Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms
Abstract
:1. Introduction
2. Materials and Methods
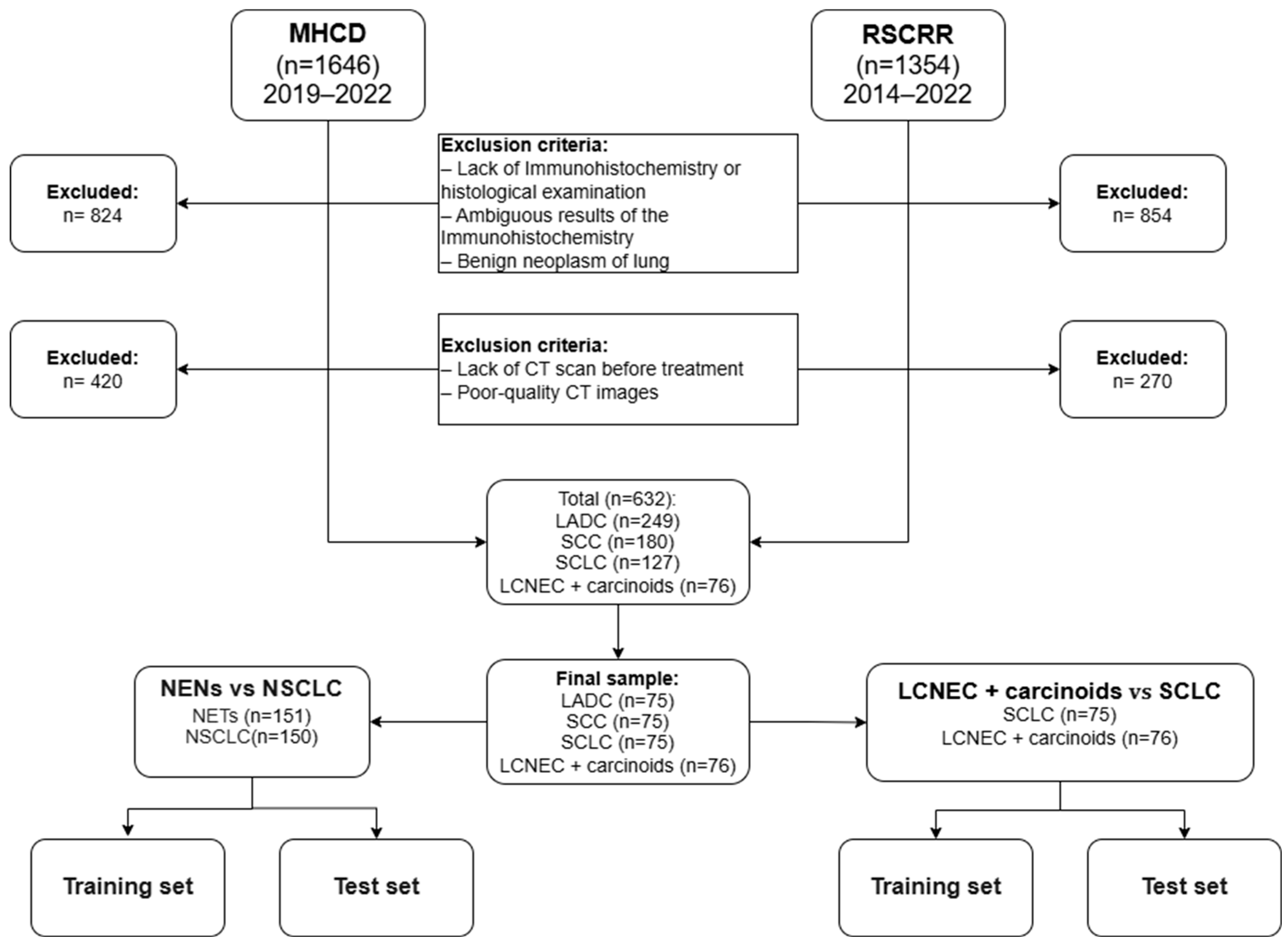
2.1. Patients’ Selection
2.2. Radiomics Feature Extraction and Radiological Signs Assessment
2.3. Model Development
2.4. Statistical Analyses
3. Results
3.1. Patient’s Characteristics
3.1.1. Model for Differentiating NENs from NSCLC
3.1.2. Model for Differentiating SCLC from Other NENs
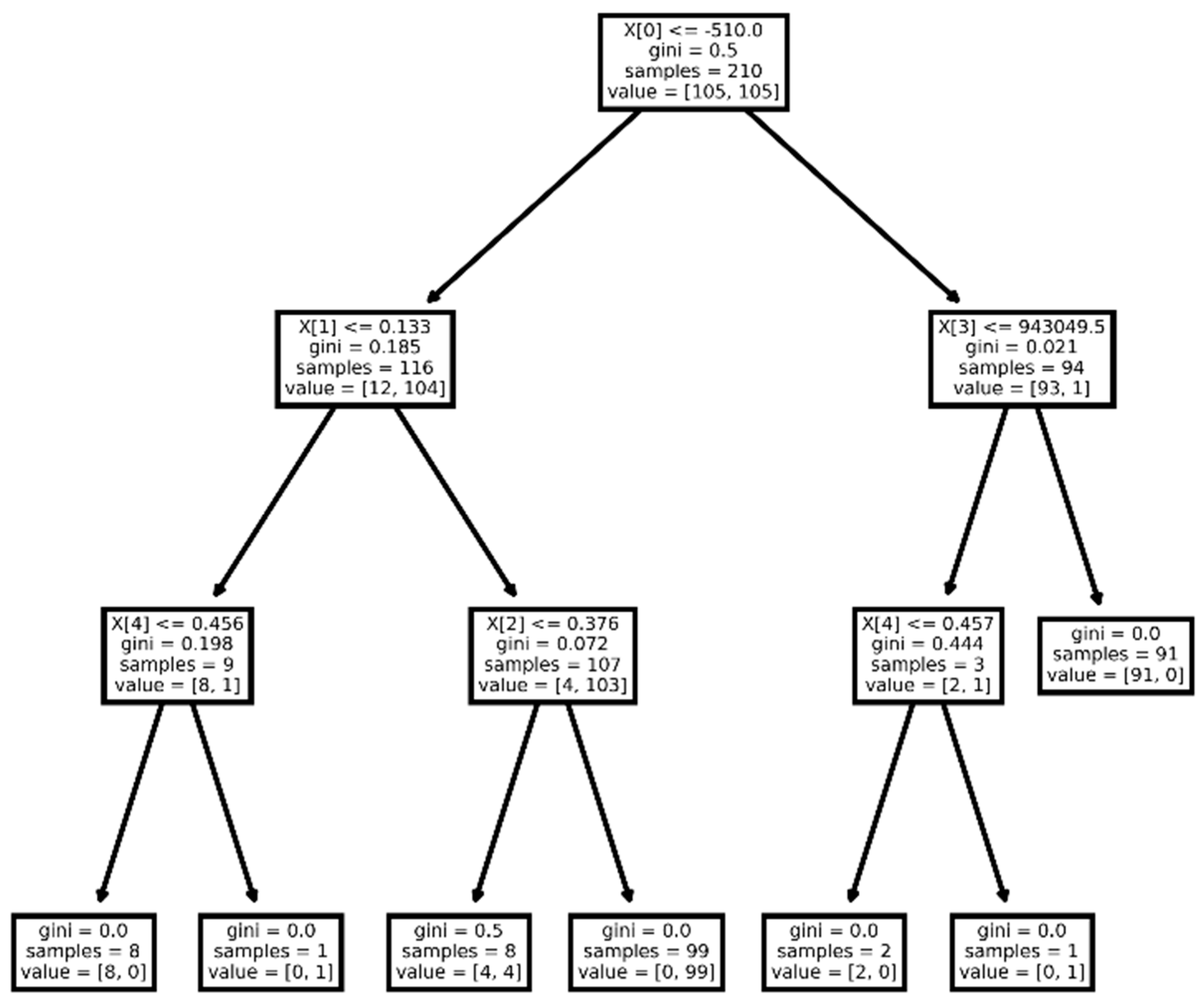
3.2. Model Construction
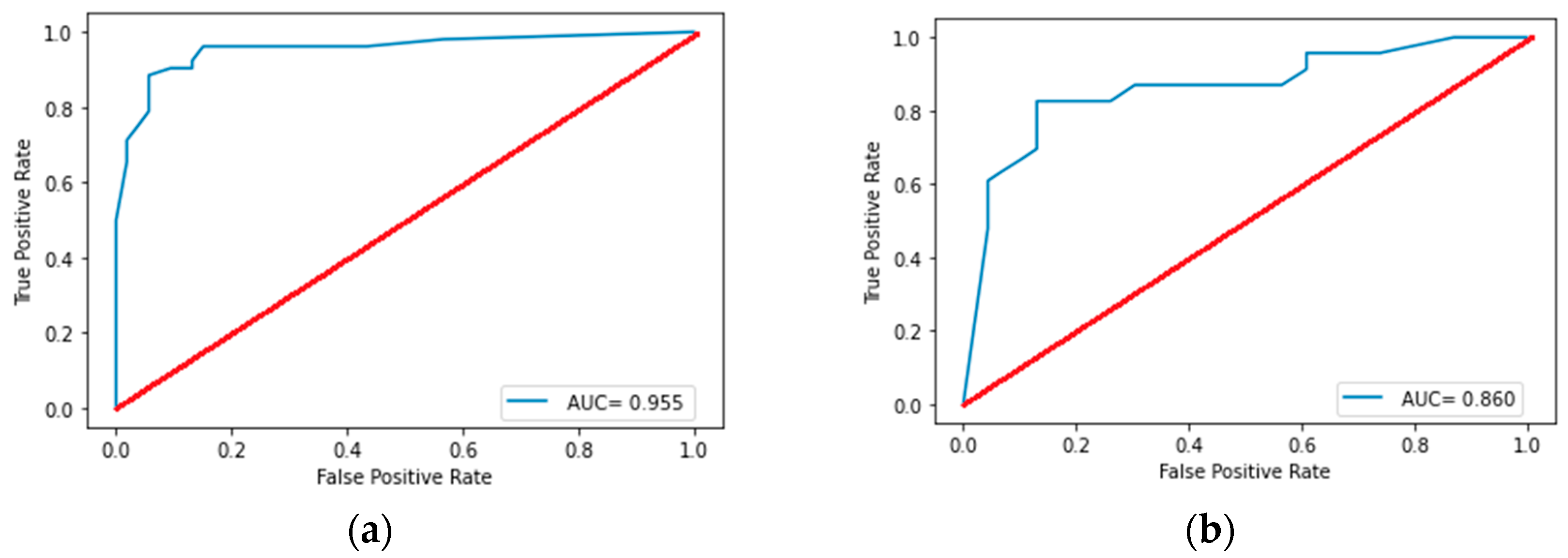
3.2.1. Model for Differentiating NENs from NSCLC
3.2.2. Model for Differentiating SCLC from Other NENs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Atypical carcinoid |
| AUC | Area under the receiver operating characteristic curve |
| CT | Computed tomography |
| GLCM | Gray level co-occurrence matrix |
| GLDM | Gray level dependence matrix |
| GLRLM | Gray level run length matrix |
| GLSZM | Gray level size zone matrix |
| IQR | Interquartile range |
| LADC | Lung adenocarcinoma |
| LC | Lung cancer |
| LCNEC | Large cell neuroendocrine carcinoma |
| MHCD | Moscow Healthcare Department |
| NENs | Neuroendocrine neoplasms |
| NETs | Neuroendocrine tumors |
| NGTDM | Neighboring gray tone difference matrix |
| NSCLC | Non-small cell lung cancer |
| RSCRR | Russian Scientific Center of Roentgenoradiology |
| SCC | Squamous cell cancer |
| SCLC | Small cell lung cancer |
| TC | Typical carcinoid |
References
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Stahel, R.; Peters, S.; Garassino, M. Thoracic Tumours Essentials for Clinicians, 2nd ed.; ESMO Press: Lugano, Switzerland, 2014; pp. 1–6. [Google Scholar]
- Hendriks, L.E.L.; Remon, J.; Faivre-Finn, C.; Garassino, M.C.; Heymach, J.V.; Kerr, K.M.; Tan, D.S.W.; Veronesi, G.; Reck, M. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Laktionov, K.K.; Breder, V.V. Rak Legkogo; GRANAT: Moscow, Russia, 2020; pp. 17–29. (In Russian) [Google Scholar]
- Savu, C.; Melinte, A.; Diaconu, C.; Stiru, O.; Gherghiceanu, F.; Tudorica, Ș.D.O.; Dumitrașcu, O.C.; Bratu, A.; Balescu, I.; Bacalbasa, N. Lung neuroendocrine tumors: A systematic literature review (Review). Exp. Ther. Med. 2022, 23, 176. [Google Scholar] [CrossRef]
- Vocino Trucco, G.; Righi, L.; Volante, M.; Papotti, M. Updates on lung neuroendocrine neoplasm classification. Histopathology 2024, 84, 67–85. [Google Scholar] [CrossRef]
- Hung, Y.P. Neuroendocrine Tumors of the Lung: Updates and Diagnostic Pitfalls. Surg. Pathol. Clin. 2019, 12, 1055–1071. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours: WHO Classification of Tumours, 5th ed.; IARC: Lyon, France, 2021. [Google Scholar]
- Ferguson, M.K.; Landreneau, R.J.; Hazelrigg, S.R.; Altorki, N.K.; Naunheim, K.S.; Zwischenberger, J.B.; Kent, M.; Yim, A.P. Long-term outcome after resection for bronchial carcinoid tumors. Eur. J. Cardiothorac. Surg. 2000, 18, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Krelbaum, T.; Yellin, A.; Bendayan, D.; Saute, M.; Glazer, M.; Kramer, M.R. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001, 119, 1647–1651. [Google Scholar] [CrossRef]
- Agra, Y.; Pelayo, M.; Sacristan, M.; Sacristán, A.; Serra, C.; Bonfill, X. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst. Rev. 2003, 4, CD001990. [Google Scholar] [CrossRef]
- Jeremic, B.; Shibamoto, Y.; Nikolic, N.; Milicic, B.; Milisavljevic, S.; Dagovic, A.; Aleksandrovic, J.; Radosavljevic-Asic, G. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J. Clin. Oncol. 1999, 17, 2092–2099. [Google Scholar] [CrossRef]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J. Thorac. Oncol. 2020, 15, 1577–1598. [Google Scholar] [CrossRef]
- PDQ Screening and Prevention Editorial Board. Lung Cancer Prevention (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2024. [Google Scholar]
- Panunzio, A.; Sartori, P. Lung Cancer and Radiological Imaging. Curr. Radiopharm. 2020, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, A.K.; Gaitanis, A.; Gkiozos, I.; Athanasiadis, E.I.; Chatziioannou, S.N.; Syrigos, K.N.; Thanos, D.; Chatziioannou, A.N.; Papanikolaou, N. Radiomics/Radiogenomics in Lung Cancer: Basic Principles and Initial Clinical Results. Cancers 2022, 14, 1657. [Google Scholar] [CrossRef]
- Cozzi, D.; Bicci, E.; Cavigli, E.; Danti, G.; Bettarini, S.; Tortoli, P.; Mazzoni, L.N.; Busoni, S.; Pradella, S.; Miele, V. Radiomics in pulmonary neuroendocrine tumours (NETs). Radiol. Med. 2022, 127, 609–615. [Google Scholar] [CrossRef]
- Bicci, E.; Cozzi, D.; Cavigli, E.; Ruzga, R.; Bertelli, E.; Danti, G.; Bettarini, S.; Tortoli, P.; Mazzoni, L.N.; Busoni, S.; et al. Reproducibility of CT radiomic features in lung neuroendocrine tumours (NETs) patients: Analysis in a heterogeneous population. Radiol. Med. 2023, 128, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Metovic, J.; Papotti, M.; Volante, M.; Molinari, F.; et al. Diagnostic Value of Conventional PET Parameters and Radiomic Features Extracted from 18F-FDG-PET/CT for Histologic Subtype Classification and Characterization of Lung Neuroendocrine Neoplasms. Biomedicines 2021, 9, 281. [Google Scholar] [CrossRef]
- Adelsmayr, G.; Janisch, M.; Müller, H.; Holzinger, A.; Talakic, E.; Janek, E.; Streit, S.; Fuchsjäger, M.; Schöllnast, H. Three dimensional computed tomography texture analysis of pulmonary lesions: Does radiomics allow differentiation between carcinoma, neuroendocrine tumor and organizing pneumonia? Eur. J. Radiol. 2023, 165, 110931. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wang, S.; Yang, S.; Zhang, G.; Xu, Y.; Yang, H.; Shan, F. CT radiomics to differentiate neuroendocrine neoplasm from adenocarcinoma in patients with a peripheral solid pulmonary nodule: A multicenter study. Front. Oncol. 2024, 17, 1420213. [Google Scholar] [CrossRef]
- Trahtenberg, A.H.; Kolbanov, K.I. “Rak legkogo” Prakticheskaya Pulmonologiya, No. 4; GEOTAR-Media: Moscow, Russia, 2008; pp. 3–9. (In Russian) [Google Scholar]
- Shulkin, I.M.; Vladzimirskij, A.V. Data-based management in imaging: Evaluation of the performance of a unified radiological information service model. Manager Zdravoohranenia. 2022, 7, 68–80. (In Russian) [Google Scholar] [CrossRef]
- Certificate of State Registration of the Computer Program No. 2025610804 Russian Federation. Dataset Curation Platform: No. 2024691653: Application. 20.12.2024: Publ. 14.01.2025 / Vasilev.Yu.A., A.V. Vladzimirsky, Omelyanskaya, O.V. [etc.]; Applicant State Budgetary Healthcare Institution of the City of Moscow “Scientific and Practical Clinical Center for Diagnostics and Telemedicine Technologies of the Moscow Healthcare Department” (GBUZ “NPKTs DiT DZM”). Available online: https://elibrary.ru/item.asp?id=80277623 (accessed on 29 March 2025).
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Volterrani, L.; Perrella, A.; Bagnacci, G.; Di Meglio, N.; Di Martino, V.; Bertelli, P.; Bellan, C.; Mazzei, M.A.; Luzzi, L. Washout-Computed Tomography Discriminates Pulmonary “Fat-poor” Hamartomas From Neuroendocrine Neoplasms: A Simple Method in the Radiomics Era. J. Thorac. Imaging 2023, 38, 278–285. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Hou, J.; Xiong, Z.; Lin, H.; Wu, S.; Yu, X. Differentiating Peripherally Located Pulmonary Noncalcified Hamartoma From Carcinoid Using CT Radiomics Approaches. J. Comput. Assist. Tomogr. 2023, 47, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Habert, P.; Decoux, A.; Chermati, L.; Gibault, L.; Thomas, P.; Varoquaux, A.; Le Pimpec-Barthes, F.; Arnoux, A.; Juquel, L.; Chaumoitre, K.; et al. Best imaging signs identified by radiomics could outperform the model: Application to differentiating lung carcinoid tumors from atypical hamartomas. Insights Imaging 2023, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Khoruzhaya, A.N.; Ahkmad, E.S.; Semenov, D.S. The role of the quality control system for diagnostics of oncological diseases in radiomics. Digit. Diagn. 2021, 2, 170–184. [Google Scholar] [CrossRef]
- Vasilev, Y.A.; Vladzimirskyy, A.V.; Omelyanskaya, O.V.; Reshetnikov, R.V.; Blokhin, I.A.; Kodenko, M.M.; Nanova, O.G. Review of meta-analyses on the use of artificial intelligence in radiology. Med. Vis. 2024, 28, 22–41. (In Russian) [Google Scholar] [CrossRef]
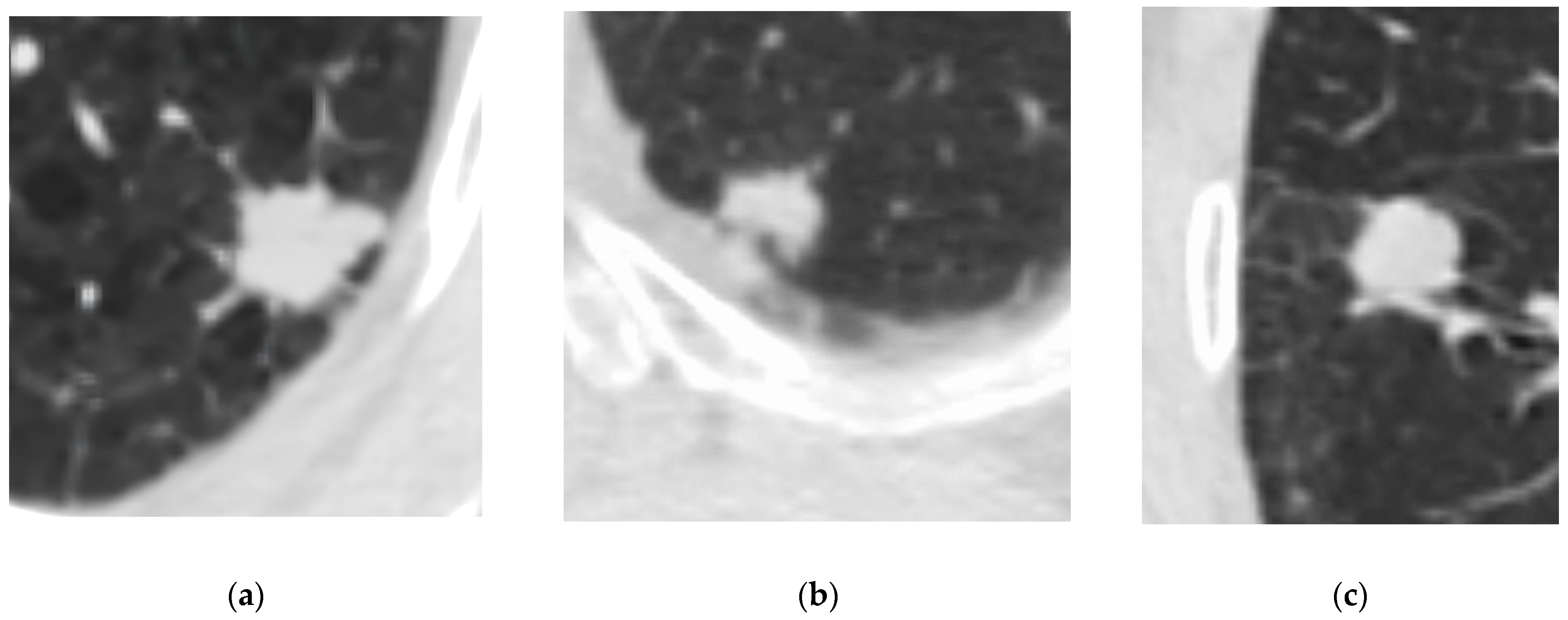
| Characteristic | Total (n = 301) | NSCLC (n = 150) | NENs (n = 151) | p-Value |
|---|---|---|---|---|
| Age (y) | 66.0 [59.0; 71.0] | 67.0 [62.7; 72.0] | 64.0 [57.0; 70.0] | 0.002 |
| Sex (male) | 194 (65.5%) | 105 (70.0%) | 89 (58.9%) | 0.054 |
| Lung (right) | 185 (61.5%) | 93 (62.0%) | 92 (60.9%) | 0.565 |
| Lobe (upper) | 155 (51.5%) | 92 (61.3%) | 63 (41.7%) | 0.001 |
| Lobe (lower) | 118 (39.2%) | 50 (33.3%) | 68 (45.0%) | <0.001 |
| Lobe (middle) | 28 (9.3%) | 8 (5.3%) | 20 (13.3%) | 0.004 |
| Spiculation | 204 (67.8%) | 133 (88.7%) | 71 (47.0%) | <0.001 |
| Pleural indentation | 201 (66.8%) | 129 (86.0%) | 72 (47.7%) | <0.001 |
| Decay cavities | 25 (8.3%) | 22 (14.7%) | 5 (3.3%) | 0.001 |
| Characteristic | Total (n = 151) | Other NENs (n = 76) | SCLC (n = 75) | p-Value |
|---|---|---|---|---|
| Age (y) | 64 [57.0; 70.0] | 64.0 [57.0; 70.0] | 64.0 [57.0; 71.0] | 0.681 |
| Sex (male) | 89 (58.9%) | 29 (38.2%) | 60 (80.0%) | <0.001 |
| Lung (right) | 92 (60.1%) | 57 (75.0%) | 35 (46.7%) | 0.001 |
| Lobe (upper) | 63 (41.7%) | 23 (30.1%) | 40 (53.3%) | 0.001 |
| Lobe (lower) | 68 (45.0%) | 37 (48.7%) | 31 (41.3%) | 0.365 |
| Lobe (middle) | 20 (13.2%) | 16 (21.1%) | 4 (5.3%) | 0.004 |
| Spiculation | 71 (47.0%) | 21 (27.6%) | 50 (66.7%) | <0.001 |
| Pleural indentation | 72 (47.7%) | 30 (39.5%) | 42 (56.0%) | <0.001 |
| Decay cavities | 5 (3.3%) | 2 (2.6%) | 3 (4.0%) | 0.681 |
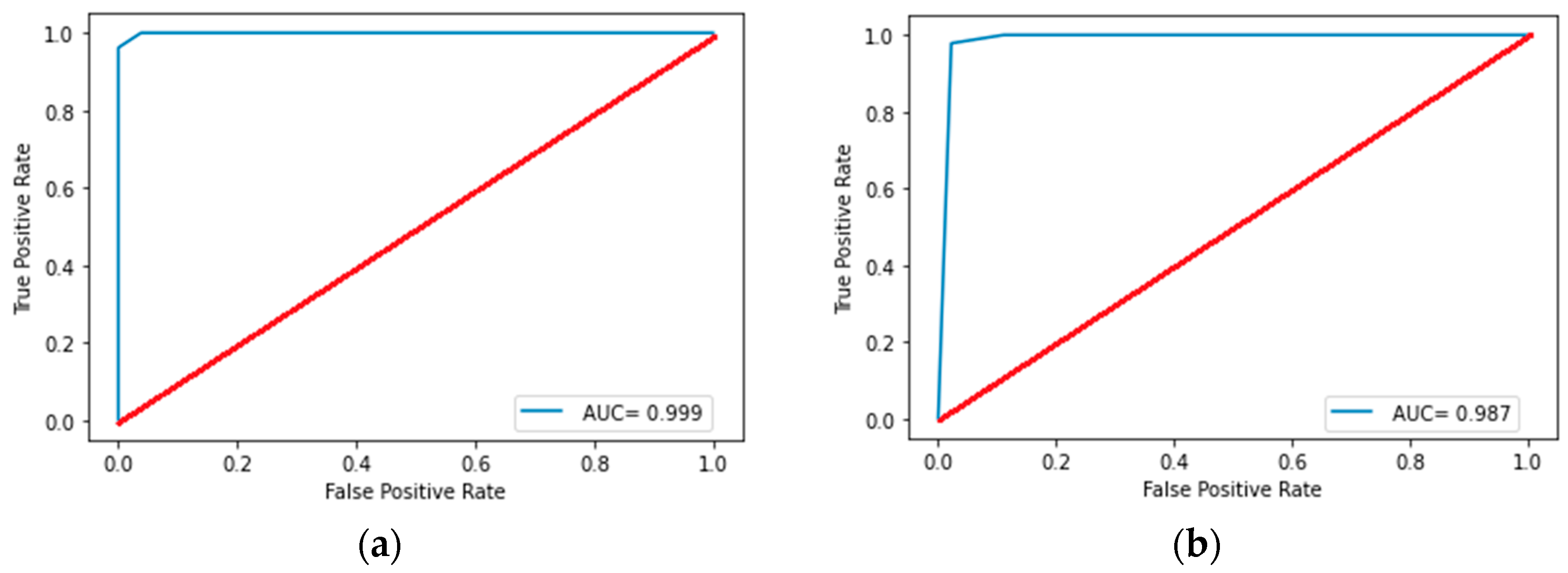
| AUC | Precision | Recall | F1-Score | Accuracy | ||
|---|---|---|---|---|---|---|
| Decision tree | Training set | 0.999 [0.983; 1.00] | 1.00 [0.983; 1.00] | 0.962 [0.926; 0.983] | 0.981 [0.952; 0.995] | 0.981 [0.952; 0.995] |
| Test set | 0.988 [0.940; 0.999] | 0.978 [0.922; 0.997] | 0.978 [0.922; 0.997] | 0.978 [0.922; 0.997] | 0.978 [0.922; 0.997] | |
| Random forest | Training set | 0.997 [0.974; 1.00] | 0.958 [0.920; 0.980] | 0.986 [0.959; 0.997] | 0.972 [0.939; 0.989] | 0.967 [0.933; 0.987] |
| Test set | 0.966 [0.906; 0.993] | 0.967 [0.906; 0.993] | 0.967 [0.906; 0.993] | 0.967 [0.906; 0.993] | 0.962 [0.905; 0.993] | |
| Logistic regression | Training set | 0.997 [0.974; 1.00] | 0.979 [0.952; 0.995] | 0.986 [0.959; 0.997] | 0.982 [0.952; 0.995] | 0.980 [0.952; 0.995] |
| Test set | 0.961 [0.891; 0.989] | 0.950 [0.891; 0.989] | 0.934 [0.861; 0.975] | 0.942 [0.875; 0.982] | 0.934 [0.861; 0.975] | |
| Gradient boosting | Training set | 0.999 [0.983; 1.00] | 0.972 [0.939; 0.989] | 1.00 [0.983; 1.00] | 0.986 [0.959; 0.997] | 0.984 [0.959; 0.997] |
| Test set | 0.972 [0.906; 0.993] | 0.952 [0.891; 0.989] | 0.967 [0.905; 0.993] | 0.959 [0.891; 0.989] | 0.953 [0.891; 0.989] |
| AUC | Precision | Recall | F1-Score | Accuracy | ||
|---|---|---|---|---|---|---|
| Random forest | Training set | 0.955 [0.892; 0.984] | 0.862 [0.786; 0.925] | 0.962 [0.905; 0.990] | 0.909 [0.832; 0.953] | 0.904 [0.832; 0.953] |
| Test set | 0.860 [0.732; 0.950] | 0.826 [0.680; 0.920] | 0.826 [0.680; 0.920] | 0.826 [0.680; 0.920] | 0.826 [0.680; 0.920] | |
| Decision tree | Training set | 0.726 [0.631; 0.809] | 0.653 [0.552; 0.741] | 0.942 [0.881; 0.979] | 0.771 [0.682; 0.849] | 0.724 [0.631; 0.809] |
| Test set | 0.739 [0.581; 0.854] | 0.657 [0.511; 0.800] | 1.00 [0.921; 1.00] | 0.793 [0.654; 0.904] | 0.739 [0.581; 0.854] | |
| Logistic regression | Training set | 0.853 [0.767; 0.911] | 0.755 [0.662; 0.833] | 0.769 [0.682; 0.849] | 0.762 [0.672; 0.841] | 0.762 [0.672; 0.841] |
| Test set | 0.747 [0.605; 0.871] | 0.667 [0.511; 0.800] | 0.609 [0.443; 0.743] | 0.636 [0.488; 0.781] | 0.652 [0.488; 0.781] | |
| Gradient boosting | Training set | 0.989 [0.949; 1.00] | 0.944 [0.811; 0.979] | 0.981 [0.934; 0.998] | 0.962 [0.906; 0.990] | 0.962 [0.906; 0.990] |
| Test set | 0.685 [0.534; 0.818] | 0.680 [0.534; 0.818] | 0.739 [0.581; 0.854] | 0.708 [0.557; 0.836] | 0.696 [0.534; 0.818] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, A.; Karelidze, D.; Ivannikov, M.; Shakhvalieva, E.; Sultanova, P.; Arzamasov, K.; Nudnov, N.; Vasilev, Y. Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms. Diagnostics 2025, 15, 874. https://doi.org/10.3390/diagnostics15070874
Borisov A, Karelidze D, Ivannikov M, Shakhvalieva E, Sultanova P, Arzamasov K, Nudnov N, Vasilev Y. Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms. Diagnostics. 2025; 15(7):874. https://doi.org/10.3390/diagnostics15070874
Chicago/Turabian StyleBorisov, Aleksandr, David Karelidze, Mikhail Ivannikov, Elina Shakhvalieva, Peri Sultanova, Kirill Arzamasov, Nikolai Nudnov, and Yuriy Vasilev. 2025. "Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms" Diagnostics 15, no. 7: 874. https://doi.org/10.3390/diagnostics15070874
APA StyleBorisov, A., Karelidze, D., Ivannikov, M., Shakhvalieva, E., Sultanova, P., Arzamasov, K., Nudnov, N., & Vasilev, Y. (2025). Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms. Diagnostics, 15(7), 874. https://doi.org/10.3390/diagnostics15070874






