Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies †
Abstract
1. Introduction
2. Methods
2.1. Panmacular SDM Treatment
2.2. Standardized Automated Perimetry Testing
2.3. Statistical Analysis
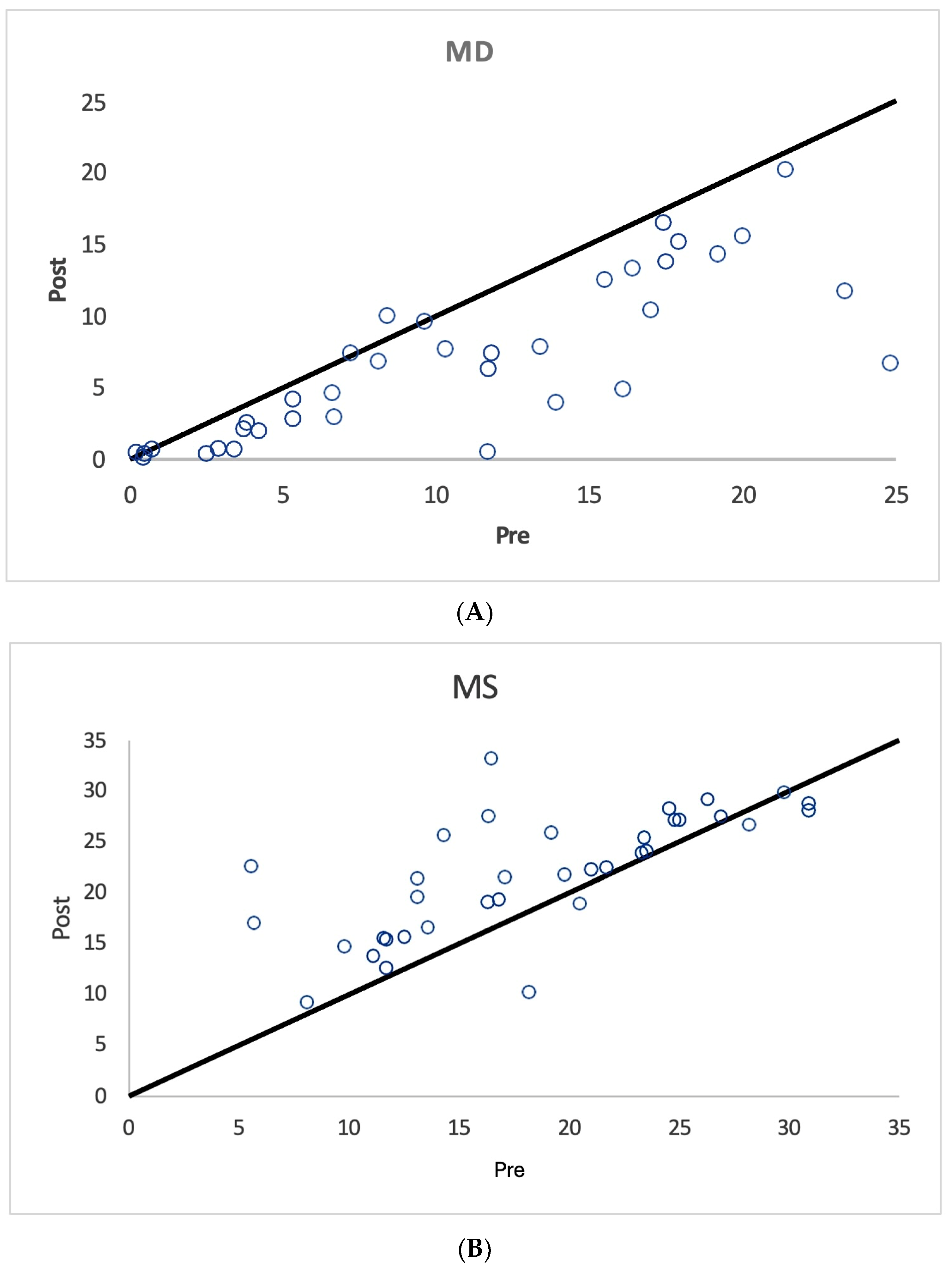
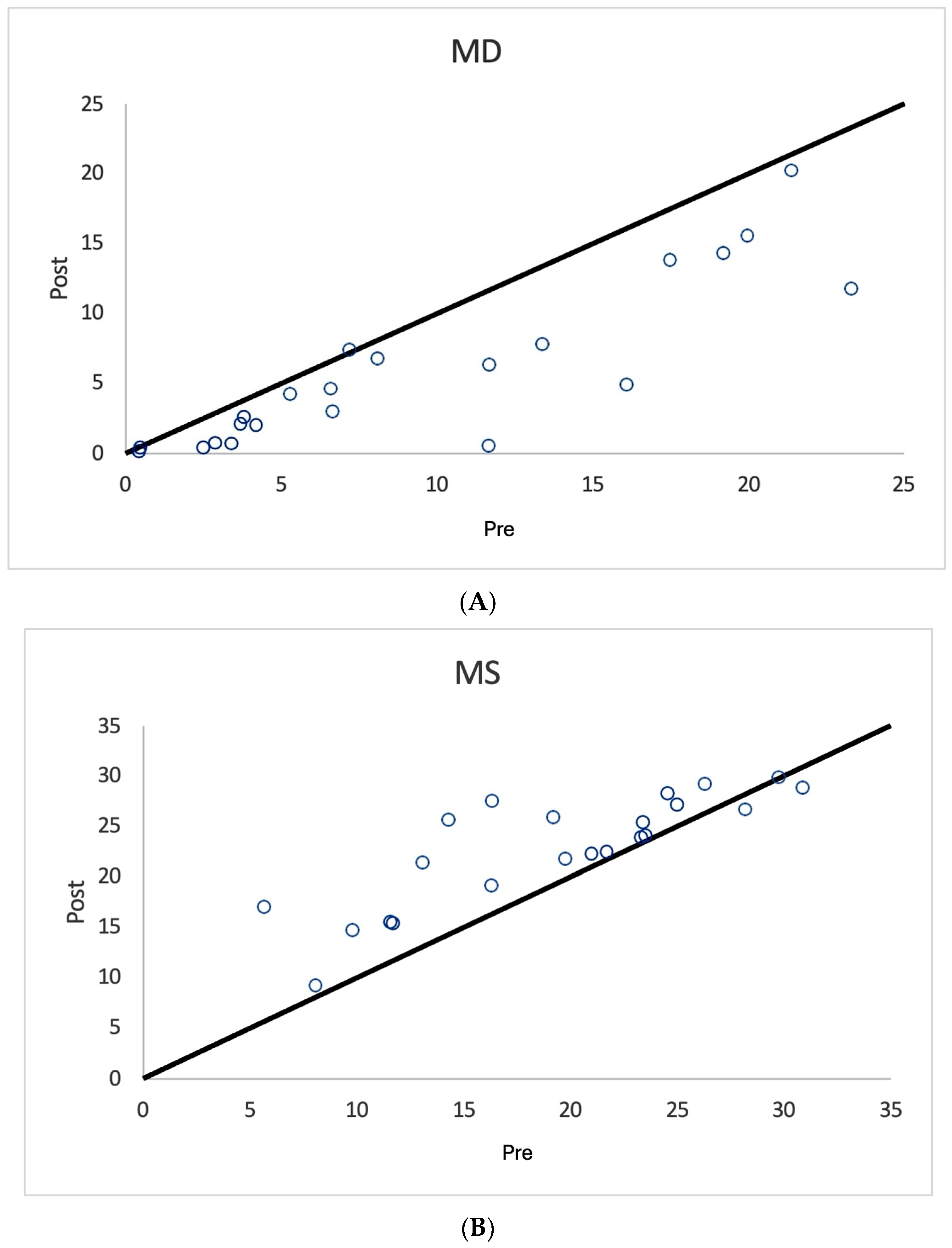
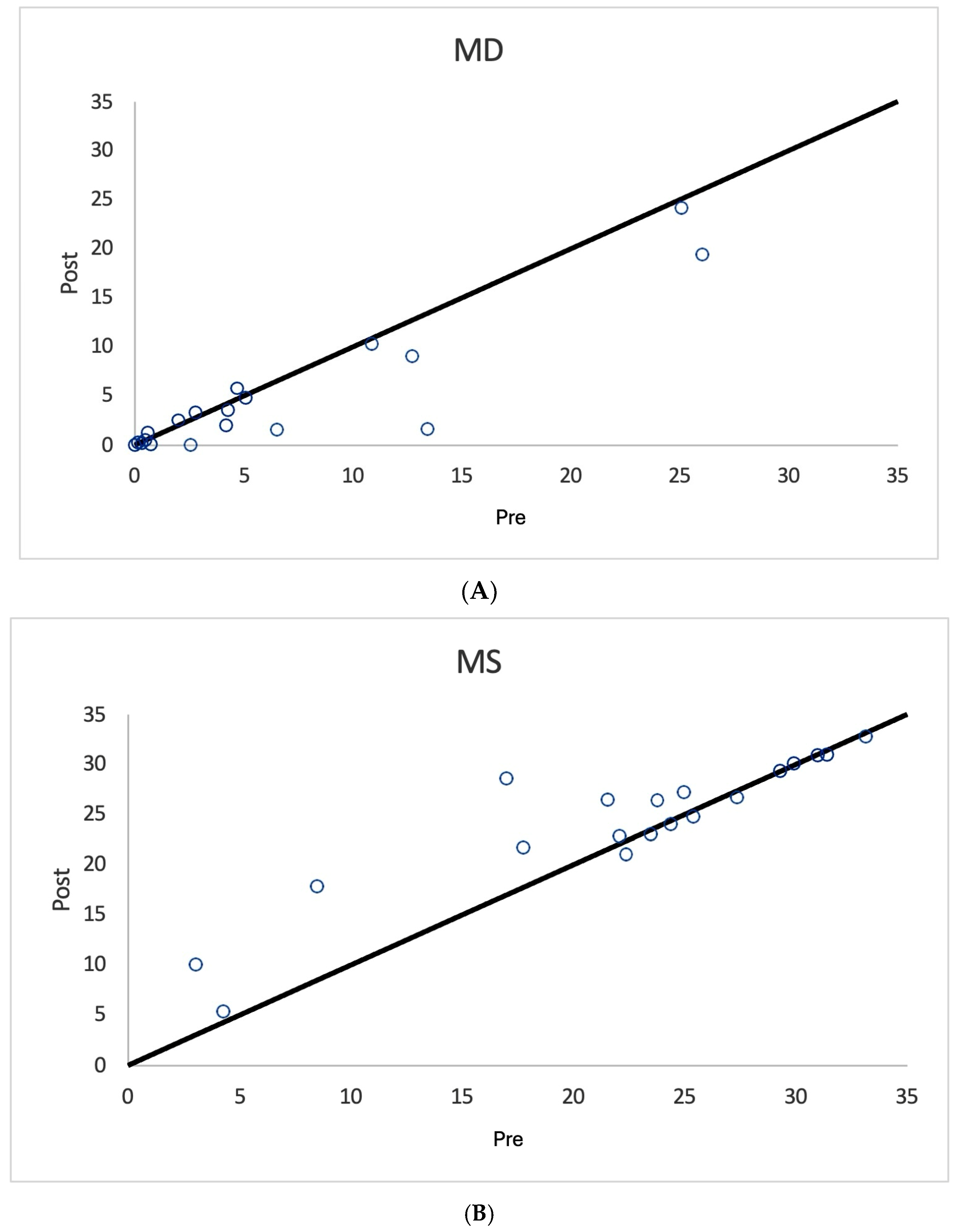
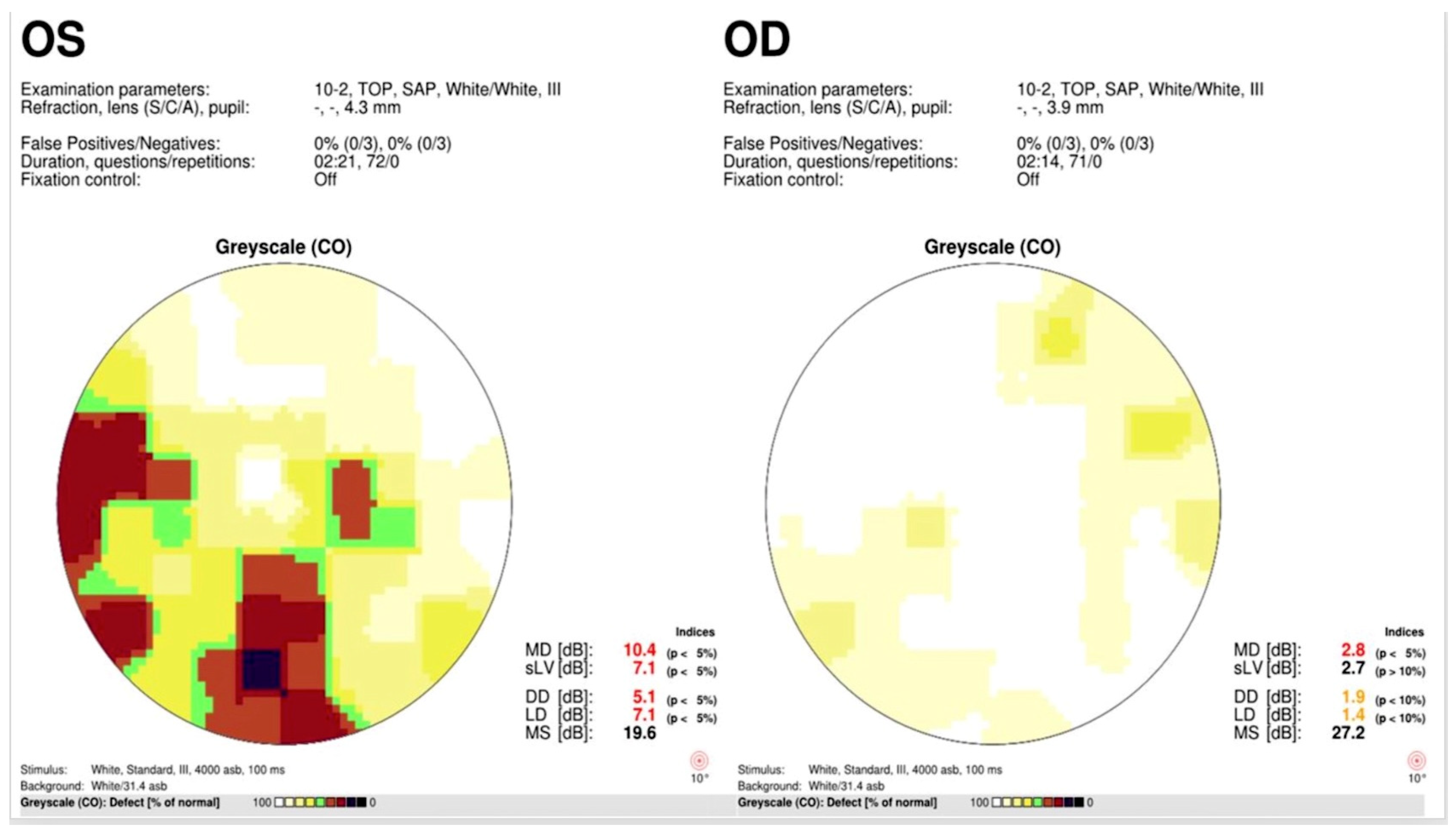
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K. Lasers in medicine. The changing role of laser-induced retinal damage—From de riguer to Nevermore. Photonics 2023, 10, 999. [Google Scholar] [CrossRef]
- Luttrull, J.K. Modern Retinal Laser Therapy: Principles and Application; Kugler Publications: Amsterdam, The Netherlands, 2023; ISBN 978-90-6299-298-0. [Google Scholar]
- Zheng, M.; Paulus, Y.M. Recent Innovations in Retinal Laser Therapy. Photonics 2025, 12, 156. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Levin, L.A. Is neuroprotection a viable therapy for glaucoma? Arch. Ophthalmol. 1999, 117, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Polo, A.D.; Sahel, J.A.; Schuman, J.S. Neuroprotection, Neuroenhancement, and Neuroregeneration of the Retina and Optic Nerve. Ophthalmol. Sci. 2022, 2, 100216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calkins, D.J.; Pekny, M.; Cooper, M.L.; Benowitz, L.; Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp. Eye Res. 2017, 157, 28–33. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Samples, J.R.; Kent, D.; Lum, B.J. Panmacular subthreshold diode micropulse laser (SDM) as neuroprotective therapy in primary open-angle glaucoma. Glaucoma Res. 2018, 2020, 281–294. [Google Scholar]
- Luttrull, J.K.; Kent, D. Modern retinal laser for neuroprotection in open-angle glaucoma. In New Concepts in Glaucoma Surgery; Samples, J.R., Ahmed, I.I.K., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2019; Volume 1, ISBN 978-1461483472/1461483476. [Google Scholar]
- Luttrull, J.K.; Tzekov, R.; Bhavan, S.V. Progressive thickening of retinal nerve fiber and ganglion cell complex layers following SDM laser Vision Protection Therapy in Open Angle Glaucoma. Ophthalmol. Ther. 2024, 13, 2903–2918. [Google Scholar] [CrossRef]
- Jampel, H.D.; Singh, K.; Lin, S.C.; Chen, T.C.; Francis, B.A.; Hodapp, E.; Samples, J.R.; Smith, S.D. Assessment of visual function in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology 2011, 118, 986–1002. [Google Scholar]
- Mowatt, G.; Burr, J.M.; Cook, J.A.; Siddiqui, M.A.R.; Ramsay, C.; Fraser, C.; Azuara-Blanco, A.; Deeks, J.J. Screening tests for detecting open-angle glaucoma: Systematic review and meta-analysis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5373–5385. [Google Scholar] [CrossRef]
- Sousa, M.C.C.; Biteli, L.G.; Dorairaj, S.; Maslin, J.S.; Leite, M.; Prata, T.S. Suitability of the Visual Field Index according to Glaucoma Severity. J. Curr. Glaucoma Pract. 2015, 9, 65–68. [Google Scholar]
- Gutstein, W.; Sinclair, S.H.; Presti, P.; North, R.V. Interactive thresholding of central acuity under contrast and luminance conditions mimicking real world environments: 1. Evaluation against LogMAR charts. J. Comput. Sci. Sys Bio 2015, 8, 225–232. [Google Scholar]
- Racette, L.; Fischer, M.; Bebie, H.; Holló, G.; Johnson, C.A.; Matsumoto, C. Visual Field Digest: A Guide to Perimetry and the Octopus Perimeter, 8th ed.; Haag-Streit: Koniz, Switzerland, 2019; ISBN 978-3-033-07419-4. [Google Scholar]
- Bengtsson, B.; Heijl, A.; Olsson, J. Evaluation of a new threshold visual field strategy, SITA, in normal subjects. Swedish Interactive Thresholding Algorithm. Acta Ophthalmol. Scand. 1998, 76, 165–169. [Google Scholar] [PubMed]
- Bengtsson, B.; Heijl, A. A visual field index for calculation of glaucoma rate of progression. Am. J. Ophthalmol. 2008, 145, 343–353. [Google Scholar] [PubMed]
- Kaufmann, H.; Flammer, J. Die Bebié-Kurve (kumulative Defektkurve) zur Differenzierung von lokalen und diffusen Gesichtsfelddefekten [The Bebié curve (cumulative defect curve) for differentiating local and diffuse visual field defects]. Fortschr. Ophthalmol. 1989, 86, 687–691. [Google Scholar] [PubMed]
- Midena, E.M.; Bini, S.M.; Martini, F.M.; Enrica, C.M.; Pilotto, E.M.; Micera, A.; Esposito, G.M.; Vujosevic, S.M. Changes of aqueous humor Muller cells’ biomarkers in human patients affected by diabetic retinopathy after subthreshold micropulse laser treatment. Retina 2020, 40, 126–134. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef]
- Caballero, S.; Kent, D.L.; Sengupta, N.; Calzi, S.L.; Shaw, L.; Beli, E.; Moldovan, L.; Dominguez, J.M.; Moorthy, R.S.; Grant, M.B. Bone Marrow-Derived Cell Recruitment to the Neurosensory Retina and Retinal Pigment Epithelial Cell Layer Following Subthreshold Retinal Phototherapy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5164–5176. [Google Scholar] [CrossRef]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar]
- Yu, P.K.; Cringle, S.J.; McAllister, I.L.; Yu, D.Y. Low power laser treatment of the retina ameliorates neovascularisation in a transgenic mouse model of retinal neovascularisation. Exp. Eye Res. 2009, 89, 791–800. [Google Scholar]
- Flaxel, C.; Bradle, J.; Acott, T.; Samples, J.R. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina 2007, 27, 629–634. [Google Scholar] [PubMed]
- Hattenbach, L.-O.; Beck, K.-F.; Pfeilschifter, J.; Koch, F.; Ohrloff, C.; Schacke, W. Pigment epithelium- derived factor is upregulated in photocoagulated human retinal pigment epithelial cells. Ophthalmic Res. 2005, 37, 341–346. [Google Scholar] [PubMed]
- Sramek, C.; Mackanos, M.; Spitler, R.; Leung, L.-S.; Nomoto, H.; Contag, C.H.; Palanker, D. Non-damaging retinal phototherapy: Dynamic range of heat shock protein expression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1780–1787. [Google Scholar]
- Kregel, K.C. Invited Review: Heat shock proteins: Modifying factors in cellular stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [PubMed]
- Manteuffel, G. Electrophysiology and anatomy of direction-specific pretectal units in Salamandra salamandra. Exp. Brain Res. 1984, 54, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Garcea, F.E.; Kristensen, S.; Almeida, J.; Mahon, B.Z. Resilience to the contralateral visual field bias as a window into object representations. Cortex 2016, 81, 14–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| No. Patients | 29 |
| Caucasian | 24 (83%) |
| Hispanic | 5 (17%) |
| Total Study Eyes | 55 |
| Total Treated Eyes | 36 |
| Bilaterally SDM-treated Eyes | 14/36 (39%) |
| Unilaterally SDM-treated eyes | 22/36 (61%) |
| Untreated Control Eyes | 19 |
| Female | 17 (59%) |
| Age (years) | 62–89 (avg 76, SD 7.5) |
| Total OD | 26 (47%) |
| Total OS | 29 (53%) |
| Total Treated Eyes OAG | 29 eyes (81%) |
| Total Treated Eyes Other OA | 7 eyes (19%) (AION 3 pts, 5 eyes, Mult Scl 1 pt 2) |
| Total Pseudophakic Eyes | 25 eyes (46%) |
| 10-2 | 26 eyes (47%) |
| 24-2 | 29 eyes (53%) |
| Pre MD | Pre PSD | Pre MS | False ± | Post MD | Post PSD | Post MS | False ± (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| All Treated Eyes n = 36 | Mean Median SD | 10.5 9.5 7.0 | 6.0 6.4 2.8 | 18.4 17.7 7.0 | 9.2 0 15.5 | 6.9 6.5 5.5 | 5.7 5.3 3.1 | 21.9 22.4 5.9 | 9.9 0 14.8 |
| Unilaterally Treated Eyes n = 22 | Mean Median SD | 9.5 6.9 7.1 | 5.4 5.4 2.6 | 19.3 20.4 7.0 | 8.5 0 16.0 | 5.9 4.4 5.7 | 4.9 4.15 2.7 | 22.8 24 5.4 | 10 0 15.6 |
| Fellow Eye Controls n = 19 | Mean Median SD | 6.46 4.2 7.7 | 4.2 3.4 3.4 | 22.2 23.8 8.5 | 3.7 0 11.7 | 4.7 2.0 6.5 | 3.8 3.18 2.9 | 24.2 26.4 6.8 | 6.0 0 14.1 |
| Study Groups | SAP Metrics | |||
|---|---|---|---|---|
| All Treated Eyes | MD | PSD | MS | |
| Mean difference | 3.651944 | 0.2786111 | −3.48528 | |
| p-value | 7.02 × 10−6 | 0.44 | 7.02 × 10−6 | |
| 95% CI | [2.25, 5.06] | [−0.45, 1.01] | [−5.21, −1.76] | |
| Unilaterally Treated Eyes | ||||
| Mean Difference | 3.612273 | 0.4277273 | −3.53909 | |
| p-value | 1.00 × 10−4 | 0.28 | 3.95 × 10−4 | |
| 95% CI | [2.04, 5.18] | [−0.37, 1.22] | [−5.29, −1.79] | |
| Unilateral Untreated Fellow Eye Controls | ||||
| Mean Difference | 1.724211 | 0.4215789 | −2.04368 | |
| p-value | 0.03 | 0.23 | 0.03 | |
| 95% CI | [0.19, 3.26] | [−0.29, 1.14] | [−3.82, −0.27] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luttrull, J.K.; Bhavan, S.V. Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies. Diagnostics 2025, 15, 912. https://doi.org/10.3390/diagnostics15070912
Luttrull JK, Bhavan SV. Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies. Diagnostics. 2025; 15(7):912. https://doi.org/10.3390/diagnostics15070912
Chicago/Turabian StyleLuttrull, Jeffrey K., and Sathy V. Bhavan. 2025. "Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies" Diagnostics 15, no. 7: 912. https://doi.org/10.3390/diagnostics15070912
APA StyleLuttrull, J. K., & Bhavan, S. V. (2025). Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies. Diagnostics, 15(7), 912. https://doi.org/10.3390/diagnostics15070912





