The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection and Variable Definitions
2.4. Outcome
2.5. Machine Learning Modeling
2.6. Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CALLY | C-reactive protein-albumin-lymphocyte index |
| CRP | C-reactive protein |
| SOFA | Sequential Organ Failure Assessment |
| qSOFA | Quick sequential organ failure assessment |
| XGBoost | eXtreme Gradient Boosting |
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Rhee, C.; Strich, J.R.; Morales, M.K.; Hohmann, S.; Menchaca, J.; Suffredini, A.F.; Danner, R.L.; Klompas, M. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest 2017, 151, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Gottlieb, M. Emergency medicine updates: Evaluation and diagnosis of sepsis and septic shock. Am. J. Emerg. Med. 2025, 90, 169–178. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Altın, N.; Hekimoğlu, C.H.; Unver Ulusoy, T.; Kuzi, S.; Sevinç, G.; Tekin, A.; Aksoy, B.R.; Şencan, I. Gram-Negative Bloodstream Infections in Healthcare: The Relationship Between Antibiotic Resistance, Mortality, and Novel Serological Biomarker. Cureus 2024, 16, e57720. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Hernández-Sarmiento, R.; Salazar, M.P.; Barrera, S.; Castilla, V.; Duque, C. The association between hypoalbuminemia and microcirculation, endothelium, and glycocalyx disorders in children with sepsis. Microcirculation 2023, 30, e12829. [Google Scholar] [CrossRef]
- Finfer, S.; Venkatesh, B.; Hotchkiss, R.S.; Sasson, S.C. Lymphopenia in sepsis-an acquired immunodeficiency? Immunol. Cell Biol. 2023, 101, 535–544. [Google Scholar] [CrossRef]
- Yang, M.; Lin, S.-Q.; Liu, X.-Y.; Tang, M.; Hu, C.-L.; Wang, Z.-W.; Zhang, Q.; Zhang, X.; Song, M.-M.; Ruan, G.-T.; et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front. Immunol. 2023, 14, 1131496. [Google Scholar] [CrossRef]
- Furukawa, K.; Tsunematsu, M.; Tanji, Y.; Ishizaki, S.; Akaoka, M.; Haruki, K.; Uwagawa, T.; Onda, S.; Matsumoto, M.; Ikegami, T. Impact of C-reactive protein-albumin-lymphocyte (CALLY) index on prognosis after hepatectomy for colorectal liver metastasis. Surg. Oncol. 2023, 47, 101911. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tang, J.; Chen, X.; Jin, Y.; Zhang, H.; Liang, R. Associations of C-reactive protein-albumin-lymphocyte (CALLY) index with cardiorenal syndrome: Insights from a population-based study. Heliyon 2024, 10, e37197. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Lan, J.; Chen, C.; Wang, Y.; Li, Z. Interpretable machine learning-based prediction of 28-day mortality in ICU patients with sepsis: A multicenter retrospective study. Front. Cell Infect. Microbiol. 2024, 14, 1500326. [Google Scholar] [CrossRef]
- Li, S.; Dou, R.; Song, X.; Lui, K.Y.; Xu, J.; Guo, Z.; Hu, X.; Guan, X.; Cai, C. Developing an Interpretable Machine Learning Model to Predict in-Hospital Mortality in Sepsis Patients: A Retrospective Temporal Validation Study. J. Clin. Med. 2023, 12, 915. [Google Scholar] [CrossRef]
- Park, S.W.; Yeo, N.Y.; Kang, S.; Ha, T.; Kim, T.-H.; Lee, D.; Kim, D.; Choi, S.; Kim, M.; Lee, D.; et al. Early Prediction of Mortality for Septic Patients Visiting Emergency Room Based on Explainable Machine Learning: A Real-World Multicenter Study. J. Korean Med. Sci. 2024, 39, e53. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Forner, J.; Dulatahu, F.; Dudda, J.; Brück, L.M.; Hoffmann, U.; Bertsch, T.; et al. C-reactive protein and procalcitonin during course of sepsis and septic shock. Ir. J. Med. Sci. 2024, 193, 457–468. [Google Scholar] [CrossRef]
- Tian, T.; Wei, B.; Wang, J. Study of C-reactive protein, procalcitonin, and immunocyte ratios in 194 patients with sepsis. BMC Emerg. Med. 2021, 21, 81. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chen, C.-J.; Shao, S.-C.; Li, C.-H.; Hsiao, C.-H.; Niu, K.-Y.; Yen, C.-C. Comparison of the Diagnostic Accuracies of Monocyte Distribution Width, Procalcitonin, and C-Reactive Protein for Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2023, 51, e106–e114. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Choi, B.; Eun, S.; Bae, G.E.; Koo, C.M.; Kim, M.K. Using the lactate-to-albumin ratio to predict mortality in patients with sepsis or septic shock: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1743–1752. [Google Scholar] [CrossRef]
- Kumar, H.G.; Kanakaraju, K.; Manikandan, V.A.C.; Patel, V.; Pranay, C. The Relationship Between Serum Albumin Levels and Sepsis in Patients Admitted to a Tertiary Care Center in India. Cureus 2024, 16, e59424. [Google Scholar] [CrossRef]
- Furukawa, M.; Kinoshita, K.; Yamaguchi, J.; Hori, S.; Sakurai, A. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern. Emerg. Med. 2019, 14, 539–548. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Bai, L.; Li, L.; Guo, J.; Li, Y. The Clinical Value of Comprehensive Nursing Intervention in Preventing Severe Lymphopenia and Improving the Survival Rate Among Patients with Sepsis. Open Access Emerg. Med. 2023, 15, 393–403. [Google Scholar] [CrossRef]
- Cilloniz, C.; Peroni, H.J.; Gabarrús, A.; García-Vidal, C.; Pericàs, J.M.; Bermejo-Martin, J.; Torres, A. Lymphopenia Is Associated with Poor Outcomes of Patients with Community-Acquired Pneumonia and Sepsis. Open Forum Infect. Dis. 2021, 8, ofab169. [Google Scholar] [CrossRef]
- Sheikh Motahar Vahedi, H.; Bagheri, A.; Jahanshir, A.; Seyedhosseini, J.; Vahidi, E. Association of Lymphopenia with Short Term Outcomes of Sepsis Patients; a Brief Report. Arch. Acad. Emerg. Med. 2019, 7, e14. [Google Scholar]
- Zhang, J.; Zhao, Q.; Liu, S.; Yuan, N.; Hu, Z. Clinical predictive value of the CRP-albumin-lymphocyte index for prognosis of critically ill patients with sepsis in intensive care unit: A retrospective single-center observational study. Front. Public. Health 2024, 12, 1395134. [Google Scholar] [CrossRef]
- Lin, T.-H.; Chung, H.-Y.; Jian, M.-J.; Chang, C.-K.; Lin, H.-H.; Yen, C.-T.; Tang, S.-H.; Pan, P.-C.; Perng, C.-L.; Chang, F.-Y.; et al. AI-Driven Innovations for Early Sepsis Detection by Combining Predictive Accuracy WITH Blood Count Analysis in an Emergency Setting: Retrospective Study. J. Med. Internet Res. 2025, 27, e56155. [Google Scholar] [CrossRef]
- Garbern, S.C.; Mamun, G.M.S.; Shaima, S.N.; Hakim, N.; Wegerich, S.; Alla, S.; Sarmin, M.; Afroze, F.; Sekaric, J.; Genisca, A.; et al. A novel digital health approach to improving global pediatric sepsis care in Bangladesh using wearable technology and machine learning. PLoS Digit. Health 2024, 3, e0000634. [Google Scholar] [CrossRef]
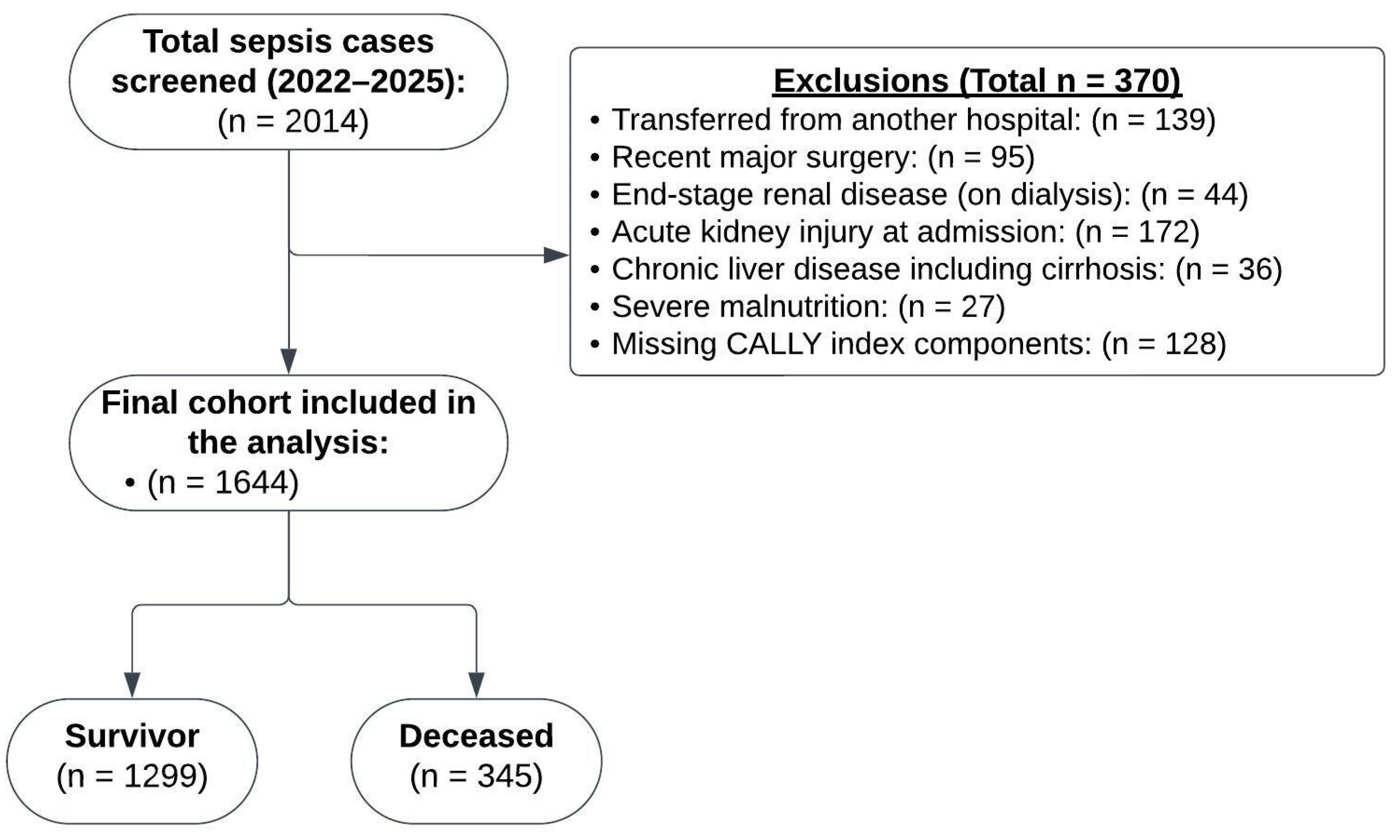

| Variable | Survivor (n = 1299) | Deceased (n = 345) | p | Mean Difference (95% CI) |
|---|---|---|---|---|
| Age (years) | 62.0 ± 12.0 | 71.2 ± 9.6 | <0.001 | 9.8 (7.91–10.34) |
| Sex (Male, n%) | 726 (55.9%) | 180 (52.2%) | 0.235 | - |
| Hypertension, n (%) | 767 (59.1%) | 219 (63.5%) | 0.157 | - |
| Diabetes, n (%) | 845 (65.1%) | 224 (64.9%) | 1.000 | - |
| CKD, n (%) | 301 (23.2%) | 103 (29.9%) | 0.013 | - |
| CAD, n (%) | 397 (30.6%) | 120 (34.8%) | 0.154 | - |
| COPD, n (%) | 254 (19.6%) | 75 (21.7%) | 0.412 | - |
| Malignancy, n (%) | 206 (15.9%) | 38 (11.0%) | 0.030 | - |
| Dementia, n (%) | 170 (13.1%) | 44 (12.8%) | 0.937 | - |
| Multiple Infections, n (%) | 197 (15.2%) | 63 (18.3%) | 0.190 | - |
| BMI, kg/m2) | 27.6 ± 3.0 | 27.8 ± 3.2 | 0.243 | - |
| Systolic BP (mmHg) | 95.9 ± 9.2 | 94.2 ± 8.0 | 0.001 | 1.7 (0.74–2.71) |
| Diastolic BP (mmHg) | 60.5 ± 8.0 | 51.8 ± 5.7 | <0.001 | 8.7 (7.92–9.40) |
| Heart Rate (bpm) | 98.2 ± 15.2 | 110.3 ± 13.0 | <0.001 | 12.1 (1.46–13.66) |
| Respiratory Rate (breaths/min) | 21.3 ± 5.1 | 27.3 ± 3.9 | <0.001 | 6 (5.48–6.47) |
| Temperature (°C) | 37.8 ± 0.5 | 38.0 ± 0.5 | <0.001 | 0.2 (0.18–0.29) |
| Oxygen Saturation (%) | 94.2 ± 3.0 | 91.2 ± 3.9 | <0.001 | 3 (2.6–3.49) |
| Glasgow Coma Scale | 12.2 ± 2.9 | 9.0 ± 3.8 | <0.001 | 3.2 (2.74–3.60) |
| qSOFA Score | 2.1 ± 0.8 | 2.2 ± 0.8 | 0.243 | - |
| SOFA Score | 12.1 ± 7.1 | 14.9 ± 7.3 | <0.001 | 2.8 (1.92–3.65) |
| Variable | Survivor (n = 1299) | Deceased (n = 345) | p | Mean Difference (95% CI) |
|---|---|---|---|---|
| White Blood Cell Count (×109/L) | 13.7 ± 2.3 | 17.4 ± 3.1 | <0.001 | 3.7 (3.35–4.06) |
| Neutrophil Count (×109/L) | 10.4 ± 2.1 | 13.2 ± 3.3 | <0.001 | 2.8 (2.43–3.16) |
| Lymphocyte Count (×109/L) | 1.2 ± 0.6 | 0.7 ± 0.7 | <0.001 | 0.5 (0.39–0.54) |
| Platelet Count (×103/µL) | 281.4 ± 203.8 | 279.9 ± 189.1 | 0.901 | - |
| Procalcitonin (ng/mL) | 4.1 ± 2.0 | 7.3 ± 3.0 | <0.001 | 3.2 (2.9–3.58) |
| C-reactive Protein (mg/L) | 129.7 [111–150.2] | 183.7 [127.4–241.2] | <0.001 | - |
| Albumin (g/dL) | 3.1 ± 0.5 | 2.4 ± 0.9 | <0.001 | 0.7 (0.56–0.77) |
| Lactate (mmol/L) | 2.6 ± 1.7 | 4.3 ± 1.9 | <0.001 | 1.7 (1.45–1.88) |
| Blood Urea Nitrogen (mg/dL) | 11.1 ± 3.5 | 13.9 ± 3.4 | <0.001 | 2.8 (2.43–3.26) |
| Creatinine (mg/dL) | 1.54 ± 0.37 | 1.59 ± 0.33 | 0.018 | 0.05 (0.01–0.90) |
| Total Bilirubin (mg/dL) | 1.5 ± 1.3 | 2.3 ± 1.6 | <0.001 | 0.8 (0.64–1.02) |
| Aspartate Aminotransferase (U/L) | 63.2 ± 48.5 | 93.3 ± 56.1 | <0.001 | 30.1 (23.6–36.6) |
| Alanine Aminotransferase (U/L) | 52.1 ± 43.0 | 69.5 ± 51.5 | <0.001 | 17.4 (11.5–23.3) |
| Glucose (mg/dL) | 192.7 ± 143.2 | 223.2 ± 166.7 | 0.002 | 30.5 (11.2–49.7) |
| Sodium (mEq/L) | 140.4 ± 17.9 | 141.7 ± 17.7 | 0.216 | - |
| Potassium (mEq/L) | 4.8 ± 1.8 | 4.6 ± 1.8 | 0.152 | - |
| Bicarbonate (mEq/L) | 20.6 ± 11.5 | 21.7 ± 11.6 | 0.136 | - |
| Cally Index | 24.3 [15.9–34.6] | 72.4 [23.3–190] | <0.001 | - |
| Model | Dataset | AUC (95% CI) | R2 | MAE | RMSE |
|---|---|---|---|---|---|
| XGBoost (Gain) | Test | 0.995 (0.991–1.000) | 0.867 | 0.063 | 0.145 |
| Train | 0.996 (0.992–1.000) | 0.973 | 0.037 | 0.076 | |
| MLP | Test | 0.988 (0.980–0.996) | 0.772 | 1.033 | 1.053 |
| Train | 0.994 (0.991–0.998) | 0.878 | 1.033 | 1.043 | |
| Random Forest | Test | 0.991 (0.987–0.993) | 0.851 | 1.011 | 1.024 |
| Train | 0.994 (0.990–0.098) | 0.982 | 1.000 | 1.002 | |
| SVM | Test | 0.992 (0.987–0.997) | 0.810 | 1.006 | 1.021 |
| Train | 0.993 (0.988–0.997) | 0.862 | 0.999 | 1.010 | |
| GLM | Test | 0.991 (0.985–0.997) | 0.810 | 1.008 | 1.024 |
| Train | 0.993 (0.989–0.997) | 0.859 | 1.000 | 1.012 |
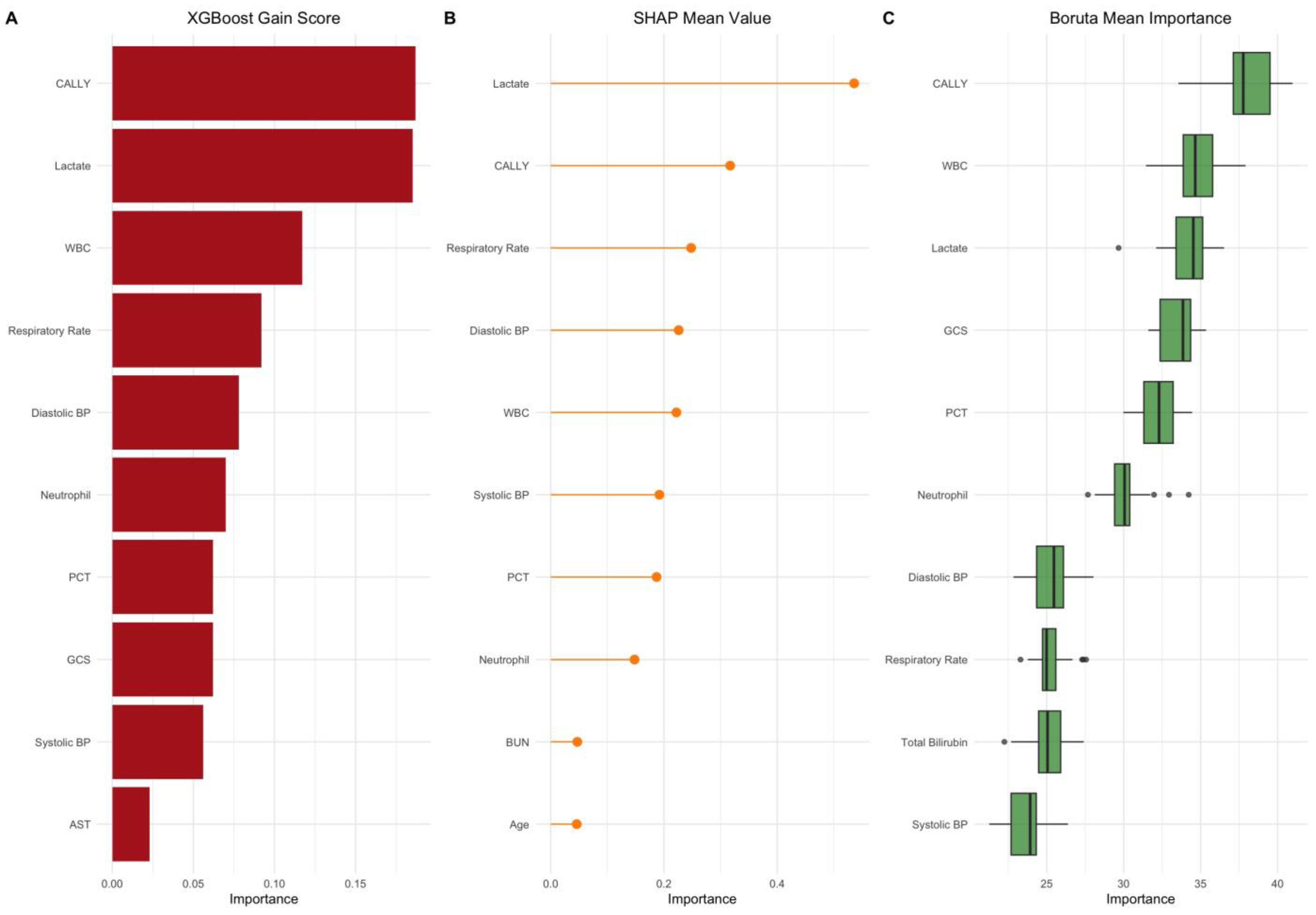
| Rank | Feature | XGBoost Gain Score | SHAP Mean Value | Boruta Mean Importance | LASSO Coefficient |
|---|---|---|---|---|---|
| 1 | CALLY Index | 0.187 | 0.317 | 37.54 | – |
| 2 | Serum Lactate (mmol/L) | 0.185 | 0.536 | 34.16 | 0.417 |
| 3 | White Blood Cell Count | 0.117 | 0.222 | 35.07 | 0.394 |
| 4 | Respiratory Rate (breaths/min) | 0.092 | 0.248 | 25.63 | 0.256 |
| 5 | Diastolic Blood Pressure (mmHg) | 0.078 | 0.226 | 25.88 | –0.121 |
| 6 | Neutrophil Count (109/L) | 0.070 | 0.148 | 30.17 | 0.208 |
| 7 | Procalcitonin (ng/mL) | 0.062 | 0.187 | 32.17 | 6.462 |
| 8 | Glasgow Coma Scale | 0.062 | 0.099 | 33.25 | –0.226 |
| 9 | Systolic Blood Pressure (mmHg) | 0.056 | 0.192 | 23.66 | 0.106 |
| 10 | Aspartate Aminotransferase (U/L) | 0.023 | 0.055 | 22.09 | 0.009 |
| 11 | Total Bilirubin (mg/dL) | 0.022 | 0.032 | 24.87 | 0.329 |
| 12 | Age (years) | 0.012 | 0.046 | 14.44 | 0.068 |
| 13 | SOFA Score | 0.011 | 0.012 | 21.65 | – |
| 14 | Blood Urea Nitrogen (mg/dL) | 0.011 | 0.047 | 14.56 | 0.239 |
| 15 | Oxygen Saturation (%) | 0.008 | 0.037 | 19.38 | –0.212 |
| Threshold | Net Benefit (CALLY Index) | Net Benefit (Treat All) | Net Benefit (Treat None) | Clinical Interpretation |
|---|---|---|---|---|
| 0.05 | 0.202 | 0.19 | 0.00 | CALLY index provides a moderate net benefit at very low-risk thresholds. |
| 0.10 | 0.12 | 0.10 | 0.00 | The highest clinical utility observed within this range. |
| 0.15 | 0.07 | 0.05 | 0.00 | CALLY remains superior to treat-all and treat-none approaches. |
| 0.20 | 0.03 | 0.02 | 0.00 | Declining net benefit, but still clinically useful. |
| 0.25 | 0.01 | 0.00 | 0.00 | Marginal benefit beyond this threshold. |
| 0.30 | 0.00 | 0.02 | 0.00 | The benefit diminishes at higher thresholds. |
| 0.35+ | 0.00 | 0.00 | 0.00 | CALLY index offers no additional benefit beyond this threshold. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıdaş, A.; Çetinkaya, R. The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity. Diagnostics 2025, 15, 1026. https://doi.org/10.3390/diagnostics15081026
Sarıdaş A, Çetinkaya R. The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity. Diagnostics. 2025; 15(8):1026. https://doi.org/10.3390/diagnostics15081026
Chicago/Turabian StyleSarıdaş, Ali, and Remzi Çetinkaya. 2025. "The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity" Diagnostics 15, no. 8: 1026. https://doi.org/10.3390/diagnostics15081026
APA StyleSarıdaş, A., & Çetinkaya, R. (2025). The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity. Diagnostics, 15(8), 1026. https://doi.org/10.3390/diagnostics15081026






