Role of Artificial Intelligence in the Diagnosis and Management of Pulmonary Embolism: A Comprehensive Review
Abstract
1. Introduction
2. What Is Artificial Intelligence?
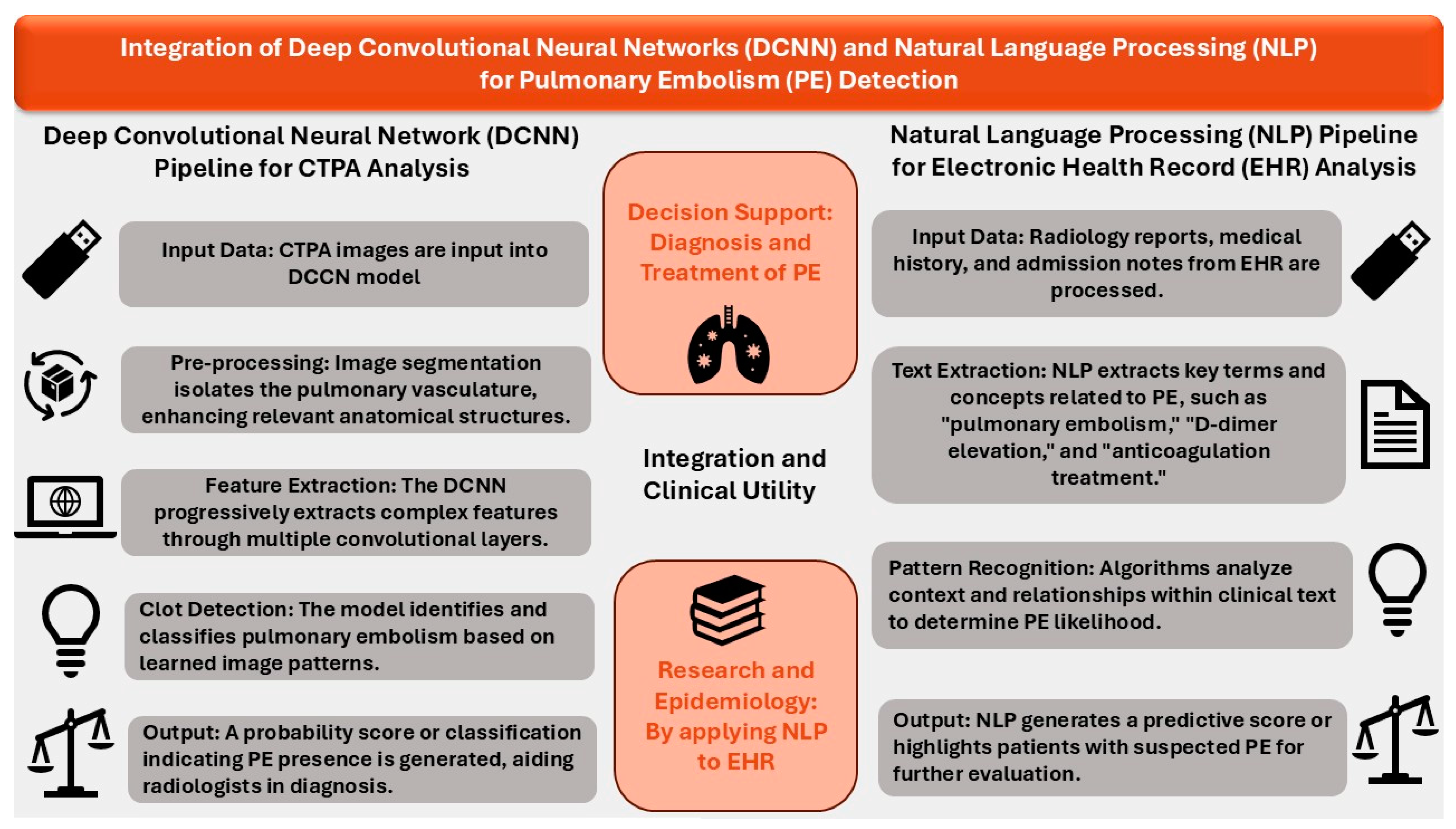
3. AI Models Using Convolutional Neural Networks (CNNs)
3.1. Enhanced CTPA-Based Diagnosis of PE Using FDA-Approved AIDOC Models
3.2. Alternate DCNN-Based Models for Improved CTPA-Based Diagnosis of PE
4. AI Models Using NLP
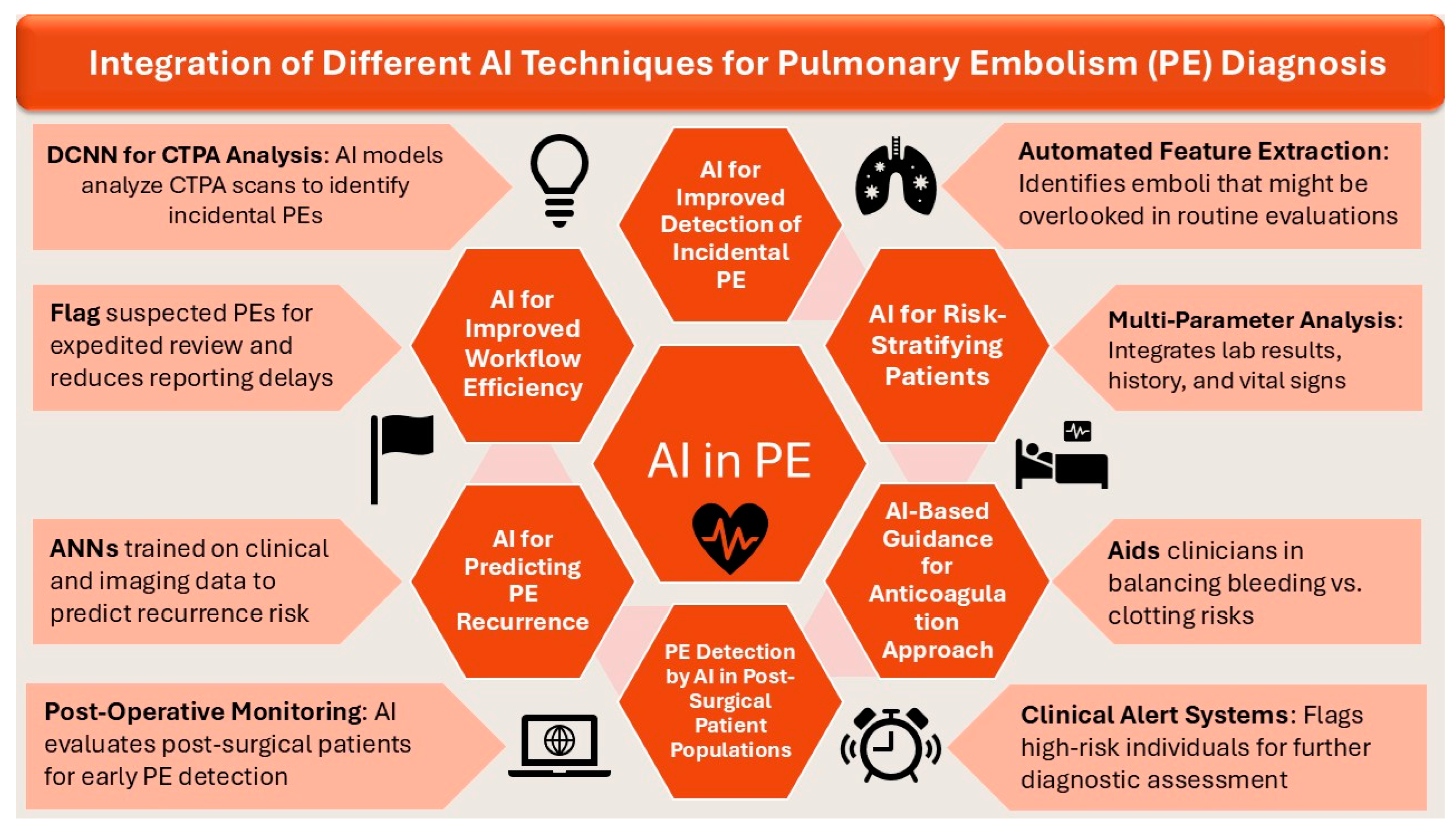
5. Role of AI in Enhanced Diagnosis and Management of PE
5.1. AI for Improved Detection of Incidental PE
5.2. AI for Improved Workflow Efficiency
5.3. AI for Predicting PE Recurrence
5.4. AI for Risk-Stratifying Patients
5.5. AI-Based Guidance for Anticoagulation Approach
5.6. PE Detection by AI in Post-Surgical Patient Populations
6. Current Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous Thromboembolism: A Public Health Concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef]
- Mahan, C.E.; Borrego, M.E.; Woersching, A.L.; Federici, R.; Downey, R.; Tiongson, J.; Bieniarz, M.C.; Cavanaugh, B.J.; Spyropoulos, A.C.; Mahan, C.E. Venous thromboembolism: Annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb. Haemost. 2012, 108, 291–302. [Google Scholar] [PubMed]
- Snyder, D.J.; Zilinyi, R.S.; Cohen, D.J.; Parikh, S.A.; Sethi, S.S. Patient-Reported Outcomes in Venous Thromboembolism: A Systematic Review of the Literature, Current Challenges, and Ways Forward. J. Am. Heart. Assoc. 2023, 12, e032146. [Google Scholar] [PubMed]
- Stein, P.D.; Matta, F.; Alrifai, A.; Rahman, A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb. Res. 2012, 130, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.K.; Schmidt, M.; Pedersen, L.; Horváth–Puhó, E.; Sørensen, H.T. 30-Year Mortality After Venous Thromboembolism. Circulation 2014, 130, 829–836. [Google Scholar] [CrossRef]
- Nguyen, E.T.; Hague, C.; Manos, D.; Memauri, B.; Souza, C.; Taylor, J.; Dennie, C. Canadian Society of Thoracic Radiology/Canadian Association of Radiologists Best Practice Guidance for Investigation of Acute Pulmonary Embolism, Part 2: Technical Issues and Interpretation Pitfalls. Can. Assoc. Radiol. J. 2022, 73, 214–227. [Google Scholar]
- Hutchinson, B.D.; Navin, P.; Marom, E.M.; Truong, M.T.; Bruzzi, J.F. Overdiagnosis of Pulmonary Embolism by Pulmonary CT Angiography. AJR Am. J. Roentgenol. 2015, 205, 271–277. [Google Scholar]
- Ayobi, A.; Chang, P.D.; Chow, D.S.; Weinberg, B.D.; Tassy, M.; Franciosini, A.; Scudeler, M.; Quenet, S.; Avare, C.; Chaibi, Y. Performance and clinical utility of an artificial intelligence-enabled tool for pulmonary embolism detection. Clin. Imaging 2024, 113, 110245. [Google Scholar]
- Chen, M.; Decary, M. Artificial intelligence in healthcare: An essential guide for health leaders. Healthc. Manag. Forum 2020, 33, 10–18. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, P.M.; Ohno-Machado, L.; Chapman, W.W. Natural language processing: An introduction. J. Am. Med. Inform. Assoc. JAMIA 2011, 18, 544–551. [Google Scholar]
- Friedman, C.; Hripcsak, G. Natural language processing and its future in medicine. Acad. Med. J. Assoc. Am. Med. Coll. 1999, 74, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Duan, N.; Liu, S.; Shum, H.Y. Progress in Neural NLP: Modeling, Learning, and Reasoning. Engineering 2020, 6, 275–290. [Google Scholar] [CrossRef]
- Chen, J.H.; Asch, S.M. Machine Learning and Prediction in Medicine—Beyond the Peak of Inflated Expectations. N. Engl. J. Med. 2017, 376, 2507–2509. [Google Scholar] [CrossRef]
- Buls, N.; Watté, N.; Nieboer, K.; Ilsen, B.; de Mey, J. Performance of an artificial intelligence tool with real-time clinical workflow integration—Detection of intracranial hemorrhage and pulmonary embolism. Phys. Med. 2021, 83, 154–160. [Google Scholar] [CrossRef]
- Ben Cheikh, A.; Gorincour, G.; Nivet, H.; May, J.; Seux, M.; Calame, P.; Thomson, V.; Delabrousse, E.; Crombé, A. How artificial intelligence improves radiological interpretation in suspected pulmonary embolism. Eur. Radiol. 2022, 32, 5831–5842. [Google Scholar] [CrossRef]
- Langius-Wiffen, E.; de Jong, P.A.; Hoesein, F.A.M.; Dekker, L.; Hoven, A.F.v.D.; Nijholt, I.M.; Boomsma, M.F.; Veldhuis, W.B. Retrospective batch analysis to evaluate the diagnostic accuracy of a clinically deployed AI algorithm for the detection of acute pulmonary embolism on CTPA. Insights Imaging 2023, 14, 102. [Google Scholar] [CrossRef]
- Zaazoue, K.A.; McCann, M.R.; Ahmed, A.K.; Cortopassi, I.O.; Erben, Y.M.; Little, B.P.; Stowell, J.T.; Toskich, B.B.; Ritchie, C.A. Evaluating the Performance of a Commercially Available Artificial Intelligence Algorithm for Automated Detection of Pulmonary Embolism on Contrast-Enhanced Computed Tomography and Computed Tomography Pulmonary Angiography in Patients with Coronavirus Disease 2019. Mayo Clin. Proc. Innov. Qual. Outcomes 2023, 7, 143–152. [Google Scholar]
- Verma, A.A.; Masoom, H.; Pou-Prom, C.; Shin, S.; Guerzhoy, M.; Fralick, M.; Mamdani, M.; Razak, F. Developing and validating natural language processing algorithms for radiology reports compared to ICD-10 codes for identifying venous thromboembolism in hospitalized medical patients. Thromb. Res. 2022, 209, 51–58. [Google Scholar]
- Petry, M.; Lansky, C.; Chodakiewitz, Y.; Maya, M.; Pressman, B. Decreased Hospital Length of Stay for ICH and PE After Adoption of an Artificial Intelligence-Augmented Radiological Worklist Triage System. Radiol. Res. Pract. 2022, 2022, 2141839. [Google Scholar] [PubMed]
- Kharawala, A.M.; Varrias, D.; Nagraj, S.; Karamanis, D.; Seo, J.; Vegivinti, C.T.R.; Barzallo, D.; Romero, G.H.; Demirhan, Y.E.; Balasubramanian, P.; et al. Abstract 14932: Prediction of Pulmonary Embolism Using Random Forest Algorithm: A Study on 917 Patients Over a Period of 1 Year in a New York City Public Hospital. Circulation 2023, 148 (Suppl. S1), A14932. [Google Scholar]
- Mora, D.; Nieto, J.A.; Mateo, J.; Bikdeli, B.; Barco, S.; Trujillo-Santos, J.; Soler, S.; Font, L.; Bosevski, M.; Monreal, M.; et al. Machine Learning to Predict Outcomes in Patients with Acute Pulmonary Embolism Who Prematurely Discontinued Anticoagulant Therapy. Thromb. Haemost. 2022, 122, 570–577. [Google Scholar] [CrossRef]
- Soffer, S.; Klang, E.; Shimon, O.; Barash, Y.; Cahan, N.; Greenspana, H.; Konen, E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15814. [Google Scholar]
- Weikert, T.; Winkel, D.J.; Bremerich, J.; Stieltjes, B.; Parmar, V.; Sauter, A.W.; Sommer, G. Automated detection of pulmonary embolism in CT pulmonary angiograms using an AI-powered algorithm. Eur. Radiol. 2020, 30, 6545–6553. [Google Scholar] [PubMed]
- Prevedello, L.M.; Erdal, B.S.; Ryu, J.L.; Little, K.J.; Demirer, M.; Qian, S.; White, R.D. Automated Critical Test Findings Identification and Online Notification System Using Artificial Intelligence in Imaging. Radiology 2017, 285, 923–931. [Google Scholar]
- Nagel, S.; Sinha, D.; Day, D.; Reith, W.; Chapot, R.; Papanagiotou, P.; Warburton, E.A.; Guyler, P.; Tysoe, S.; Fassbender, K.; et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int. J. Stroke Off. J. Int. Stroke. Soc. 2017, 12, 615–622. [Google Scholar]
- Ebrahimian, S.; Digumarthy, S.R.; Homayounieh, F.; Bizzo, B.C.; Dreyer, K.J.; Kalra, M.K. Predictive values of AI-based triage model in suboptimal CT pulmonary angiography. Clin. Imaging 2022, 86, 25–30. [Google Scholar]
- Jin, Z.G.; Zhang, H.; Tai, M.H.; Yang, Y.; Yao, Y.; Guo, Y.T. Natural Language Processing in a Clinical Decision Support System for the Identification of Venous Thromboembolism: Algorithm Development and Validation. J. Med. Internet Res. 2023, 25, e43153. [Google Scholar]
- Woller, B.; Daw, A.; Aston, V.; Lloyd, J.; Snow, G.; Stevens, S.M.; Woller, S.C.; Jones, P.; Bledsoe, J. Natural Language Processing Performance for the Identification of Venous Thromboembolism in an Integrated Healthcare System. Clin. Appl. Thromb. 2021, 27, 10760296211013108. [Google Scholar]
- Gálvez, J.A.; Pappas, J.M.; Ahumada, L.; Martin, J.N.; Simpao, A.F.; Rehman, M.A.; Witmer, C. The use of natural language processing on pediatric diagnostic radiology reports in the electronic health record to identify deep venous thrombosis in children. J. Thromb. Thrombolysis 2017, 44, 281–290. [Google Scholar] [CrossRef]
- Wiklund, P.; Medson, K.; Elf, J. Incidental pulmonary embolism in patients with cancer: Prevalence, underdiagnosis and evaluation of an AI algorithm for automatic detection of pulmonary embolism. Eur. Radiol. 2023, 33, 1185–1193. [Google Scholar] [CrossRef]
- Wiklund, P.; Medson, K.; Elf, J. Unreported incidental pulmonary embolism in patients with cancer: Radiologic natural history and risk of recurrent venous thromboembolism and death. Thromb. Res. 2023, 224, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Batra, K.; Xi, Y.; Bhagwat, S.; Espino, A.; Peshock, R.M. Radiologist Worklist Reprioritization Using Artificial Intelligence: Impact on Report Turnaround Times for CTPA Examinations Positive for Acute Pulmonary Embolism. AJR Am. J. Roentgenol. 2023, 221, 324–333. [Google Scholar] [CrossRef]
- Rothenberg, S.A.; Savage, C.H.; Elkassem, A.A.; Singh, S.; Abozeed, M.; Hamki, O.; Junck, K.; Tridandapani, S.; Li, M.; Li, Y.; et al. Prospective Evaluation of AI Triage of Pulmonary Emboli on CT Pulmonary Angiograms. Radiology 2023, 309, e230702. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.D.; Annichino-Bizzacchi, J.M.; Romano, A.V.C.; Maciel Filho, R. Artificial neural networks for prediction of recurrent venous thromboembolism. Int. J. Med. Inf. 2020, 141, 104221. [Google Scholar] [CrossRef]
- Rodger, M.A.; Kahn, S.R.; Wells, P.S.; Anderson, D.A.; Chagnon, I.; Le Gal, G.; Solymoss, S.; Crowther, M.; Perrier, A.; White, R.; et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale. Can. 2008, 179, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Heinze, G.; Jandeck, L.M.; Kyrle, P.A. Risk Assessment of Recurrence in Patients with Unprovoked Deep Vein Thrombosis or Pulmonary Embolism. Circulation 2010, 121, 1630–1636. [Google Scholar] [CrossRef]
- Tosetto, A.; Iorio, A.; Marcucci, M.; Baglin, T.; Cushman, M.; Eichinger, S.; Palareti, G.; Poli, D.; Tait, R.C.; Douketis, J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: A proposed prediction score (DASH). J. Thromb. Haemost. 2012, 10, 1019–1025. [Google Scholar] [CrossRef]
- Kim, J.S.; Merrill, R.K.B.; Arvind, V.B.; Kaji, D.B.; Pasik, S.D.B.; Nwachukwu, C.C.B.; Vargas, L.B.; Osman, N.S.B.; Oermann, E.K.; Caridi, J.M.; et al. Examining the Ability of Artificial Neural Networks Machine Learning Models to Accurately Predict Complications Following Posterior Lumbar Spine Fusion. Spine 2018, 43, 853–860. [Google Scholar] [CrossRef]
- Chen, M.C.; Ball, R.L.; Yang, L.; Moradzadeh, N.; Chapman, B.E.; Larson, D.B.; Langlotz, C.P.; Amrhein, T.J.; Lungren, M.P. Deep Learning to Classify Radiology Free-Text Reports. Radiology 2018, 286, 845–852. [Google Scholar] [PubMed]
- Ma, H.; Sheng, W.; Li, J.; Hou, L.; Yang, J.; Cai, J.; Xu, W.; Zhang, S. A novel hierarchical machine learning model for hospital-acquired venous thromboembolism risk assessment among multiple-departments. J. Biomed. Inform. 2021, 122, 103892. [Google Scholar]
- Park, J.I.; Kim, D.; Lee, J.A.; Zheng, K.; Amin, A. Personalized Risk Prediction for 30-Day Readmissions with Venous Thromboembolism Using Machine Learning. J. Nurs. Scholarsh. 2021, 53, 278–287. [Google Scholar] [PubMed]
- Yan, Y.; Yu, Z.; Ding, L.-P.; Zhou, M.; Zhang, C.; Pan, M.-M.; Zhang, J.-Y.; Wang, Z.-Y.; Gao, F.; Li, H.-Y.; et al. Machine Learning to Dynamically Predict In-Hospital Venous Thromboembolism After Inguinal Hernia Surgery: Results From the CHAT-1 Study. Clin. Appl. Thromb. 2023, 29, 10760296231171082. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Chen, J.; Li, J.; Che, Y.; Tang, J.; Li, H.; Li, J.; Ma, Y. Current prevalence of perioperative early venous thromboembolism and risk factors in Chinese adult patients with inguinal hernia (CHAT-1). Sci. Rep. 2020, 10, 12667. [Google Scholar] [CrossRef]
- Stuck, A.K.; Spirk, D.; Schaudt, J.; Kucher, N. Risk assessment models for venous thromboembolism in acutely ill medical patients. A systematic review. Thromb. Haemost. 2017, 117, 801–808. [Google Scholar]
- Golemi, I.; Salazar Adum, J.P.; Tafur, A.; Caprini, J. Venous thromboembolism prophylaxis using the Caprini score. Disease-a-Month 2019, 65, 249–298. [Google Scholar] [CrossRef]
- Shohat, N.; Ludwick, L.; Sherman, M.B.; Fillingham, Y.; Parvizi, J. Using machine learning to predict venous thromboembolism and major bleeding events following total joint arthroplasty. Sci. Rep. 2023, 13, 2197. [Google Scholar]

| Author and Year | AI Model | Details of Population | Number of CT Scans | Diagnostic Performance |
|---|---|---|---|---|
| Buls, et al. [16] | AIDOC Version 1.3 | All consecutive CTPA scans performed between 1 July 2019 and 1 February 2020 for any reason | 448 CTPAs | Sensitivity: 73% Specificity: 95% PPV: 73% NPV: 94% |
| Cheikh, et al. [17] | AIDOC version 1.0 | All consecutive adults with suspected PE obtaining CTPA between 21 September 2019 and 24 December 2019 | 1202 CTPAs | Sensitivity: 92.6% Specificity: 95.8% PPV: 80.4% NPV: 98.6% |
| Langius-Wiffen, et al. [18] | AIDOC (version not specified) | All consecutive adults with suspected PE obtaining CTPA between 24 February 2018 and 31 December 2020 | 3316 CTPAs | Sensitivity: 96.8% Specificity: 99.9% PPV: 99.7% NPV: 99.1% |
| Zaazoue, et al. [19] | AIDOC (version not specified) | Hospitalized adult COVID-19 patients receiving contrast enhanced chest CTs. All scans positive for PE (527) and selected matched controls (977) were included | 1504 contrast-enhanced CT cans | Sensitivity: 93.2% Specificity: 99.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser, A.M.; Vyas, R.; Morgan, A.A.; Kalaiger, A.M.; Kharawala, A.; Nagraj, S.; Agarwal, R.; Maliha, M.; Mangeshkar, S.; Singh, N.; et al. Role of Artificial Intelligence in the Diagnosis and Management of Pulmonary Embolism: A Comprehensive Review. Diagnostics 2025, 15, 889. https://doi.org/10.3390/diagnostics15070889
Naser AM, Vyas R, Morgan AA, Kalaiger AM, Kharawala A, Nagraj S, Agarwal R, Maliha M, Mangeshkar S, Singh N, et al. Role of Artificial Intelligence in the Diagnosis and Management of Pulmonary Embolism: A Comprehensive Review. Diagnostics. 2025; 15(7):889. https://doi.org/10.3390/diagnostics15070889
Chicago/Turabian StyleNaser, Ahmad Moayad, Rhea Vyas, Ahmed Ashraf Morgan, Abdul Mukhtadir Kalaiger, Amrin Kharawala, Sanjana Nagraj, Raksheeth Agarwal, Maisha Maliha, Shaunak Mangeshkar, Nikita Singh, and et al. 2025. "Role of Artificial Intelligence in the Diagnosis and Management of Pulmonary Embolism: A Comprehensive Review" Diagnostics 15, no. 7: 889. https://doi.org/10.3390/diagnostics15070889
APA StyleNaser, A. M., Vyas, R., Morgan, A. A., Kalaiger, A. M., Kharawala, A., Nagraj, S., Agarwal, R., Maliha, M., Mangeshkar, S., Singh, N., Satish, V., Mathai, S., Palaiodimos, L., & Faillace, R. T. (2025). Role of Artificial Intelligence in the Diagnosis and Management of Pulmonary Embolism: A Comprehensive Review. Diagnostics, 15(7), 889. https://doi.org/10.3390/diagnostics15070889









