.1. 2024. Available online: https://CRAN.R-project.org/package=haplo.stats (accessed on 25 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 25 March 2025).
- Don, E.E.; Middelkoop, M.A.; Hehenkamp, W.J.K.; Mijatovic, V.; Griffioen, A.W.; Huirne, J.A.F. Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids-A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7011. [Google Scholar] [CrossRef]
- Kasius, A.; Smit, J.G.; Torrance, H.L.; Eijkemans, M.J.; Mol, B.W.; Opmeer, B.C.; Broekmans, F.J.M. Endometrial thickness and pregnancy rates after IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 530–541. [Google Scholar]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar]
- Chloe, J.; Peach, V.M.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.O.; Jeon, Y.J.; An, H.J.; Lee, H.A.; Kim, J.H.; Ahn, E.H.; Lee, W.S.; Kim, N.K. Association between vascular endothelial growth factor promoter polymorphisms and the risk of recurrent implantation failure. Exp. Ther. Med. 2018, 15, 2109–2119. [Google Scholar] [PubMed]
- Vagnini, L.D.; Nascimento, A.M.; Canas, M.C.; Renzi, A.; Oliveira-Pelegrin, G.R.; Petersen, C.G.; Mauri, A.L.; Oliveira, J.B.; Baruffi, R.L.; Cavagna, M.; et al. The relationship between vascular endothelial growth factor 1154G/A polymorphism and recurrent implantation failure. Med. Princ. Pract. 2015, 24, 533–537. [Google Scholar] [PubMed]
- Jung, Y.W.; Kim, J.O.; Rah, H.; Kim, J.H.; Kim, Y.R.; Lee, Y.; Lee, W.S.; Kim, N.K. Genetic variants of vascular endothelial growth factor are associated with recurrent implantation failure in Korean women. Reprod. Biomed. Online 2016, 32, 190–196. [Google Scholar]
- Mrozikiewicz, A.E.; Kurzawinska, G.; Walczak, O.M.; Ozegowska, K.; Jedrzejczak, P. Polymorphic Variants of Genes Encoding Angiogenesis-Related Factors in InfertileWomen with Recurrent Implantation Failure. Int. J. Mol. Sci. 2023, 24, 4267. [Google Scholar] [CrossRef]
- Lambrechts, D.; Storkebaum, E.; Morimoto, M.; Del-Favero, J.; Desmet, F.; Marklund, S.L.; Wyns, S.; Thijs, V.; Andersson, J.; van Marion, I.; et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 2003, 34, 383–394. [Google Scholar]
- Shahbazi, M.; Fryer, A.A.; Pravica, V.; Brogan, I.J.; Ramsay, H.M.; Hutchinson, I.V.; Harden, P.N. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J. Am. Soc. Nephrol. 2002, 13, 260–264. [Google Scholar]
- Ben Salem, A.; Megdich, F.; Kacem, O.; Souayeh, M.; Ben Ali, F.H.; Hizem, S.; Janhai, F.; Ajina, M.; Abu-Elmagd, M.; Assidi, M.; et al. Vascular endothelial growth factor (VEGFA) gene variation in polycystic ovary syndrome in a Tunisian women population. BMC Genom. 2016, 17, 748. [Google Scholar]
- Maziotis, E.; Kalampokas, T.; Giannelou, P.; Grigoriadis, S.; Rapani, A.; Anifantakis, M.; Kotsifaki, A.; Pantou, A.; Triantafyllidou, O.; Tzanakaki, D.; et al. Commercially Available Molecular Approaches to Evaluate Endometrial Receptivity: A Systematic Review and Critical Analysis of the Literature. Diagnostics 2022, 12, 2611. [Google Scholar] [CrossRef]


| Sequences of Primers | Conc. of Primers (μM) | MgCl2 (mM) | Anneal. Temp. (C) | PCR (bp) | Restriction Enzyme/ Digest. Temp./Time | Alleles |
|---|---|---|---|---|---|---|
| VEGF-936C/T | ||||||
| FW: 5′-AAGGAAGAGGAGACTCTGCGCAGAGC-3′ RV: 5′-TAAATGTATGTATGTGGGTGGGTGTGTCTACAG-3′ | 0.3 | 2.0 | 68.2 | 208 | NlaIII/37 °C/3 h | C936: 208 bp T936: 122, 86 bp |
| VEGF-634C/G | ||||||
| FW: 5′-ATTTATTTTTGCTTGCCATT-3′ RV: 5′-GTCTGTCTGTCTGTCCGTCA-3′ | 0.4 | 2.0 | 53.7 | 304 | BsmFI/65 °C/1 h | C634: 304 bp G634: 193, 111 bp |
| VEGF-2578C/A | ||||||
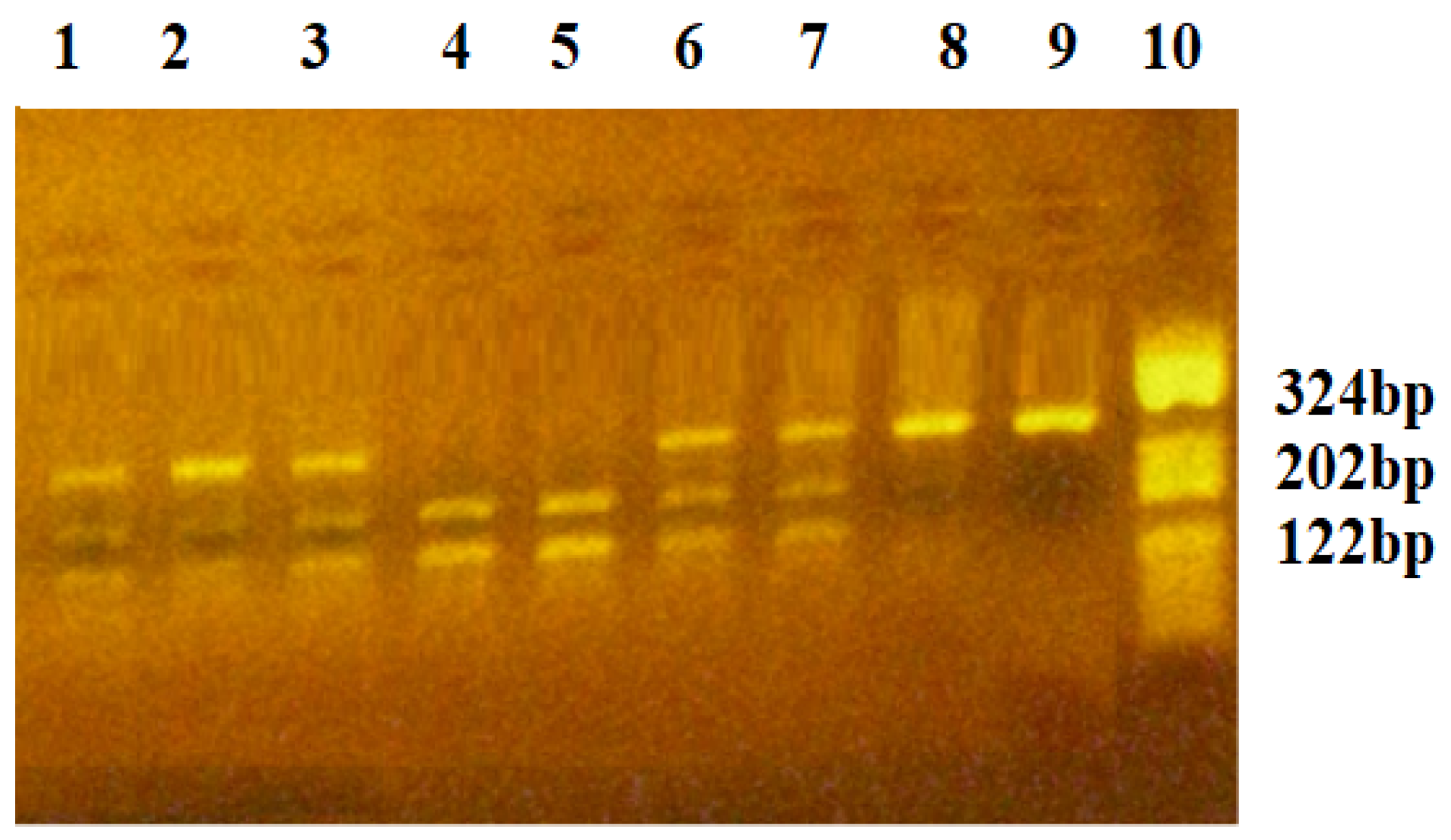
| FW: 5′-GGATGGGGCTGACT AGGTAAGC-3′ Rv: 5′-AGCCCCCTTTTCCT CCAAC-3′ | 0.2 | 2.0 | 67.2 | 324 | BglII/37 °C/3 h | C2758: 324 bp A2758: 202, 122 bp |
| Characteristics | Control Women (n1 = 44) | Infertile Women with RIF (n2 = 41) | p-Value |
|---|---|---|---|
| Age, years (1) | 33.16 (4.03) | 35.51 (3.33) | 0.0045 * |
| BMI, kg/m2 (1) | 23.09 (1.40) | 23.51 (1.79) | 0.2287 |
| Smoking, n (%) (3) | 6 (13.6) | 5 (12.2) | 0.8432 |
| Previous implantation failures (2) | - | 4 [3, 4] | - |
| Infertility diagnosis, n (%) (3) | 0.0451 * | ||
| Male | 7 (15.9) | 10 (24.4) | |
| Idiopathic | 14 (31.8) | 17 (41.5) | |
| Trompe | 9 (20.5) | 11 (26.8) | |
| PCOS | 14 (31.8) | 3 (7.3) | |
| Infertility duration, years (1) | 3.16 (1.95) | 6.05 (1.75) | <0.0001 * |
| Thickness of endometrium (1) | 8.95 (1.65) | 9.36 (2.36) | 0.3613 |
| Endometrium volume (2) | 4.10 [3.10, 6.13] | 4.68 [3.20, 5.10] | 0.8741 |
| Severe menorrhagia, n (%) (3) | 3 (6.8) | 7 (17.1) | 0.1858 |
| Severe dysmenorrhea, n (%) (3) | 6 (13.6) | 13 (31.7) | 0.0457 * |
| Adenomyosis, n (%) (3) | 14 (31.8) | 16 (39.0) | 0.4872 |
| Testosterone (ng/mL) (2) | 0.40 [0.20, 0.68] | 0.30 [0.20, 0.40] | 0.0550 |
| Progesterone (ng/mL) (2) | 0.30 [0.20, 0.40] | 0.30 [0.20, 0.30] | 0.0487 * |
| FSH (mUI/mL) (2) | 7.10 [6.20, 8.15] | 7.30 [6.20, 8.10] | 0.6036 |
| LH * (mUI/mL) (2) | 6.70 [6.20, 7.33] | 5.60 [5.10, 6.80] | 0.0008 * |
| Estradiol (ng/mL) (2) | 63.00 [54.50, 72.00] | 59.00 [46.00, 72.00] | 0.1956 |
| AMH (ng/mL) (1) | 2.07 (1.08) | 1.51 (1.01) | 0.0144 * |
| Genotypes | Control Group (n1 = 44) | RIF Group (n2 = 41) | OR [95% CI] | p | pFDR (a) | Adjusted OR (b) [95% CI] | p | pFDR (a) | AIC |
|---|---|---|---|---|---|---|---|---|---|
| VEGF–936C/T | 0.0711 | 0.1599 | 0.1059 | 0.2383 | 112.9 | ||||
| CC | 33 (75.0) | 21 (51.2) | Reference | Reference | |||||
| CT | 9 (20.5) | 17 (41.5) | 2.97 [1.12, 7.88] | 2.91 [1.05, 8.08] | |||||
| TT | 2 (4.5) | 3 (7.3) | 2.36 [0.36, 15.31] | 1.85 [0.28, 12.33] | |||||
| Dominant (CC vs. CT+TT) | 11 (25.0) | 20 (48.8) | 2.86 [1.14, 7.15] | 0.0222 * | 0.0667 | 2.70 [1.04, 7.00] | 0.0383 * | 0.1149 | 111.1 |
| Recessive (CC+CT vs. TT) | 2 (4.5) | 3 (7.3) | 1.66 [0.26, 10.46] | 0.5876 | 0.8620 | 1.30 [0.20, 8.40] | 0.7849 | 0.9964 | 115.3 |
| HWE p-value | 0.2141 | 1.000 | |||||||
| VEGF–634C/G | 0.8620 | 0.8620 | 0.9561 | 0.9964 | 117.3 | ||||
| CC | 30 (68.2) | 27 (65.9) | Reference | Reference | |||||
| CG | 11 (25.0) | 12 (29.3) | 1.21 [0.46, 3.20] | 1.06 [0.38, 2.93] | |||||
| GG | 3 (6.8) | 2 (4.9) | 0.74 [0.11, 4.77] | 0.78 [0.11, 5.39] | |||||
| Dominant (CC vs. CG+GG) | 14 (31.8) | 14 (24.2) | 1.11 [0.45, 2.75] | 0.8195 | 0.8620 | 1.00 [0.39, 2.59] | 0.9964 | 0.9964 | 115.4 |
| Recessive (CC+CG vs. GG) | 3 (6.8) | 2 (4.9) | 0.70 [0.11, 4.42] | 0.7029 | 0.8620 | 0.76 [0.11, 5.19] | 0.7809 | 0.9964 | 115.3 |
| HWE p-value | 0.1744 | 0.6265 | |||||||
| VEGF–2578C/A | 0.0192 * | 0.0667 | 0.0163 * | 0.0734 | 109.2 | ||||
| CC | 20 (45.5) | 12 (29.3) | Reference | Reference | |||||
| CA | 19 (43.2) | 14 (34.1) | 1.23 [0.45, 3.32] | 1.05 [0.37, 3.01] | |||||
| AA | 5 (11.4) | 15 (36.6) | 5.00 [1.45, 17.27] | 5.28 [1.42, 19.65] | |||||
| Dominant (CC vs. CA+AA) | 24 (54.6) | 29 (70.7) | 2.01 [0.82, 4.94] | 0.1223 | 0.2201 | 1.86 [0.73, 4.76] | 0.1923 | 0.3461 | 113.7 |
| Recessive (CC+CA vs. AA) | 5 (11.4) | 15 (36.6) | 4.50 1.46, 13.89] | 0.0054 * | 0.0486 | 5.15 [1.55, 17.09] | 0.0041 * | 0.0369 * | 107.2 |
| HWE p-value | 1.000 | 0.0582 |
| Estimated Haplotypes | HF in All Sample | HF in Control Group | HF in RIF Group | OR (a) [95% CI] | p-Value | OR (b) [95% CI] | p |
|---|---|---|---|---|---|---|---|
| Three-locus haplotypes (VEGF-936C/T-634C/G-2578C/A) | |||||||
| C-C-C | 0.3841 | 0.4566 | 0.3462 | Reference haplotype | Reference haplotype | ||
| C-C-A | 0.2970 | 0.2561 | 0.2988 | 1.07 [0.49, 2.32] | 0.8683 | 1.05 [0.47, 2.33] | 0.9031 |
| C-G-A | 0.0255 | 0.0251 | 0.0487 | NA | <0.0001 | NA | 0.5036 |
| C-G-C | 0.0817 | 0.1145 | 0.0258 | NA | NA | NA | <0.0001 |
| T-C-A | 0.0523 | 0.0127 | 0.1351 | 12.39 [1.39, 110.44] | 0.0241 * | 8.51 [0.93, 78.01] | 0.0582 |
| T-C-C | 0.0725 | 0.0815 | 0.0248 | NA | <0.0001 | NA | <0.0001 |
| T-G-A | 0.0547 | 0.0357 | 0.0540 | NA | 0.7921 | 8.48 [0.12, 621.75] | 0.3293 |
| T-G-C | 0.0323 | 0.0179 | 0.0666 | NA | <0.0001 | NA | <0.0001 |
| Two-locus haplotypes | |||||||
| VEGF 936C/T-634C/G (1) | |||||||
| C-C | 0.6791 | 0.7096 | 0.6500 | Reference haplotype | Reference haplotype | ||
| C-G | 0.1091 | 0.1427 | 0.0695 | 0.39 [0.11, 1.39] | 0.1479 | 0.32 [0.09, 1.13] | 0.0772 |
| T-C | 0.1267 | 0.0973 | 0.1549 | 1.43 [0.50, 4.05] | 0.5003 | 1.11 [0.38, 3.28] | 0.8519 |
| T-G | 0.0850 | 0.0505 | 0.1256 | 3.66 [0.90, 14.89] | 0.0700 | 4.22 [0.98, 18.12] | 0.0531 |
| VEGF 634C/G-2578C/A (2) | |||||||
| C-C | 0.4582 | 0.5390 | 0.3713 | Reference haplotype | Reference haplotype | ||
| C-A | 0.3477 | 0.2679 | 0.4336 | 1.87 [0.96, 3.67] | 0.0671 | 1.81 [0.90, 3.63] | 0.0965 |
| G-A | 0.0817 | 0.0617 | 0.1030 | 2.96 [0.66, 13.37] | 0.1577 | 3.10 [0.72, 13.31] | 0.1276 |
| G-C | 0.1124 | 0.1315 | 0.0921 | 0.74 [0.20, 2.76] | 0.6556 | 0.64 [0.18, 2.30] | 0.4935 |
| VEGF 936C/T-2578C/A (3) | |||||||
| C-C | 0.4611 | 0.5450 | 0.3642 | Reference haplotype | Reference haplotype | ||
| C-A | 0.3271 | 0.3073 | 0.3553 | 1.51 [0.75, 3.03] | 0.2506 | 1.52 [0.73, 3.14] | 0.2592 |
| T-A | 0.1023 | 0.0222 | 0.1813 | 12.23 [1.79, 83.62] | 0.0107 * | 9.56 [1.67, 54.84] | 0.0113 * |
| T-C | 0.1095 | 0.1255 | 0.0992 | 1.14 [0.39, 3.30] | 0.8129 | 1.10 [0.38, 3.15] | 0.8603 |
| Characteristics | RIF Group | Control Group | ||||
|---|---|---|---|---|---|---|
| VEGF–936C/T genotypes | CC (n1 = 21) | CT+TT (n2 = 20) | p | CC (n1 = 33) | CT+TT (n2 = 11) | p |
| Testosterone (ng/mL) (a) | 0.3 [0.2, 0.4] | 0.3 [0.2, 0.3] | 0.3986 | 0.3 [0.2, 0.6] | 0.4 [0.3, 1.1] | 0.5467 |
| Progesterone (ng/mL) (a) | 0.3 [0.2, 0.3] | 0.25 [0.2, 0.325] | 0.8278 | 0.3 [0.3, 0.4] | 0.3 [0.2, 0.4] | 0.5077 |
| FSH (mUI/mL) (a) | 7.9 [6.9, 8.8] | 6.6 [6.1, 7.9] | 0.0948 | 7.1 [6.2, 8.1] | 7.1 [6.3, 8.9] | 0.6643 |
| LH (mUI/mL) (b) | 5.4 [4.8, 6.4] | 6.3 [5.2, 6.8] | 0.3145 | 6.7 [6.3, 7.1] | 7.1 [6.1, 8.6] | 0.8068 |
| Estradiol (ng/mL) (a) | 61.0 [49.0, 72.0] | 55.5 [42.0, 73.8] | 0.6665 | 63.0 [53.0, 72.0] | 64.0 [55.0, 75.0] | 0.7446 |
| AMH (ng/mL) (b) | 1.4 (1.2) | 1.6 (0.8) | 0.5674 | 1.9 (1.0) | 2.3 (1.3) | 0.371 |
| VEGF–634C/G genotypes | CC (n1 = 27) | CG+GG (n2 = 14) | CC (n1 = 30) | CG+GG (n2 = 14) | ||
| Testosterone (ng.mL) (a) | 0.2 [0.2, 0.4] | 0.3 [0.3, 0.4] | 0.0707 | 0.5 [0.3, 1.1] | 0.3 [0.2, 0.4] | 0.0167 * |
| Progesterone (ng/mL) (a) | 0.3 [0.2, 0.3] | 0.3 [0.3, 0.4] | 0.3819 | 0.3 [0.3, 0.4] | 0.3 [0.2, 0.38] | 0.4017 |
| FSH (mUI/mL) (a) | 7.4 [6.8, 8,9] | 6.4 [6.1, 7.9] | 0.1605 | 6.8 [6.1, 8.1] | 7.5 [6.9, 9.5] | 0.0553 |
| LH (mUI/mL) (b) | 5.7 [4.9, 6.9] | 5.5 [5.2, 6.4] | 0.8256 | 6.6 [6.1, 7.1] | 7.0 [6.4, 8.1] | 0.1362 |
| Estradiol (ng/mL) (a) | 5.7 [4.9, 6.9] | 5.5 [5.2, 6.4] | 0.8688 | 61.5 [53.5, 72.8] | 64.0 [61.5, 71.3] | 0.8697 |
| AMH (ng/mL) (b) | 1.4 (1.1) | 1.7 (0.8) | 0.4374 | 2.3 (1.0) | 1.5 (0.98) | 0.0140 * |
| VEGF–2578C/A genotypes | CC (n1 = 12) | CA+AA (n2 = 29) | CC (n1 = 20) | CA+AA (n2 = 24) | ||
| Testosterone (ng.mL) (a) | 0.3 [0.2, 0.6] | 0.3 [0.2, 0.4] | 0.5077 | 0.6 [0.2, 1.0] | 0.3 [0.2, 0.4] | 0.2837 |
| Progesterone (ng/mL) (a) | 0.3 [0.2, 0.3] | 0.3 [0.2, 0.3] | 0.8227 | 0.3 [0.3, 0.4] | 0.3 [0.2, 0.4] | 0.5160 |
| FSH (mUI/mL) (a) | 6.9 [5.9, 7.5] | 7.9 [6.4, 8.8] | 0.1215 | 6.9 [6.0, 8.4] | 7.1 [6.4, 7.9] | 0.9812 |
| LH (mUI/mL) (b) | 6.0 [5.4, 6.8] | 5.4 [4.9, 6.8] | 0.3436 | 6.6 [6.3, 7.5] | 6.9 [6.2, 7.3] | 0.9906 |
| Estradiol (ng/mL) (a) | 55.0 [45.0, 69.0] | 61.0 [48.0, 79.0] | 0.4910 | 63.0 [55.0, 78.0] | 63.5 [52.0, 72.0] | 0.6368 |
| AMH (ng/mL) (b) | 1.6 (1.3) | 1.5 (0.9) | 0.7190 | 2.2 (1.2) | 1.9 (0.9) | 0.4631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procopciuc, L.M.; Iancu, M.; Caracostea, G.V.; Goidescu, I.; Staicu, A.; Lucaciu, R.L.; Hangan, A.C.; Gog Bogdan, S.; Surcel, M. Vascular Endothelial Growth Factor Variants (936C/T, 634C/G, 2578A/C) and Their Genotype–Haplotype Association with Recurrent Implantation Failure in Infertile Women: A Single-Center Analytical Study. Diagnostics 2025, 15, 868. https://doi.org/10.3390/diagnostics15070868
Procopciuc LM, Iancu M, Caracostea GV, Goidescu I, Staicu A, Lucaciu RL, Hangan AC, Gog Bogdan S, Surcel M. Vascular Endothelial Growth Factor Variants (936C/T, 634C/G, 2578A/C) and Their Genotype–Haplotype Association with Recurrent Implantation Failure in Infertile Women: A Single-Center Analytical Study. Diagnostics. 2025; 15(7):868. https://doi.org/10.3390/diagnostics15070868
Chicago/Turabian StyleProcopciuc, Lucia Maria, Mihaela Iancu, Gabriela Valentina Caracostea, Iulian Goidescu, Adelina Staicu, Roxana Liana Lucaciu, Adriana Corina Hangan, Sidonia Gog Bogdan, and Mihai Surcel. 2025. "Vascular Endothelial Growth Factor Variants (936C/T, 634C/G, 2578A/C) and Their Genotype–Haplotype Association with Recurrent Implantation Failure in Infertile Women: A Single-Center Analytical Study" Diagnostics 15, no. 7: 868. https://doi.org/10.3390/diagnostics15070868
APA StyleProcopciuc, L. M., Iancu, M., Caracostea, G. V., Goidescu, I., Staicu, A., Lucaciu, R. L., Hangan, A. C., Gog Bogdan, S., & Surcel, M. (2025). Vascular Endothelial Growth Factor Variants (936C/T, 634C/G, 2578A/C) and Their Genotype–Haplotype Association with Recurrent Implantation Failure in Infertile Women: A Single-Center Analytical Study. Diagnostics, 15(7), 868. https://doi.org/10.3390/diagnostics15070868







.jpg)


