Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions—A Review on Its Frequency and Possible Causes
Abstract
1. Introduction
2. Method/Search Strategy
3. Late Phase Washout in CEUS of Benign FLLs
4. Various Common and Rare Benign FLLs with Washout and LP Hypoenhancement
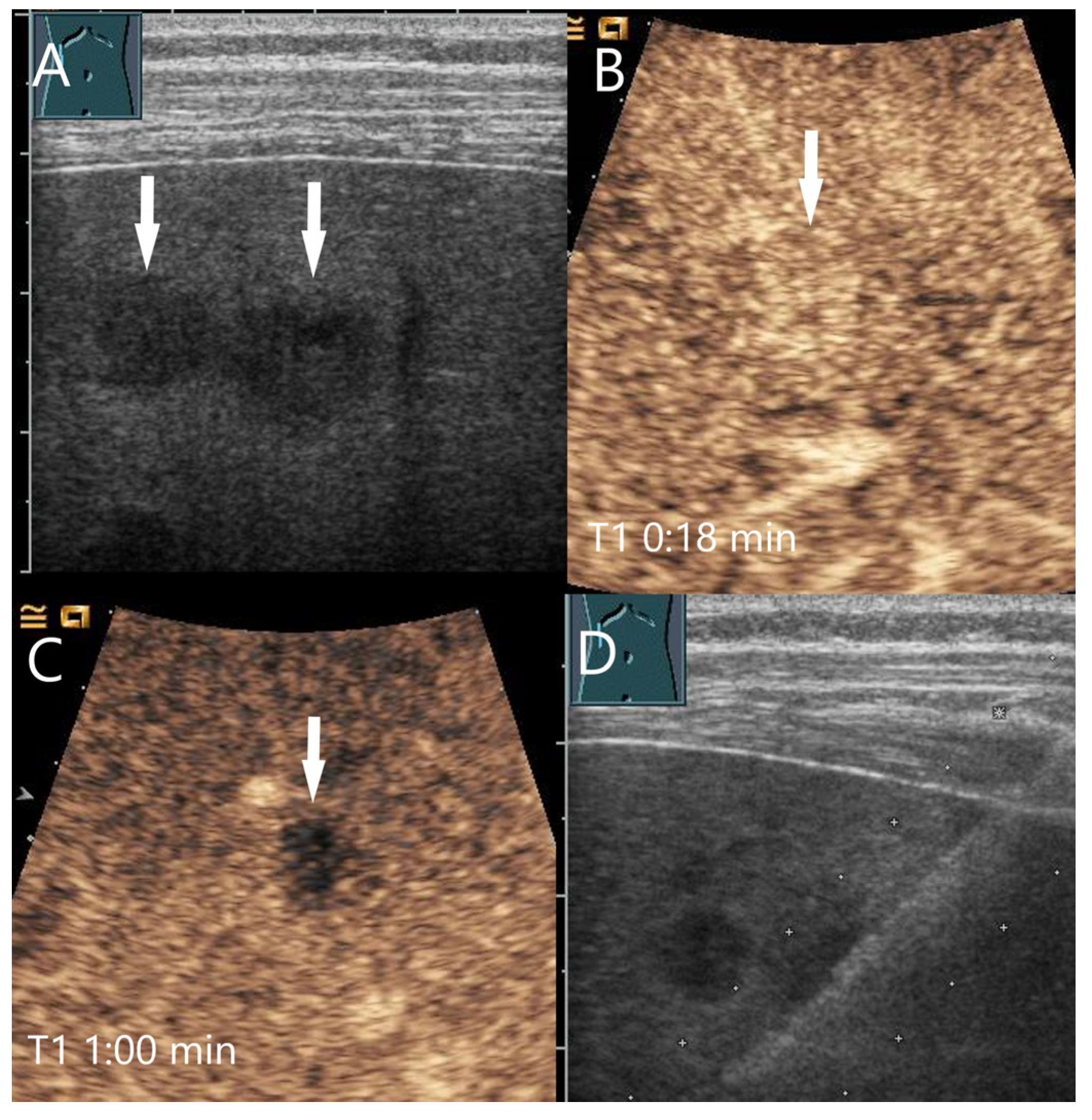
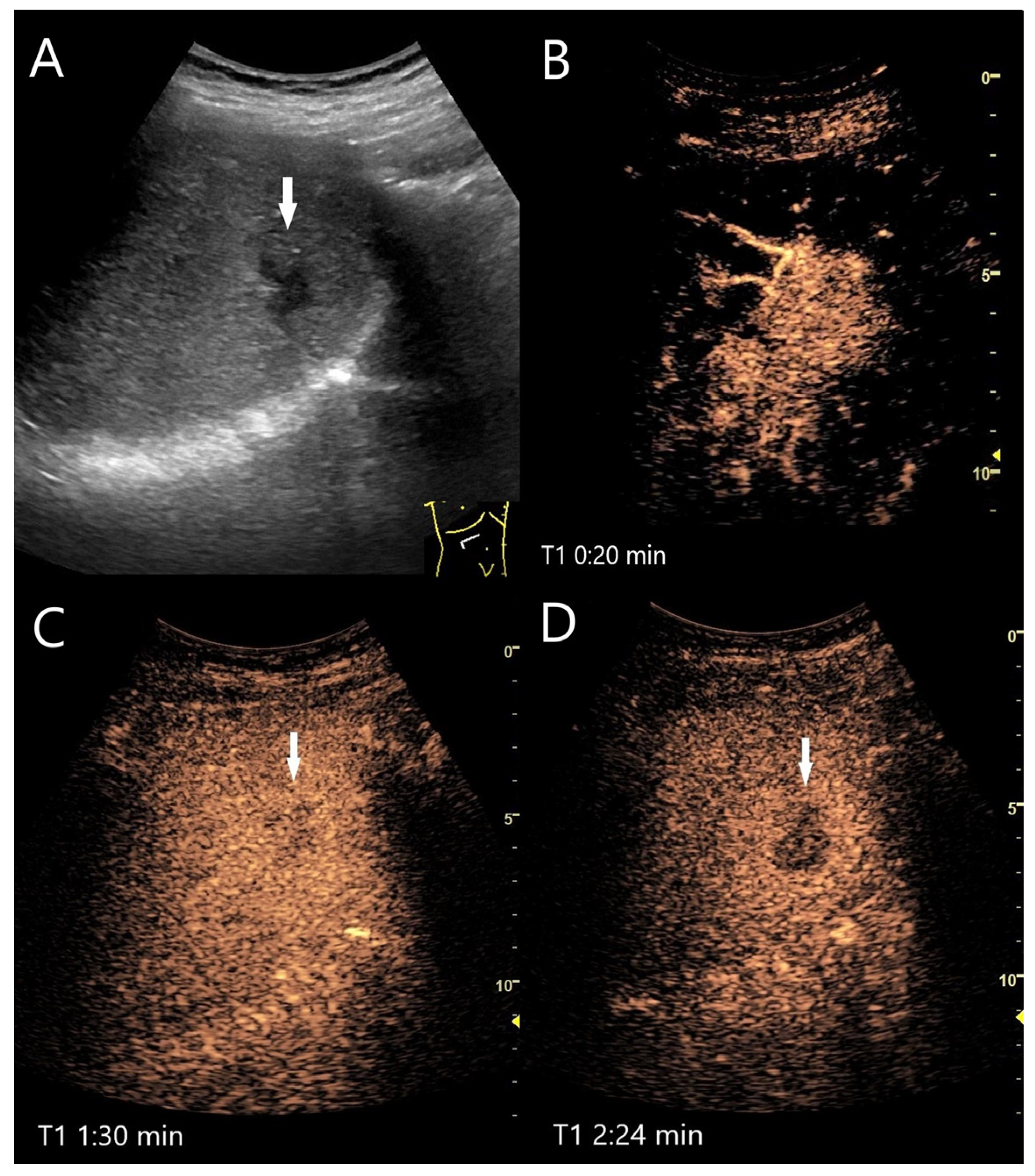
4.1. Hemangioma
4.2. Focal Nodular Hyperplasia (FNH)
| Study | FLL (n) | LP Washout | Comments |
|---|---|---|---|
| Ding 2005 [52] | Benign lesions n = 51 | n = 11/51 (22%) | |
| Hemangioma n = 27 | n = 3/27 (11%) | ||
| FNH n = 16 | n = 2/16 (12.5%) | ||
| Kim 2008 [41] | FNH n = 43 | PVP: Reader 1: 6/43 (14%) Reader 2: 4/43 (9%) | Two readers, hypoenhancement in PVP, no data about LP. |
| HCA n = 19 | PVP: Reader 1: 10/19 (53%) Reader 2: 7/19 (37%) | Two readers, hypoenhancement in PVP, no data about LP. | |
| Strobel 2009 [25] DEGUM-Multicenter-Study | Hemangioma n = 242 | 22.9% | The frequency of hypoenhancement is given as a percentage in this paper. The total number of FNH and hemangiomas allows conclusions to be drawn about the number of patients. |
| FNH n = 170 | 6.4% | ||
| Piscaglia 2010 [46] | FNH n = 90 | n = 3/90 (3.3%) | “Faintly” hypoechoic. |
| Bhayana 2010 [4] | Hypervascular benign FLL n = 74 (overall n = 146 FLL) | 36% of all benign FLL | Washout occurred in 36% of benign and 97% of malignant FLL. The onset of washout after injection was defined as <30 s/after 30–75 s/75–180 s/<180 s. |
| Hemangioma | n = 6/29 (21%) | Mostly (83%) > 180 s. | |
| HCA | n = 5/7 (71%) | Mostly (71%) 75–180 s. | |
| FNH | n = 9/31 (29%) | Mostly (56%) 75–180 s. | |
| Wang 2013 [53] | FNH n = 85 | n = 22/85 (26%) | In 18%, the hypoenhancement was present in PVP. |
| Bertin 2014 [47] | FNH n = 94 | n = 5/94 (5.3%) | Start of washout in the PVP n = 1/5 (20%). Start in the LP n = 4/5 (80%). |
| Roche 2015 [43] | FNH n = 43 | Reader 1: 4/43 (9%) Reader 2: 4/43 (9%) | 31% of all FNH with concomitant steatosis showed washout from the PVP onwards. All FNH with washout had steatosis hepatis at the same time. There was no relation to the size of the lesion. The washout is described as portal venous. Two readers. |
| HCA n = 20 | Reader 1: 9/20 (45%) Reader 2: 7/20 (35%) | Hypoenhancement from the PVP was more frequent with HCA > 35 mm (83%) than < 35 mm (29%). The washout is described as portal venous. Two readers. | |
| Kong 2015 [42] | FNH n = 28 | n = 3/28 (11%) | |
| HCA n = 10 | n = 6/10 (60%) | ||
| Taimr 2017 [51] | FNH n = 181 | n = 8/181 (4%) | |
| HCA n = 143 | n = 21/143 (15%) | ||
| Fang 2019 [27] | Hypoechoic hepatic hemangioma n = 101 | Hypoenhanced or mild fade n = 6/101 (6%) | Center mild fade in PVP in 4/101 (4%). |
4.3. Hepatocellular Adenoma
| Study | FLL (n) | LP Washout | Comments |
|---|---|---|---|
| Laumonier 2012 [62] | All HCA n = 38 | n = 14/38 (37%) of all HCA | PVP was defined as the interval between 45 and 70 s, and the late PVP was observed up to 5 min after injection. |
| H-HCA n = 16 | n = 2/16 (12%) | In 56%, hypoenhancement started in PVP. | |
| I-HCA n = 17 | n = 11/17 (65%) | In 12%, hypoenhancement started in PVP. | |
| U-HCA n = 4 | n = 1/4 (25%) | In this one case, the hypoenhancement started only in the LP, not PVP. | |
| ß-Catenin–Activated HCA n = 1 | n = 0/1 (0%) | This kind of HCA has an increased risk of malignancy but was without hypoenhancement. | |
| Garcovic 2019 [64] Italian multicenter study | All HCA n = 19 | n = 11/19 (58%) | |
| I-HCA n = 14 | n = 7/14 (50%) | In 3 HCAs, washout started in PVP, in 4 in the LP. | |
| β-catenin-activated HCA n = 1 | n = 1/1 (100%) | The washout started in the PVP. | |
| U-HCA n = 4 | n = 3/4 (75%) | In 2 HCAs, the washout started in the PVP, in 1 in the LP. | |
| Chen 2020 [63] | All HCA n = 53 | n = 28/53 (52.8%) | Start of hypoenhancement in PVP: n = 22/53 (41.5%); n = 11/53 (20.8%) central hypoenhanced area. |
| H-HCA n = 12 | n = 1/12 (8.3%) | Start of hypoenhancement in PVP; no central hypoenhanced area. | |
| ß-catenin activated HCAs n = 8 | n = 7/8 (87.5%) | Start of hypoenhancement in PVP: n = 6/8 (75%); n = 1/8: central hypoenhanced area. | |
| I-HCAs n = 31 | n = 19/31 (61,3%) | Start of hypoenhancement in PVP: n = 14/31 (45.2%); n = 9/31 (29%) central hypoenhanced area. | |
| U-HCAs n = 2 | n = 1/2 (50%) | Start of hypoenhancement in PVP: n = 1/2 (50%); n = 1/2 central hypoenhanced area). |
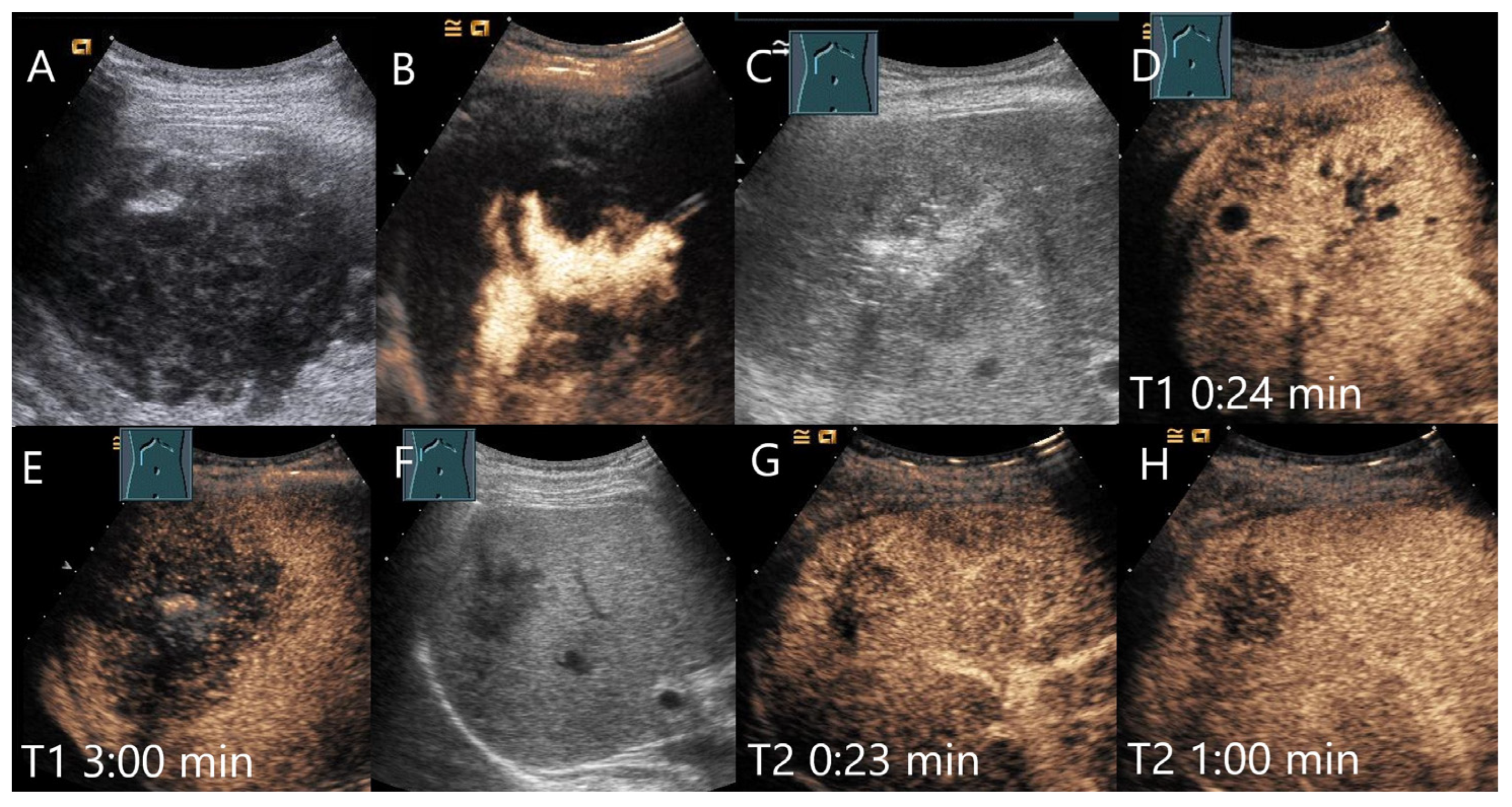
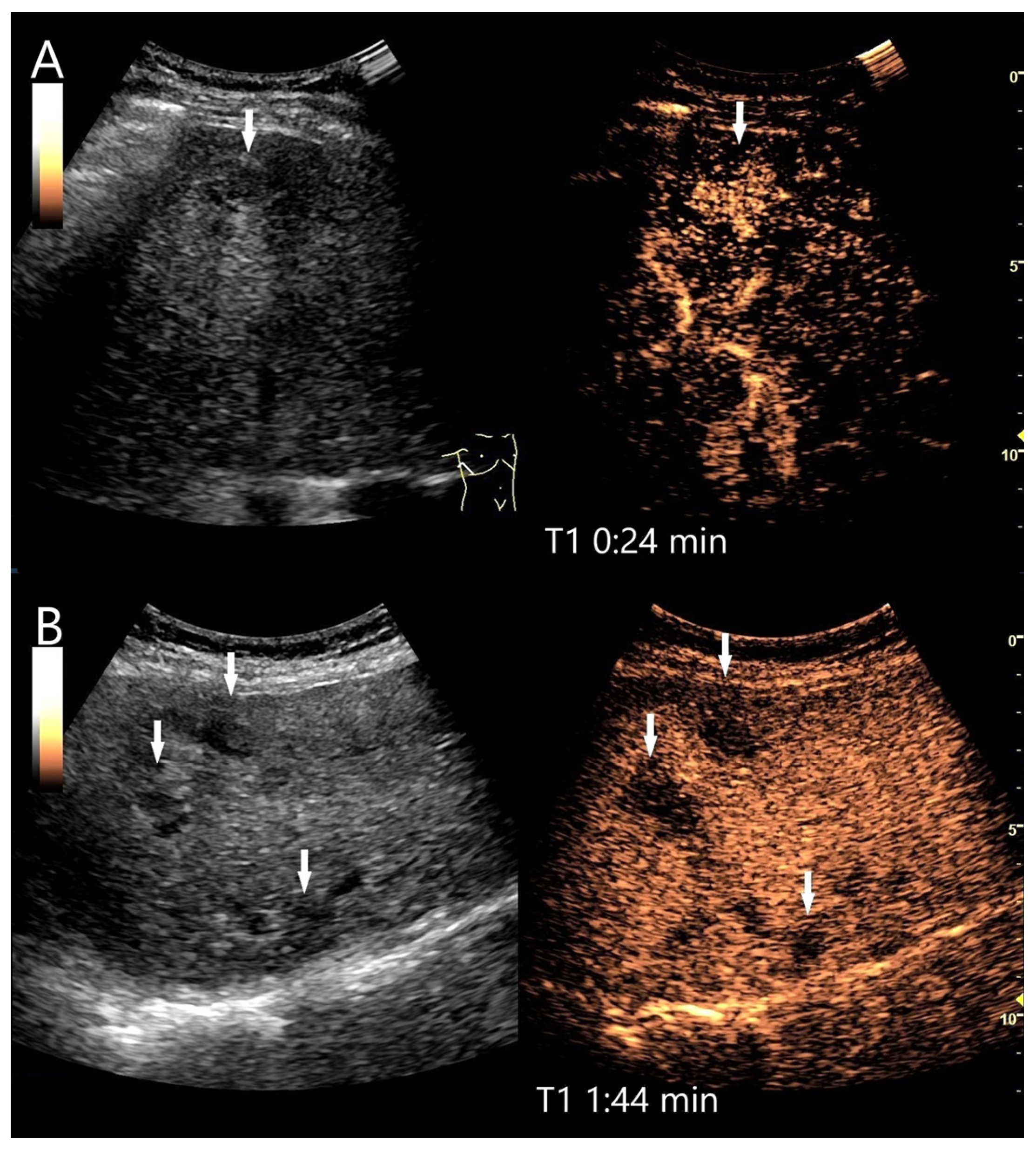
4.4. Inflammatory Lesions
4.4.1. Bacterial (Pyogenic) Liver Abscesses
4.4.2. Mycotic Abscesses
4.4.3. Actinomycetes Abscesses
4.4.4. Parasitic Abscesses
Toxocariasis (Visceral Larva Migrans)
Fasciolosis Hepatica
Paragonimus
Amebic Abscesses
4.4.5. Granulomatous Inflammation
Sarcoidosis
Tuberculosis
4.4.6. Inflammatory Pseudotumor
4.5. Angiomyolipoma, Perivascular Epithelioid Cell Neoplasms (PEComas), and Epithelioid Angiomyolipomas (EAML)
4.5.1. Hepatic Angiomyolipoma (HAML)
4.5.2. Perivascular Epithelioid Cell Neoplasms (PEComas) and Epithelioid Angiomyolipomas (EAMLs)
4.6. Lipoma
4.7. Peliosis
4.8. Cholangiocellular Adenoma
4.9. Extramedullary Hematopoiesis
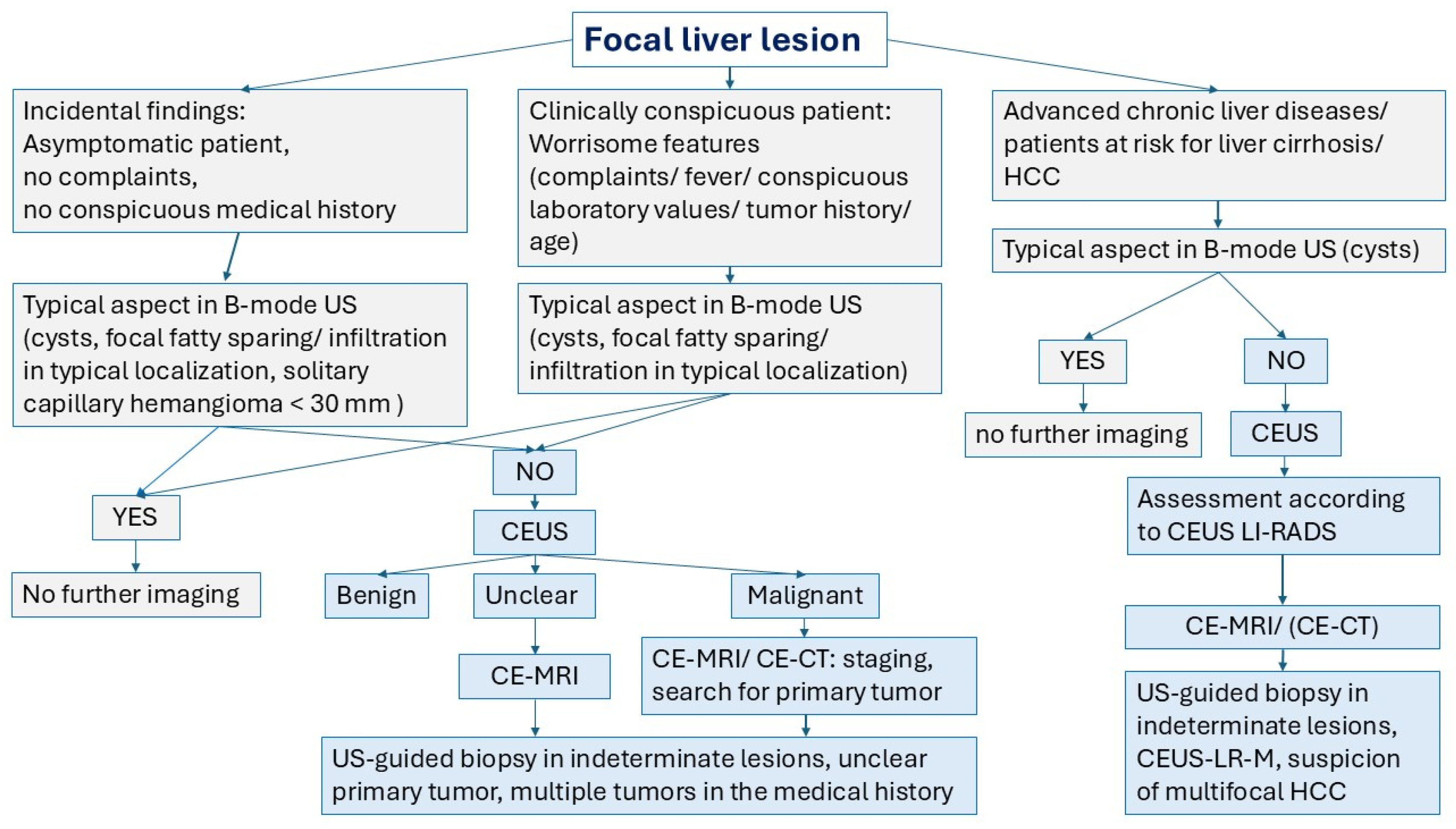
5. Summary of the Typical Appearance of Benign FLL and Possible Causes of Washout
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| AP | arterial phase |
| APHE | arterial phase hyperenhancement |
| ß-HCA | ß-catenin mutated hepatocellular adenoma |
| CDI | color Doppler imaging |
| CE-CT | contrast-enhanced computed tomography |
| CE-MRI | contrast-enhanced magnetic resonance imaging |
| CEUS | contrast-enhanced ultrasound |
| DEGUM | German society of ultrasound in medicine |
| DILI | drug induced liver injury |
| EAML | epithelioid angiomyolipoma |
| EMH | extramedullary hematopoiesis |
| EUS | endoscopic ultrasound |
| FLL | focal liver lesion |
| FNH | focal nodular hyperplasia |
| HAML | hepatic angiomyolipoma |
| HCA | hepatocellular adenoma |
| H-HCA | hepatocyte nuclear factor 1-α HCA |
| HCC | hepatocellular carcinoma |
| IgG4 | immunoglobulin G4 |
| I-HCA | inflammatory HCA |
| IPT | inflammatory pseudotumor |
| LP | late phase |
| MI | mechanical index |
| NRH | nodular regenerative hyperplasia |
| NSG | necrotizing sarcoid granulomatosis |
| PBC | primary biliary cirrhosis |
| PEComa | perivascular epithelioid cell neoplasm |
| PH | peliosis hepatis |
| p.i. | post injection |
| PVP | portal venous phase |
| sh-HCA | sonic hedgehog activated HCA |
| T1 | timer 1 |
| T2 | timer 2 |
| UCA | ultrasound contrast agent |
| U-HCA | unclassified HCA |
| US | ultrasound |
References
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Minami, Y.; Choi, B.I.; Lee, W.J.; Chou, Y.-H.; Jeong, W.K.; Park, M.-S.; Kudo, N.; Lee, M.W.; Kamata, K.; et al. The AFSUMB Consensus Statements and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound using Sonazoid. Ultrasonography 2020, 39, 191–220. [Google Scholar] [CrossRef]
- Kong, W.-T.; Ji, Z.-B.; Wang, W.-P.; Cai, H.; Huang, B.-J.; Ding, H. Evaluation of Liver Metastases Using Contrast-Enhanced Ultrasound: Enhancement Patterns and Influencing Factors. Gut Liver 2016, 10, 283–287. [Google Scholar] [CrossRef]
- Bhayana, D.; Kim, T.K.; Jang, H.-J.; Burns, P.N.; Wilson, S.R. Hypervascular Liver Masses on Contrast-Enhanced Ultrasound: The Importance of Washout. Am. J. Roentgenol. 2010, 194, 977–983. [Google Scholar] [CrossRef]
- Kong, W.; Wang, W.; Huang, B.; Ding, H.; Mao, F. Value of wash-in and wash-out time in the diagnosis between hepatocellular carcinoma and other hepatic nodules with similar vascular pattern on contrast-enhanced ultrasound. J. Gastroenterol. Hepatol. 2014, 29, 576–580. [Google Scholar] [CrossRef]
- Shin, S.K.; Choi, D.J.; Kim, J.H.; Kim, Y.S.; Kwon, O.S. Characteristics of contrast-enhanced ultrasound in distinguishing small (≤3 cm) hepatocellular carcinoma from intrahepatic cholangiocarcinoma. Medicine 2018, 97, e12781. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-J.; Wang, W.; Lu, M.-D.; Xie, X.-Y.; Xu, H.-X.; Xu, Z.-F.; Chen, L.-D.; Wang, Z.; Liang, J.-Y.; Huang, Y.; et al. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer 2015, 4, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Wildner, D.; Bernatik, T.; Greis, C.; Seitz, K.; Neurath, M.F.; Strobel, D. CEUS in Hepatocellular Carcinoma and Intrahepatic Cholangiocellular Carcinoma in 320 Patients—Early or Late Washout Matters: A Subanalysis of the DEGUM Multicenter Trial. Ultraschall Med. 2015, 36, 132–139. [Google Scholar] [CrossRef]
- Kono, Y.; Lyshchik, A.; Cosgrove, D.; Dietrich, C.F.; Jang, H.-J.; Kim, T.K.; Piscaglia, F.; Willmann, J.K.; Wilson, S.R.; Santillan, C.; et al. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): The official version by the American College of Radiology (ACR). Ultraschall Med. 2017, 38, 85–86. [Google Scholar] [CrossRef]
- Meitner-Schellhaas, B.; Jesper, D.; Goertz, R.S.; Zundler, S.; Strobel, D. Washout appearance of hepatocellular carcinomas using standardized contrast-enhanced ultrasound (CEUS) including an extended late phase observation—Real-world data from the prospective multicentre DEGUM study. Clin. Hemorheol. Microcirc. 2023, 84, 413–424. [Google Scholar] [CrossRef]
- Kim, T.K.; Noh, S.Y.; Wilson, S.R.; Kono, Y.; Piscaglia, F.; Jang, H.J.; Lyshchik, A.; Dietrich, C.F.; Willmann, J.K.; Vezeridis, A.; et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017—A review of important differences compared to the CT/MRI system. Clin. Mol. Hepatol. 2017, 23, 280–289. [Google Scholar] [CrossRef]
- Wilson, S.R.; Lyshchik, A.; Piscaglia, F.; Cosgrove, D.; Jang, H.J.; Sirlin, C.; Dietrich, C.F.; Kim, T.K.; Willmann, J.K.; Kono, Y. CEUS LI-RADS: Algorithm, implementation, and key differences from CT/MRI. Abdom. Radiol. 2018, 43, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kim, T.K.; Burns, P.N.; Wilson, S.R. Enhancement Patterns of Hepatocellular Carcinoma at Contrast-enhanced US: Comparison with Histologic Differentiation. Radiology 2007, 244, 898–906. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, X.-C.; Luo, Y.; Li, Y.-Z.; Zhou, X. Efficacy of Contrast-Enhanced Ultrasound Washout Rate in Predicting Hepatocellular Carcinoma Differentiation. Ultrasound Med. Biol. 2015, 41, 1553–1560. [Google Scholar] [CrossRef]
- Qin, X.; Hu, X.; Xiao, W.; Zhu, C.; Ma, Q.; Zhang, C. Preoperative Evaluation of Hepatocellular Carcinoma Differentiation Using Contrast-Enhanced Ultrasound-Based Deep-Learning Radiomics Model. J. Hepatocell. Carcinoma 2023, 10, 157–168. [Google Scholar] [CrossRef]
- Bernatik, T.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.F.; Strobel, D. Unclear Focal Liver Lesions in Contrast-Enhanced Ultrasonography—Lessons to be Learned from the DEGUM Multicenter Study for the Characterization of Liver Tumors. Ultraschall Med. 2010, 31, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Seitz, K.; Greis, C.; Schuler, A.; Bernatik, T.; Blank, W.; Dietrich, C.; Strobel, D. Frequency of Tumor Entities among Liver Tumors of Unclear Etiology Initially Detected by Sonography in the Noncirrhotic or Cirrhotic Livers of 1349 Patients. Results of the DEGUM multicenter study. Ultraschall Med. 2011, 32, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Bernatik, T.; Blank, W.; Schuler, A.; Greis, C.; Dietrich, C.; Seitz, K. Diagnostic accuracy of CEUS in the differential diagnosis of small (</= 20 mm) and subcentimetric (</= 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011, 32, 593–597. [Google Scholar] [CrossRef]
- Seitz, K.; Strobel, D.; Bernatik, T.; Blank, W.; Friedrich-Rust, M.; Herbay, A.; Dietrich, C.F.; Strunk, H.; Kratzer, W.; Schuler, A. Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions—Prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreilandertreffen 2008, Davos. Ultraschall Med. 2009, 30, 383–389. [Google Scholar] [CrossRef]
- Strobel, D.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.; Herbay, A.; Friedrich-Rust, M.; Kunze, G.; Becker, D.; Will, U.; et al. Contrast-enhanced Ultrasound for the Characterization of Focal Liver Lesions—Diagnostic Accuracy in Clinical Practice (DEGUM multicenter trial). Ultraschall Med. 2008, 29, 499–505. [Google Scholar] [CrossRef]
- Wang, F.; Numata, K.; Nihonmatsu, H.; Chuma, M.; Ideno, N.; Nozaki, A.; Ogushi, K.; Tanab, M.; Okada, M.; Luo, W.; et al. Added Value of Ultrasound-Based Multimodal Imaging to Diagnose Hepatic Sclerosed Hemangioma before Biop-sy and Resection. Diagnostics 2022, 12, 2818. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.-Y.; Chen, L.-D.; Zhou, L.-Y.; Wang, Z.; Liu, G.-J.; Huang, Y.; Li, W.; Liu, J.-Y.; Xie, X.-Y.; Lu, M.-D.; et al. Focal Lesions in Fatty Liver: If Quantitative Analysis Facilitates the Differentiation of Atypical Benign from Malignant Lesions. Sci. Rep. 2016, 6, 18640. [Google Scholar] [CrossRef] [PubMed]
- Sirli, R.; Sporea, I.; Săndulescu, D.L.; Popescu, A.; Dănilă, M.; Săftoiu, A.; Spârchez, Z.; Badea, R. Contrast enhanced ultrasound for the diagnosis of liver hemangiomas—Results of a Romanian multicentre study. Med. Ultrason. 2015, 17, 444–450. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Mertens, J.C.; Braden, B.; Schuessler, G.; Ott, M.; Ignee, A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas†. Hepatology 2007, 45, 1139–1145. [Google Scholar] [CrossRef]
- Strobel, D.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.F.; von Herbay, A.; Friedrich-Rust, M.; Bernatik, T. Tumor-Specific Vascularization Pattern of Liver Metastasis, Hepatocellular Carcinoma, Hemangioma and Focal Nodular Hyperplasia in the Differential Diagnosis of 1349 Liver Lesions in Contrast-Enhanced Ultrasound (CEUS). Ultraschall Med. 2009, 30, 376–382. [Google Scholar] [CrossRef]
- Fang, L.; Zhu, Z.; Huang, B.; Ding, H.; Mao, F.; Li, C.; Zeng, M.; Zhou, J.; Wang, L.; Wang, W.; et al. A comparative study of contrast enhanced ultrasound and contrast enhanced magnetic resonance imaging for the detection and characterization of hepatic hemangiomas. Biosci. Trends 2015, 9, 104–110. [Google Scholar] [CrossRef]
- Fang, L.; Huang, B.-J.; Ding, H.; Mao, F.; Li, C.-L.; Zeng, M.-S.; Zhou, J.-J.; Chen, Y.; Wang, W.-P. Contrast-enhanced ultrasound (CEUS) for the diagnosis of hypoechoic hepatic hemangioma in clinical practice. Clin. Hemorheol. Microcirc. 2019, 72, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wermke, W. Sonographische Differentialdiagnose. Lebererkrankungen. Lehrbuch und Systematischer Atlas; Deutscher Ärzte-Verlag: Köln, Germany, 2006. [Google Scholar]
- Kallenbach, M.; Qvartskhava, N.; Weigel, C.; Dorffel, Y.; Berger, J.; Kunze, G.; Luedde, T. Contrast-enhanced ultrasound (CEUS) for characterisation of focal liver lesions. Z. Gastroenterol. 2024, 62, 952–970. [Google Scholar]
- Kim, K.W.; Kim, T.K.; Han, J.K.; Kim, A.Y.; Lee, H.J.; Choi, B.I. Hepatic Hemangiomas with Arterioportal Shunt: Findings at Two-Phase CT. Radiology 2001, 219, 707–711. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, A.Y.; Kim, T.K.; Kim, S.Y.; Kim, M.-J.; Park, M.-S.; Park, S.H.; Lee, K.H.; Kim, J.K.; Kim, P.-N.; et al. Hepatic Hemangiomas with Arterioportal Shunt: Sonographic Appearances with CT and MRI Correlation. Am. J. Roentgenol. 2006, 187, W406–W414. [Google Scholar] [CrossRef]
- Akahoshi, S.; Yamamura, K.; Sato, N.; Oda, E.; Kinoshita, K.; Yuki, H.; Motohara, T.; Deguchi, A.; Komohara, Y.; Beppu, T. A hepatic sclerosed hemangioma with drastic changes in contrast-enhanced ultrasonography. Clin. J. Gastroenterol. 2020, 13, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Franci, L.; Grechi, C.; Giangregorio, F. Contrast-enhanced sonography in the diagnosis of hepatic hemangiomas: Atypical appearance due to the washout of microbubbles. J. Clin. Ultrasound 2013, 41, 361–365. [Google Scholar] [CrossRef]
- Fukukura, Y.; Nakashima, O.; Kusaba, A.; Kage, M.; Kojiro, M. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J. Hepatol. 1998, 29, 470–475. [Google Scholar] [CrossRef]
- Wanless, I.R.; Mawdsley, C.; Adams, R. On the Pathogenesis of Focal Nodular Hyperplasia of the Liver. Hepatology 1985, 5, 1194–1200. [Google Scholar] [CrossRef]
- Brancatelli, G.; Federle, M.P.; Katyal, S.; Kapoor, V. Hemodynamic Characterization of Focal Nodular Hyperplasia Using Three-Dimensional Volume-Rendered Multidetector CT Angiography. Am. J. Roentgenol. 2002, 179, 81–85. [Google Scholar] [CrossRef]
- Gaiani, S.; Piscaglia, F.; Serra, C.; Bolondi, L. Hemodynamics in focal nodular hyperplasia. J. Hepatol. 1999, 31, 576. [Google Scholar] [CrossRef]
- Şirli, R.; Sporea, I.; Popescu, A.; Dănilă, M.; Săndulescu, D.L.; Săftoiu, A.; Moga, T.; Spârchez, Z.; Cijevschi, C.; Mihai, C.; et al. Contrast-enhanced ultrasound for the assessment of focal nodular hyperplasia—Results of a multicentre study. Med. Ultrason. 2021, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Schuessler, G.; Trojan, J.; Fellbaum, C.; Ignee, A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br. J. Radiol. 2005, 78, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, M.; Sirli, R.; Sporea, I.; Bota, S.; Danila, M.; Popescu, A.; Timar, B.; Buzas, R.; Mazilu, O.; Ardelean, O.; et al. The value of Contrast-Enhanced Ultrasound in the characterization of vascular pattern of solid pancreatic lesions. Med. Ultrason. 2015, 17, 16–21. [Google Scholar] [CrossRef][Green Version]
- Kim, T.K.; Jang, H.-J.; Burns, P.N.; Murphy-Lavallee, J.; Wilson, S.R. Focal Nodular Hyperplasia and Hepatic Adenoma: Differentiation with Low-Mechanical-Index Contrast-Enhanced Sonography. Am. J. Roentgenol. 2008, 190, 58–66. [Google Scholar] [CrossRef]
- Kong, W.-T.; Wang, W.-P.; Huang, B.-J.; Ding, H.; Mao, F.; Si, Q. Contrast-Enhanced Ultrasound in Combination with Color Doppler Ultrasound Can Improve the Diagnostic Performance of Focal Nodular Hyperplasia and Hepatocellular Adenoma. Ultrasound Med. Biol. 2015, 41, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.; Pigneur, F.; Tselikas, L.; Roux, M.; Baranes, L.; Djabbari, M.; Costentin, C.; Calderaro, J.; Laurent, A.; Rahmouni, A.; et al. Differentiation of focal nodular hyperplasia from hepatocellular adenomas with low-mecanical-index contrast-enhanced sonography (CEUS): Effect of size on diagnostic confidence. Eur. Radiol. 2015, 25, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Taibbi, A.; Matranga, D.; Malizia, G.; Lagalla, R.; Midiri, M. Hepatic focal nodular hyperplasia: Contrast-enhanced ultrasound findings with emphasis on lesion size, depth and liver echogenicity. Eur. Radiol. 2010, 20, 2248–2256. [Google Scholar] [CrossRef]
- de Figueiredo, G.N.; Mueller-Peltzer, K.; Schwarze, V.; Marschner, C.; Zhang, L.; Rübenthaler, J.; Siepmann, T.; Illigens, B.; Clevert, D. Long-term study analysis of contrast-enhanced ultrasound in the diagnosis of focal nodular hyperplasia. Clin. Hemorheol. Microcirc. 2020, 74, 441–452. [Google Scholar] [CrossRef]
- Piscaglia, F.; Venturi, A.; Mancini, M.; Giangregorio, F.; Vidili, G.; Magnolfi, F.; Mirarchi, M.; Fornari, F.; Bolondi, L. Diagnostic Features of Real-Time Contrast-Enhanced Ultrasound in Focal Nodular Hyperplasia of the Liver. Ultraschall Med. 2010, 31, 276–282. [Google Scholar] [CrossRef]
- Bertin, C.; Egels, S.; Wagner, M.; Huynh-Charlier, I.; Vilgrain, V.; Lucidarme, O. Contrast-enhanced ultrasound of focal nodular hyperplasia: A matter of size. Eur. Radiol. 2014, 24, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-W.; Yang, J.-J.; Lin, X.-Y.; Xue, E.-S.; He, Y.-M.; Gao, S.-D.; Yang, L.; Yu, L.-Y. Effect of fatty liver background on contrast-enhanced ultrasonographic appearance of focal nodular hyperplasia. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 610–615. [Google Scholar]
- Theophil, S. Wertung der Diagnose “Fokal Noduläre Hyperplasie“ Durch Betroffene Frauen in der Generativen Phase. Ph.D. Thesis, Medizinische Fakultät der Charité, Berlin, Germany, 2010. [Google Scholar]
- Bauditz, J.; Fondis, K.; Wermke, W. Sonomorphologie und Kontrastverhalten von Fokal Nodulären Hyperplasien der Leber im Langzeitverlauf. Ultraschall Med. 2006, 27, V_16_1. [Google Scholar] [CrossRef]
- Taimr, P.; Bröker, M.E.; Dwarkasing, R.S.; Hansen, B.E.; de Knegt, R.J.; De Man, R.A.; Ijzermans, J.N. A Model-Based Prediction of the Probability of Hepatocellular Adenoma and Focal Nodular Hyperplasia Based on Characteristics on Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2017, 43, 2144–2150. [Google Scholar] [CrossRef]
- Ding, H.; Wang, W.P.; Huang, B.J.; Wei, R.X.; He, N.A.; Qi, Q.; Li, C.L. Imaging of focal liver lesions: Low-mechanical-index real-time ultrasonography with SonoVue. J. Ultrasound Med. 2005, 24, 285–297. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.-D.; Lu, M.-D.; Liu, G.-J.; Shen, S.-L.; Xu, Z.-F.; Xie, X.-Y.; Wang, Y.; Zhou, L.-Y. Contrast-enhanced ultrasound features of histologically proven focal nodular hyperplasia: Diagnostic performance compared with contrast-enhanced CT. Eur. Radiol. 2013, 23, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Haring, M.P.D.; Elfrink, A.K.E.; Oudmaijer, C.A.J.; Andel, P.C.M.; Furumaya, A.; de Jong, N.; Willems, C.J.J.M.; Huits, T.; Sijmons, J.M.L.; Belt, E.J.T.; et al. A nationwide assessment of hepatocellular adenoma resection: Indications and pathological discordance. Hepatol. Commun. 2023, 7, e2110. [Google Scholar] [CrossRef] [PubMed]
- Haring, M.P.; de Haas, R.J.; van Vilsteren, F.G.; Klaase, J.M.; Duiker, E.W.; Blokzijl, H.; de Jong, K.P.; de Meijer, V.E.; Cuperus, F.J.; de Boer, Y.; et al. Variation in the management of benign liver tumors: A European survey and case vignette study. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102094. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of benign liver tumours. J. Hepatol. 2016, 65, 386–398. [Google Scholar] [CrossRef]
- Marrero, J.A.; Ahn, J.; Rajender Reddy, K.; Americal College of Gastroenterology. ACG clinical guideline: The diagnosis and management of focal liver lesions. Am. J. Gastroenterol. 2014, 109, 1328–1347, quiz 48.. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Tannapfel, A.; Jang, H.-J.; Kim, T.K.; Burns, P.N.; Dong, Y. Ultrasound Imaging of Hepatocellular Adenoma Using the New Histology Classification. Ultrasound Med. Biol. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Nault, J.-C.; Couchy, G.; Balabaud, C.; Morcrette, G.; Caruso, S.; Blanc, J.-F.; Bacq, Y.; Calderaro, J.; Paradis, V.; Ramos, J.; et al. Molecular Classification of Hepatocellular Adenoma Associates with Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology 2017, 152, 880–894.e6. [Google Scholar] [CrossRef]
- Nault, J.-C.; Paradis, V.; Cherqui, D.; Vilgrain, V.; Zucman-Rossi, J. Molecular classification of hepatocellular adenoma in clinical practice. J. Hepatol. 2017, 67, 1074–1083. [Google Scholar] [CrossRef]
- Manichon, A.-F.; Bancel, B.; Durieux-Millon, M.; Ducerf, C.; Mabrut, J.-Y.; Lepogam, M.-A.; Rode, A. Hepatocellular Adenoma: Evaluation with Contrast-Enhanced Ultrasound and MRI and Correlation with Pathologic and Phenotypic Classification in 26 Lesions. HPB Surg. 2012, 2012, 418745. [Google Scholar] [CrossRef]
- Laumonier, H.; Cailliez, H.; Balabaud, C.; Possenti, L.; Zucman-Rossi, J.; Bioulac-Sage, P.; Trillaud, H. Role of Contrast-Enhanced Sonography in Differentiation of Subtypes of Hepatocellular Adenoma: Correlation with MRI Findings. Am. J. Roentgenol. 2012, 199, 341–348. [Google Scholar] [CrossRef]
- Chen, K.; Dong, Y.; Zhang, W.; Han, H.; Mao, F.; Zhang, Q.; Zheng, Z.; He, W.; Wang, W.-P. Analysis of contrast-enhanced ultrasound features of hepatocellular adenoma according to different pathological molecular classifications. Clin. Hemorheol. Microcirc. 2020, 76, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, M.; Faccia, M.; Meloni, F.; Bertolini, E.; de Sio, I.; Calabria, G.; Francica, G.; Vidili, G.; Riccardi, L.; Zocco, M.A.; et al. Contrast-enhanced ultrasound patterns of hepatocellular adenoma: An Italian multicenter experience. J. Ultrasound 2019, 22, 157–165. [Google Scholar] [CrossRef]
- Liu, G.J.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Zheng, Y.L.; Liang, J.Y.; Wang, W. Real-time contrast-enhanced ultrasound imaging of infected focal liver lesions. J. Ultrasound Med. 2008, 27, 657–666. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Chen, S.; Liu, X.; Gu, P. Diagnosis efficacy of CEUS for hepatic inflammatory lesions. J. Clin. Lab. Anal. 2020, 34, e23231. [Google Scholar] [CrossRef]
- Popescu, A.; Sporea, I.; Şirli, R.; Dănilă, M.; Mare, R.; Taşcău, O.G.; Moga, T. Does Contrast Enhanced Ultrasound improve the management of liver abscesses? A single centre experience. Med. Ultrason. 2015, 17, 451–455. [Google Scholar]
- Francica, G. Intracavitary contrast-enhanced ultrasound in ultrasound-guided percutaneous management of abdominal fluid collections/abscesses by a single clinician: An example of point-of-care ultrasound. J. Ultrasound 2020, 23, 175–181. [Google Scholar] [CrossRef]
- Kunze, G.; Staritz, M.; Köhler, M. Contrast-Enhanced Ultrasound in Different Stages of Pyogenic Liver Abscess. Ultrasound Med. Biol. 2015, 41, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Dobek, A.; Kobierecki, M.; Ciesielski, W.; Grząsiak, O.; Kosztowny, K.; Fabisiak, A.; Białek, P.; Stefańczyk, L. Comparative efficacy of contrast-enhanced ultrasound versus B-mode ultrasound in the diagnosis and monitoring of hepatic abscesses. Pol. J. Radiol. 2024, 89, e470–e479. [Google Scholar] [CrossRef] [PubMed]
- Dobek, A.; Kobierecki, M.; Kosztowny, K.; Grząsiak, O.; Fabisiak, A.; Falenta, K.; Stefańczyk, L. Utility of Contrast-Enhanced Ultrasound in Optimizing Hepatic Abscess Treatment and Monitoring. J. Clin. Med. 2024, 13, 5046. [Google Scholar] [CrossRef]
- Boccatonda, A.; D’Ardes, D.; Cocco, G.; Cipollone, F.; Schiavone, C. Ultrasound and hepatic abscess: A successful alliance for the internist. Eur. J. Intern. Med. 2019, 68, e19–e21. [Google Scholar] [CrossRef]
- Sun, K.; Zhu, W.; Luo, Y.; Li, Y.; Zhou, X. Transient Segmental Enhancement of Pyogenic Liver Abscess: A Comparison Between Contrast-Enhanced Ultrasound and Computed Tomography. J. Comput. Assist. Tomogr. 2018, 42, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Wei, H.; Zhang, Y.; Gao, G.; Deng, B.; Huang, L.; Wang, Q.; Zheng, X.; Luo, X. The Effect of Contrast-enhanced Ultrasound via Vessels and Surgical Drains Guidance Percutaneous Catheter Drainage in the Treatment of Pyogenic Liver Abscess. Curr. Med. Imaging 2024, 20, e15734056261616. [Google Scholar] [CrossRef]
- Francica, G. Pyogenic liver abscess: Contrast-enhanced ultrasound allows morpho-evolutive classification and guides personalized management. Explor. Med. 2022, 3, 289–299. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, K.; Zhao, Y.; Pan, M.; Zhang, C.; Wei, S. Sarcomatoid hepatocellular carcinoma mimicking hepatic abscess: A case report. Medicine 2020, 99, e22489. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, N.; Thomsen, T.; Kabaalioglu, A.; Srivastava, D.; Rösch, S.S.; Dietrich, C.F. Ultrasound and contrast-enhanced ultrasound (CEUS) in infective liver lesions. Z. Gastroenterol. 2021, 59, 1309–1321. [Google Scholar] [CrossRef]
- Gorg, C.; Bert, T.; Klassen, E.; Neesse, A.; Barth, P.; Neubauer, A. Contrast enhanced sonographic patterns of hepatic candidiasis. Z. Gastroenterol. 2010, 48, 678–682. [Google Scholar]
- Wang, H.; Yu, H.; Bai, D.; Yao, D.; Han, Y.; Shi, Y.; Wang, Z. Value of diffusion-weighted imaging in diagnosis and therapy response assessment of hepatic fungal infection in patients with acute leukemia. Immun. Inflamm. Dis. 2023, 11, e843. [Google Scholar] [CrossRef]
- Feier, D.; Socaciu, M.A.; Anton, O.; Al Hajjar, N.; Badea, R. The combined role of intravenous contrast enhanced ultrasound (CEUS) and computed tomography (CT) in liver abscess diagnosis. Chirurgia 2012, 107, 343–351. [Google Scholar]
- Akrak, Ö.; Müderrisoǧlu, I.; Bedirli, A.; Ince, Ö.; Canöz, Ö. Abdominal Actinomycosis appearing as an Intra-abdominal Tumoral Mass. Turk. J. Med. Sci. 2003, 33, 53–55. [Google Scholar]
- Garner, J.P.; Macdonald, M.; Kumar, P.K. Abdominal actinomycosis. Int. J. Surg. 2007, 5, 441–448. [Google Scholar] [CrossRef]
- Ignee, A.; Möller, K.; Thees-Laurenz, R.; Zadeh, E.S.; Görg, C.; Correas, J.M.; Chaubal, N.; Sansone, V.; Jenssen, C.; Dong, Y.; et al. Comments and illustrations of the WFUMB CEUS liver guidelines: Rare focal liver lesions—Infectious (bacterial). Med. Ultrason. 2023, 25, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Cretu, C.; Dong, Y. Imaging of toxocariasis. Adv. Parasitol. 2020, 109, 165–187. [Google Scholar] [PubMed]
- Dietrich, C.F.; Kabaalioglu, A.; Brunetti, E.; Richter, J. Fasciolosis. Z. Gastroenterol. 2015, 53, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Atkinson, N.S.; Lee, W.J.; Kling, K.; Neumayr, A.; Braden, B.; Richter, J.; Akpata, R.; Southisavath, P.; Schreiber-Dietrich, D.; et al. Never seen before? Opisthorchiasis and Clonorchiasis. Zeitschrift Gastroenterol. 2018, 56, 1513–1520. [Google Scholar] [CrossRef]
- Lu, Q.; Ling, W.W.; Ma, L.; Huang, Z.X.; Lu, C.L.; Luo, Y. Contrast-enhanced ultrasonographic findings of hepatic paragonimiasis. World J. Gastroenterol. 2013, 19, 2087–2091. [Google Scholar] [CrossRef]
- Ralls, P.; Barnes, P.; Radin; Colletti, P.; Halls, J. Sonographic features of amebic and pyogenic liver abscesses: A blinded comparison. Am. J. Roentgenol. 1987, 149, 499–501. [Google Scholar] [CrossRef]
- Marenga, G.; Traficante, S.; Ragonici, S.; Vincenzi, C.; Rocchetti, M.; De Rito, G.; Fonsi, G.B.; Messineo, D. Successful Diagnosis of a Longstanding Giant Amoebic Liver Abscess Using Contrast-Enhanced Ultrasonography (CEUS): A Case Report in a Western Country. Am. J. Case Rep. 2019, 20, 493–498. [Google Scholar] [CrossRef]
- Mironova, M.; Gopalakrishna, H.; Franco, G.R.; Holland, S.M.; Koh, C.; Kleiner, D.E.; Heller, T. Granulomatous liver diseases. Hepatol. Commun. 2024, 8, e0392. [Google Scholar] [CrossRef]
- Tana, C.; Schiavone, C.; Ticinesi, A.; Ricci, F.; Giamberardino, M.A.; Cipollone, F.; Silingardi, M.; Meschi, T.; Dietrich, C.F. Ultrasound imaging of abdominal sarcoidosis: State of the art. World J. Clin. Cases 2019, 7, 809–818. [Google Scholar] [CrossRef]
- Tana, C.; Dietrich, C.F.; Schiavone, C. Hepatosplenic Sarcoidosis: Contrast-Enhanced Ultrasound Findings and Implications for Clinical Practice. BioMed Res. Int. 2014, 2014, 926203. [Google Scholar] [CrossRef]
- Grzelak, P.; Augsburg, Ł.; Majos, A.; Stefańczyk, L.; Górski, P.; Piotrowski, W.; Antczak, A. Diagnostic Potential of Contrast-Enhanced Ultrasound (CEUS) In the Assessment of Spleen and Liver Granulomas in the Course of Sarcoidosis. Adv. Respir. Med. 2013, 81, 424–428. [Google Scholar] [CrossRef]
- Stang, A.; Keles, H.; Hentschke, S.; von Seydewitz, C.U.; Dahlke, J.; Malzfeldt, E.; Braumann, D. Differentiation of Benign From Malignant Focal Splenic Lesions Using Sulfur Hexafluoride–Filled Microbubble Contrast-Enhanced Pulse-Inversion Sonography. Am. J. Roentgenol. 2009, 193, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, R.; Grima, P.; Tundo, P. Characterization of nontraumatic focal splenic lesions using contrast-enhanced sonography. J. Clin. Ultrasound 2011, 39, 310–315. [Google Scholar] [CrossRef]
- Tana, C.; Iannetti, G.; D’alessandro, P.; Tana, M.; Mezzetti, A.; Schiavone, C. Pitfalls of contrast-enhanced ultrasound (CEUS) in the diagnosis of splenic sarcoidosis. J. Ultrasound 2013, 16, 75–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Essop, A.R.; Posen, J.A.; Hodkinson, J.H.; Segal, I. Tuberculosis Hepatitis: A Clinical Review of 96 Cases. QJM Int. J. Med. 1984, 53, 465–477. [Google Scholar] [CrossRef]
- Hickey, A.J.; Gounder, L.; Moosa, M.-Y.S.; Drain, P.K. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect. Dis. 2015, 15, 209. [Google Scholar] [CrossRef]
- Das, C.J.; Rednam, N.; Vora, Z.; Aggarwal, A.; Chandrashekhara, S.H.; Kundra, V. Abdominal visceral tuberculosis: A malignancy mimic. Abdom. Radiol. 2023, 48, 2705–2715. [Google Scholar] [CrossRef]
- Yu, R.-S.; Zhang, S.-Z.; Wu, J.-J.; Li, R.-F. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J. Gastroenterol. 2004, 10, 1639–1642. [Google Scholar] [CrossRef]
- Cao, B.S.; Li, X.L.; Li, N.; Wang, Z.Y. The nodular form of hepatic tuberculosis: Contrast-enhanced ultrasonographic findings with pathologic correlation. J. Ultrasound Med. 2010, 29, 881–888. [Google Scholar] [CrossRef]
- Hu, N.; Wu, Y.; Tang, M.; Luo, T.; Yuan, S.; Li, C.; Lei, P. Case report: Hepatic tuberculosis mimicking hepatocellular carcinoma in a patient with cirrhosis induced by hepatitis B virus. Front. Med. 2022, 9, 1005680. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.-B.; Ni, S.-L.; Zhang, S.-N.; Shen, C.-B.; Lu, M.-Q. A case of multiple macronodular hepatic tuberculosis difficult to differentiate from hepatocellular carcinoma with intrahepatic metastasis: CT-guided fine needle aspiration biopsy confirmed the diagnosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8240–8244. [Google Scholar] [PubMed]
- Alsaif, H.S.; Hassan, A.; Refai, O.; Awary, K.; Kussaibi, H.; Ismail, M.H.; Alghnimi, I. Concomitant hepatic tuberculosis and hepatocellular carcinoma: A case report and review of the literature. BMC Surg. 2021, 21, 2. [Google Scholar] [CrossRef]
- Kale, A.; Patil, P.S.; Chhanchure, U.; Deodhar, K.; Kulkarni, S.; Mehta, S.; Tandon, S. Hepatic tuberculosis masquerading as malignancy. Hepatol. Int. 2022, 16, 463–472. [Google Scholar] [CrossRef]
- Choudhury, A.; Shukla, J.; Mahajan, G.; Jha, D.K.; Gupta, P.; Sharma, V. Hepatic tuberculosis: Myriad of hues. GERMS 2021, 11, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Schininà, V.; Albarello, F.; Cristofaro, M.; Di Stefano, F.; Fusco, N.; Cuzzi, G.; Arend, S.M.; Goletti, D.; Rizzi, E.B. Diagnostic imaging of hepatic tuberculosis: Case series. Int. J. Tuberc. Lung Dis. 2018, 22, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Forgione, A.; Tovoli, F.; Ravaioli, M.; Renzulli, M.; Vasuri, F.; Piscaglia, F.; Granito, A. Contrast-Enhanced Ultrasound LI-RADS LR-5 in Hepatic Tuberculosis: Case Report and Literature Review of Imaging Features. Gastroenterol. Insights 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Li, W.; Wang, Y.; Xiong, K.; Zhang, Z.; Fan, L. Diagnostic yield of Xpert MTB/RIF on contrast-enhanced ultrasound-guided pleural biopsy specimens for pleural tuberculosis. Int. J. Infect. Dis. 2021, 108, 89–95. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Zhang, L.; Ni, T. The value of histopathologic examination and Xpert (MTB/RIF) assay in diagnosis of cervical lymph node tuberculosis after coarse needle biopsy guided by CEUS: A retrospective analysis of 612 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1951–1957. [Google Scholar] [CrossRef]
- Zen, Y.; Fujii, T.; Sato, Y.; Masuda, S.; Nakanuma, Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod. Pathol. 2007, 20, 884–894. [Google Scholar] [CrossRef]
- Liao, M.; Wang, C.; Zhang, B.; Jiang, Q.; Liu, J.; Liao, J. Distinguishing Hepatocellular Carcinoma from Hepatic Inflammatory Pseudotumor Using a Nomogram Based on Contrast-Enhanced Ultrasound. Front. Oncol. 2021, 11, 737099. [Google Scholar] [CrossRef]
- Gesualdo, A.; Tamburrano, R.; Gentile, A.; Giannini, A.; Palasciano, G.; Palmieri, V.O. A Diagnosis of Inflammatory Pseudotumor of the Liver by Contrast Enhaced Ultrasound and Fine-Needle Biopsy: A Case Report. Eur. J. Case Rep. Intern. Med. 2017, 4, 000495. [Google Scholar] [CrossRef] [PubMed]
- Renzing, N.; Ebsen, M.; Schwerk, W. Inflammatory pseudotumours of the liver associated with Crohn’s disease: A possible pitfall in contrast-enhanced ultrasound. Z. Gastroenterol. 2011, 49, 827–831. [Google Scholar] [CrossRef]
- Guarino, B.; Catalano, O.; Corvino, A.; Corvino, F.; Amore, A.; Petrillo, A. Hepatic inflammatory pseudotumor: Educational value of an incorrect diagnosis at contrast-enhanced ultrasound. J. Med. Ultrason. 2015, 42, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-T.; Wang, W.-P.; Shen, H.-Y.; Xue, H.-Y.; Liu, C.-R.; Huang, D.-Q.; Wu, M. Hepatic inflammatory pseudotumor mimicking malignancy: The value of differential diagnosis on contrast enhanced ultrasound. Med. Ultrason. 2021, 23, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-T.; Wang, W.-P.; Cai, H.; Huang, B.-J.; Ding, H.; Mao, F. The analysis of enhancement pattern of hepatic inflammatory pseudotumor on contrast-enhanced ultrasound. Abdom. Imaging 2014, 39, 168–174. [Google Scholar] [CrossRef]
- Chen, K.; Luo, M.; He, Y.; Huang, D.; Tang, M.; Shi, J.; Qin, H.; Deng, M.; Wang, W.; Kong, W. Clinical and Multimodal Imaging Features of Hepatic Inflammatory Pseudotumors: A Two-Center Retrospective Study. J. Ultrasound Med. 2024, 44, 691–701. [Google Scholar] [CrossRef]
- Schuessler, G.; Fellbaum, C.; Fauth, F.; Jacobi, V.; Schmidt-Matthiesen, A.; Ignee, A.; Dietrich, C.F. The infammatory pseudotumour—An unusual liver tumour. Ultraschall Med. 2006, 27, 273–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.-X.; Liao, Y.; Yu, Y.; Guo, R.; Han, X.; Lan, L.; Zhou, J. Contrast-enhanced ultrasound features of hepatic angiomyolipoma: Comparison with AFP-negative and non-viral hepatocellular carcinoma. Ultrasound Int. Open 2024, 10, a23186654. [Google Scholar] [CrossRef]
- Fan, P.-L.; Ji, Z.-B.; Cao, J.-Y.; Xu, C.; Dong, Y.; Wang, W.-P. Baseline and contrast-enhanced ultrasound features of hepatic epithelioid angiomyolipoma. Clin. Hemorheol. Microcirc. 2022, 80, 447–461. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhuang, Y.; Peng, J.; Huang, F.; Zhang, S. Hepatic perivascular epithelioid cell neoplasm: A clinical and pathological experience in diagnosis and treatment. Mol. Clin. Oncol. 2017, 6, 487–493. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, J.M.; Ying, J.Q.; Ge, Y.P. Characteristics and treatment strategy of hepatic angiomyolipoma: A series of 94 patients collected from four institutions. J. Gastrointestin. Liver Dis. 2011, 20, 65–69. [Google Scholar] [PubMed]
- Li, T.; Wang, L.; Yu, H.-H.; Sun, H.-C.; Qin, L.-X.; Ye, Q.-H.; Fan, J.; Tang, Z.-Y. Hepatic angiomyolipoma: A retrospective study of 25 cases. Surg. Today 2008, 38, 529–535. [Google Scholar] [CrossRef]
- Boccatonda, A.; Marcellini, M.M.; Ruggeri, E.; Felicani, C.; Brighenti, A.; Loiacono, R.; Ercolani, G.; Serra, C. Ceus features of liver pecoma: A case report and literature review. J. Ultrasound 2024, 28, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-X.; Wang, W.-P.; Ding, H.; Huang, B.-J.; Li, C.-L.; Fan, P.-L.; Hou, J.; He, N.-A. Hepatic Angiomyolipoma: Contrast Patterns with SonoVue-enhanced Real-time Gray-scale Ultrasonography. Asian Pac. J. Cancer Prev. 2012, 13, 493–497. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Hua, X.; Cai, P.; Zhong, H.; Guo, Y.; Ding, S.; Yan, X. Real-time contrast-enhanced ultrasonography of resected and immunohistochemically proven hepatic angiomyolipomas. Abdom. Imaging 2010, 35, 676–682. [Google Scholar] [CrossRef]
- Matrood, S.; Görg, C.; Zadeh, E.S.; Alhyari, A. Hepatic perivascular epithelioid cell tumor (PEComa): Contrast-enhanced ultrasound (CEUS) characteristics—A case report and literature review. Clin. J. Gastroenterol. 2023, 16, 444–449. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Y.; Liu, S.; Zhang, M.; Qiao, B. Hepatic perivascular epithelioid cell tumor: A retrospective analysis of 36 cases. Front. Oncol. 2024, 14, 1416254. [Google Scholar] [CrossRef]
- Tan, Y.; Xie, X.; Lin, Y.; Huang, T.; Huang, G. Hepatic epithelioid angiomyolipoma: Clinical features and imaging findings of contrast-enhanced ultrasound and CT. Clin. Radiol. 2017, 72, 339.e1–339.e6. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, X.; Li, S.; Li, K. Contrast-Enhanced Ultrasound Findings and Differential Diagnosis of Hepatic Epithelioid Angiomyolipoma Compared with Hepatocellular Carcinoma. Ultrasound Med. Biol. 2020, 46, 1403–1411. [Google Scholar] [CrossRef]
- Panahova, S.; Rempp, H.; Sipos, B.; Malek, N.P.; Boozari, B. Primary perivascular epitheloid cell tumour (PEComa) of the liver—Is a new entity of the liver tumors? Z. Gastroenterol. 2015, 53, 399–408. [Google Scholar] [PubMed]
- Dong, Y.; Guggisberg, E.; Wang, W.-P.; Zadeh, E.S.; Görg, C.; Möller, K.; Berzigotti, A.; Chaubal, N.; Cui, X.W.; De Molo, C.; et al. Comments and illustrations of the WFUMB CEUS liver guidelines: Rare benign focal liver lesion, part II. Med. Ultrason. 2024, 26, 168–177. [Google Scholar] [CrossRef]
- Zou, M.-H.; Huang, Q.; Zou, Q.; Jiang, Y.; Ju, J.-X.; Zhou, H.-C.; Jiao, J.; Zheng, R.-Q. Clinical and Contrast-enhanced Ultrasound Characteristics of Epithelioid and Classic Hepatic Angiomyolipoma: Comparison with Alpha-fetoprotein–negative Hepatocellular Carcinoma. Ultrasound Med. Biol. 2021, 47, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Abhirup, B.; Kaushal, K.; Sanket, M.; Ganesh, N. Malignant hepatic perivascular epithelioid cell tumor (PEComa)—Case report and a brief review. J. Egypt. Natl. Cancer Inst. 2015, 27, 239–242. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.-W.; Hong, D.-F.; Tao, R.; Chen, Y.; Shang, M.-J.; Zhang, Y.-H. Primary hepatic epithelioid angiomyolipoma: A malignant potential tumor which should be recognized. World J. Gastroenterol. 2016, 22, 4908–4917. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Kwiatkowski, D.J. Perivascular epithelioid cell neoplasms: Pathology and pathogenesis. Hum. Pathol. 2010, 41, 1–15. [Google Scholar] [CrossRef]
- Bruneton, J.-N.; Kerboul, P.; Drouillard, J.; Menu, Y.; Normand, F.; Santini, N. Hepatic lipomas: Ultrasound and computed tomographic findings. Gastrointest. Radiol. 1987, 12, 299–303. [Google Scholar] [CrossRef]
- Xu, H.-X.; Liu, G.-J.; Lu, M.-D.; Xie, X.-Y.; Xu, Z.-F.; Zheng, Y.-L.; Liang, J.-Y. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J. Clin. Ultrasound 2006, 34, 261–272. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.-P.; Lim, A.; Lee, W.J.; Clevert, D.-A.; Höpfner, M.; Tannapfel, A.; Dietrich, C.F. Ultrasound findings in peliosis hepatis. Ultrasonography 2021, 40, 546–554. [Google Scholar] [CrossRef]
- Dave, Y.A.; Gupta, A.; Shah, M.M.; Carpizo, D. Liver haematoma as a presentation of peliosis hepatis. BMJ Case Rep. 2019, 12, e226737. [Google Scholar] [CrossRef]
- Spiesecke, P.; Pahl, S.; Fischer, T.; Lerchbaumer, M.H. Solitary peliosis hepatis mimics a liver metastasis on contrast-enhanced ultrasound. Radiol. Case Rep. 2023, 18, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, D.; Palmieri, A.; Pedullà, G.; Pasta, V.; D’orazi, V.; Grazi, G.L. Peliosis hepatis: Personal experience and literature review. World J. Gastroenterol. 2015, 21, 13188–13194. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Holman, M.J.; Riley, T.R.; Abendroth, C.S.; Langhoff, E.G.; Yang, H.C. Peloisis hepatis due to Bartonella henselae in transplantation: A hemato-hepato-renal syndrome. Transplantation 1998, 65, 1000–1003. [Google Scholar] [CrossRef]
- Gazineo, J.L.D.; Trope, B.M.; Maceira, J.P.; May, S.B.; Coelho, J.M.C.d.O.; Lambert, J.S.; Nogueira, S.A. Bacillary angiomatosis: Description of 13 cases reported in five reference centers for AIDS treatment in Rio de Janeiro, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, W.; Luo, R.; Song, D.; Wang, X.; Gao, Q.; Fan, J.; Zhou, J.; Rao, S.; Wang, X. Newly detected liver nodules with a history of colorectal cancer: Are they metastatic? Review of 2,632 cases in a single center. Ann. Transl. Med. 2021, 9, 1079. [Google Scholar] [CrossRef]
- Biswas, S.; Gogna, S.; Patel, P. A Fatal Case of Intra-Abdominal Hemorrhage Following Diagnostic Blind Percutaneous Liver Biopsy in a Patient with Peliosis Hepatis. Gastroenterol. Res. 2017, 10, 318–321. [Google Scholar] [CrossRef]
- Xu, H.X.; Xie, X.Y.; Lu, M.D.; Liu, G.J.; Xu, Z.F.; Liang, J.Y.; Chen, L.D. Unusual benign focal liver lesions: Findings on real-time contrast-enhanced sonography. J. Ultrasound. Med. 2008, 27, 243–254. [Google Scholar] [CrossRef]
- Schuldes, M.; Weickert, U. Contrast-enhanced ultrasound in suspected liver metastases. Dtsch. Med. Wochenschr. 2011, 136, 1255–1256. [Google Scholar] [CrossRef]
- Grønlykke, L.; Tarp, B.; Dutoit, S.H.; Wilkens, R. Peliosis hepatis: A complicating finding in a case of biliary colic. BMJ Case Rep. 2013, 2013, bcr2013200539. [Google Scholar] [CrossRef]
- Loizides, A.; Glodny, B.; Zoller, H.; Zelger, B.G.; Junker, D.; Henninger, B.; Putzer, D.; Gruber, H. Contrast enhanced ultrasound of a rare case of Peliosis hepatis. Med. Ultrason. 2017, 19, 114–116. [Google Scholar] [CrossRef]
- Allaire, G.S.; Rabin, L.; Ishak, K.G.; Sesterhenn, I.A. Bile duct adenoma. A study of 152 cases. Am. J. Surg. Pathol. 1988, 12, 708–715. [Google Scholar] [CrossRef]
- Ignee, A.; Piscaglia, F.; Ott, M.; Salvatore, V.; Dietrich, C.F. A benign tumour of the liver mimicking malignant liver disease—Cholangiocellular adenoma. Scand. J. Gastroenterol. 2009, 44, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, L. Villous adenoma of extrahepatic bile duct: Contrast-enhanced sonography findings. J. Clin. Ultrasound 2008, 36, 39–41. [Google Scholar] [CrossRef]
- Tefas, C.; Tanţău, M.; Szenftleben, A.; Chiorean, L.; Badea, R. Villous adenoma of the common hepatic duct: The importance of contrast-enhanced ultrasound and endoscopic retrograde cholangiopancreatography for relevant diagnosis. A case report and review of the literature. Med. Ultrason. 2015, 17, 553–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Leo, E.K.; Shah, C.P.; Grajo, J.R.; Liu, X.; Parekh, H. Extramedullary Hematopoiesis in Mismatch Repair Deficient Colon Cancer Patient on Adjuvant Chemotherapy. Cureus 2021, 13, e12899. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Shetty, A.; Mellnick, V.; Pickhardt, P.; Bhalla, S.; Menias, C. Extramedullary haematopoiesis: Radiological imaging features. Clin. Radiol. 2016, 71, 807–814. [Google Scholar] [CrossRef]
- Bradley, M.J.; Metreweli, C. Ultrasound appearances of extramedullary haematopoiesis in the liver and spleen. Br. J. Radiol. 1990, 63, 816–818. [Google Scholar] [CrossRef]
- Alhyari, A.; Zadeh, E.S.; Algíbez, A.M.; Berzigotti, A.; Görg, C.; Trenker, C.; Jenssen, C.; Lim, A.; Möller, K.; Dong, Y.; et al. Comments and illustrations of the WFUMB CEUS liver guidelines: Rare benign hematological focal liver lesions (hepatic extramedullary hematopoiesis, Hemophagocytic lymphohistiocytosis, reactive lymphoid hyperplasia). Med. Ultrason. 2024, 27, 73–81. [Google Scholar] [CrossRef]
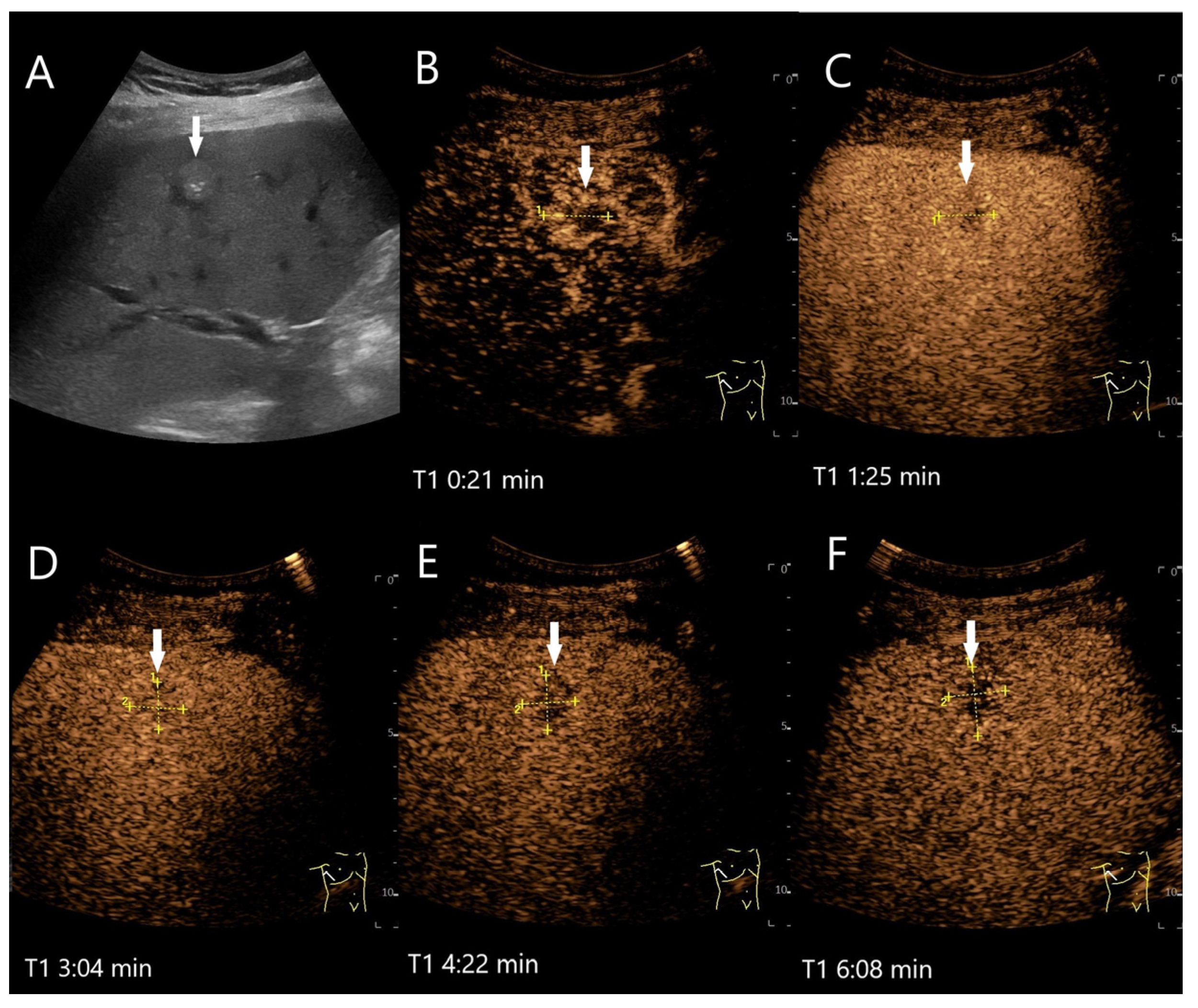
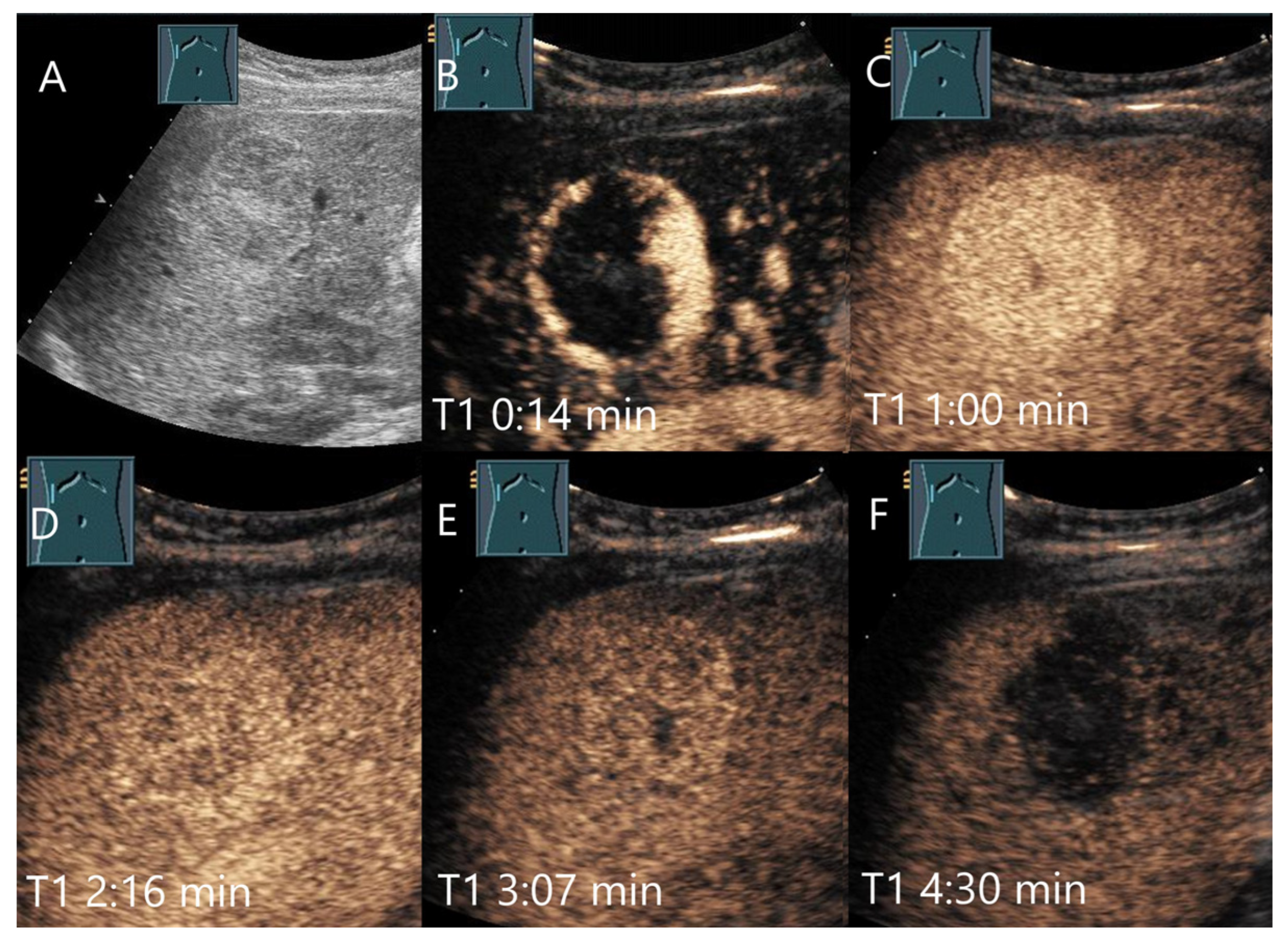
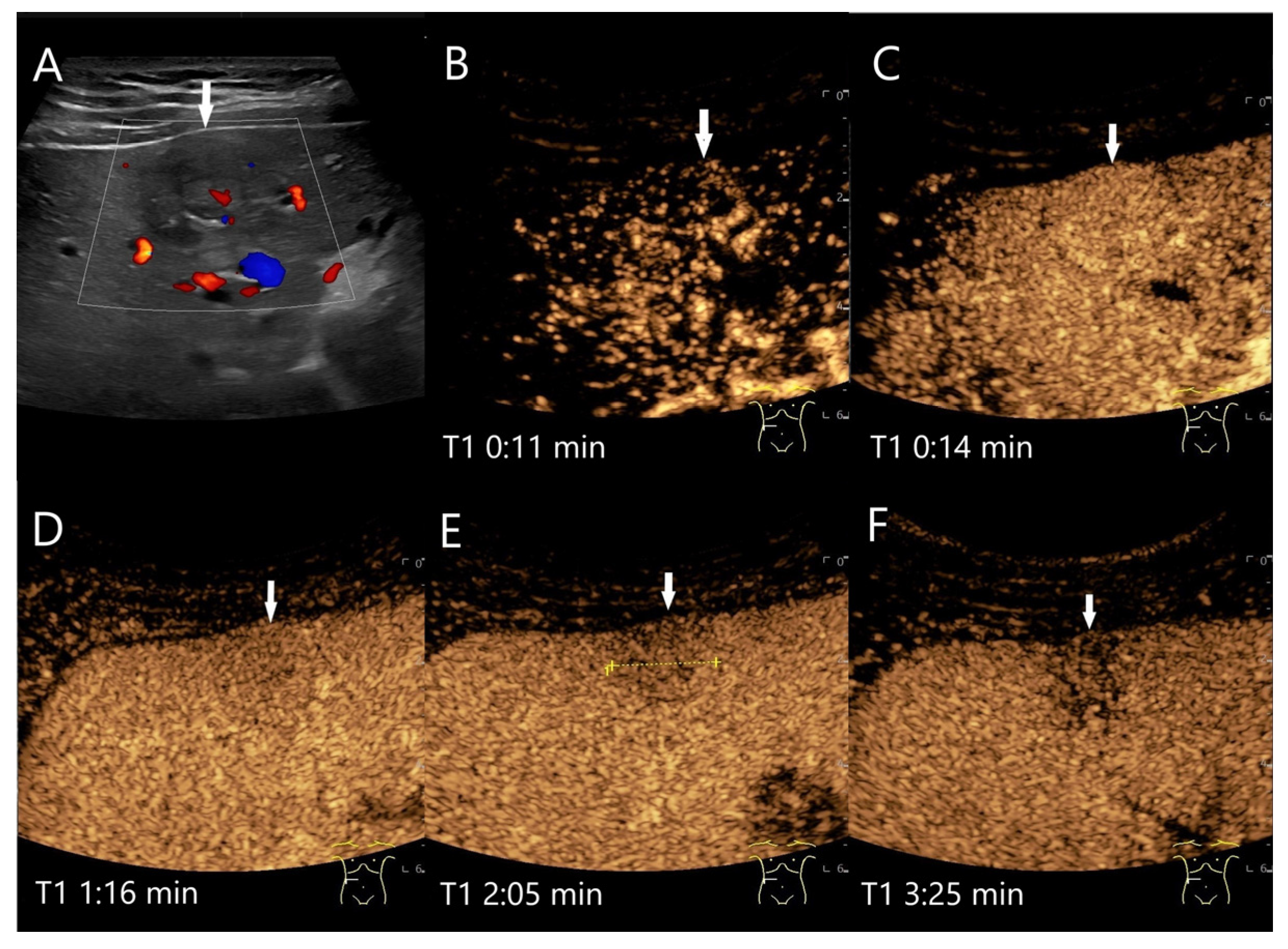
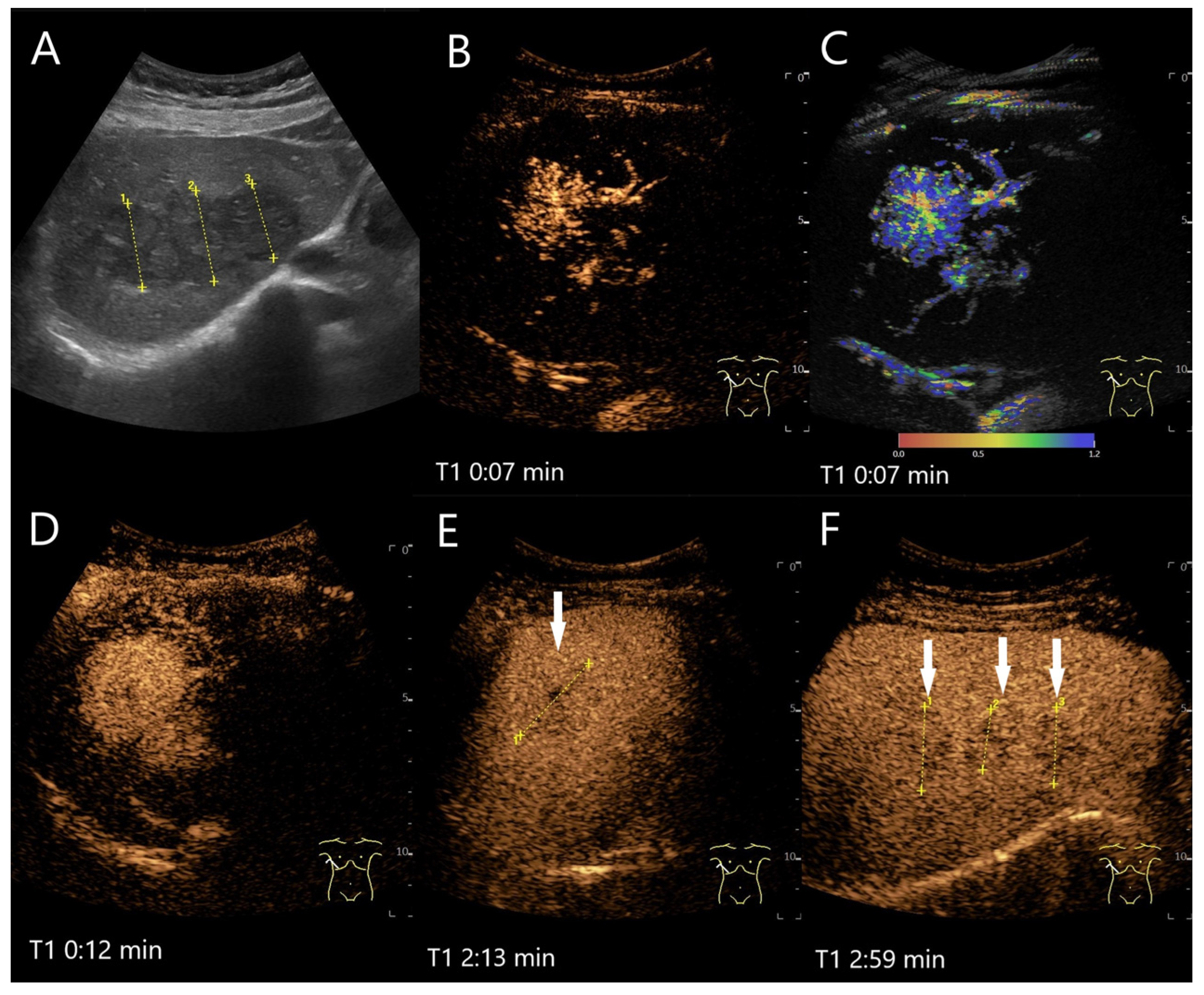
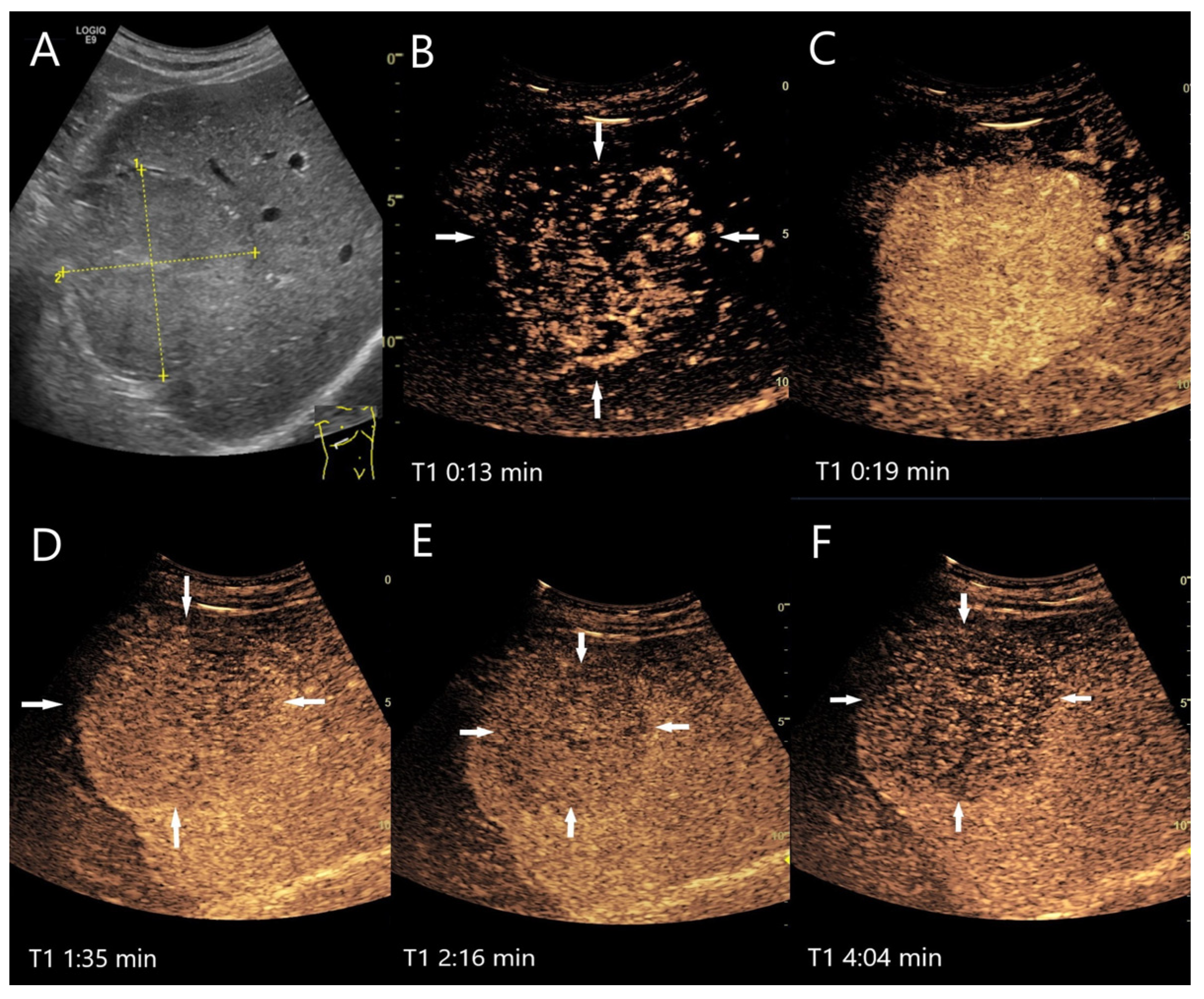
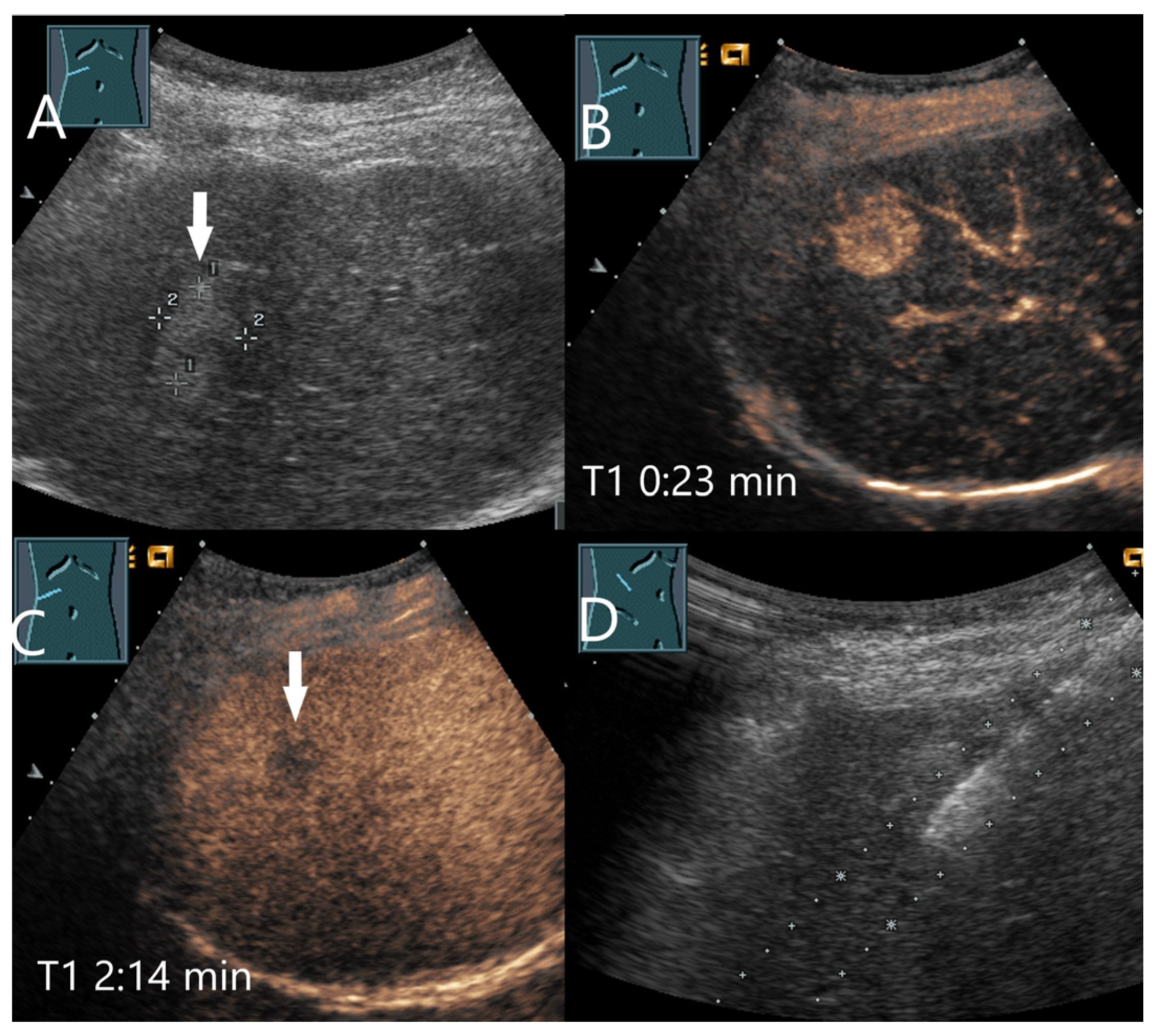
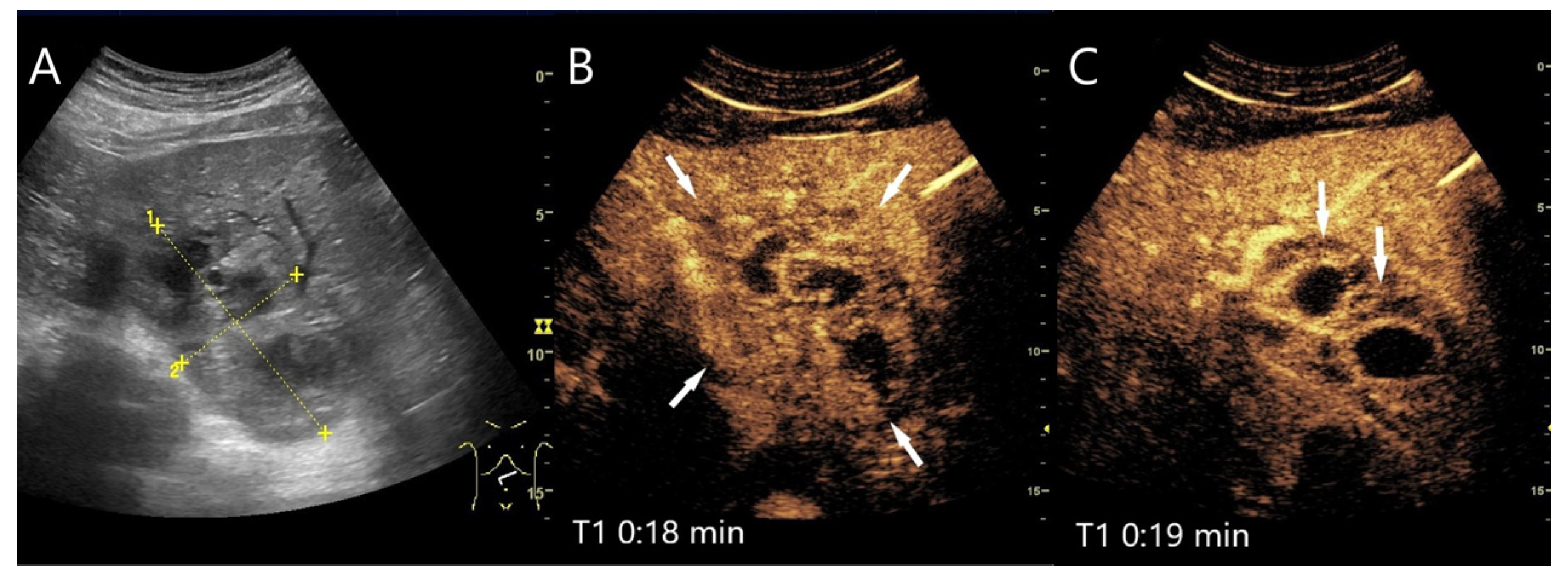

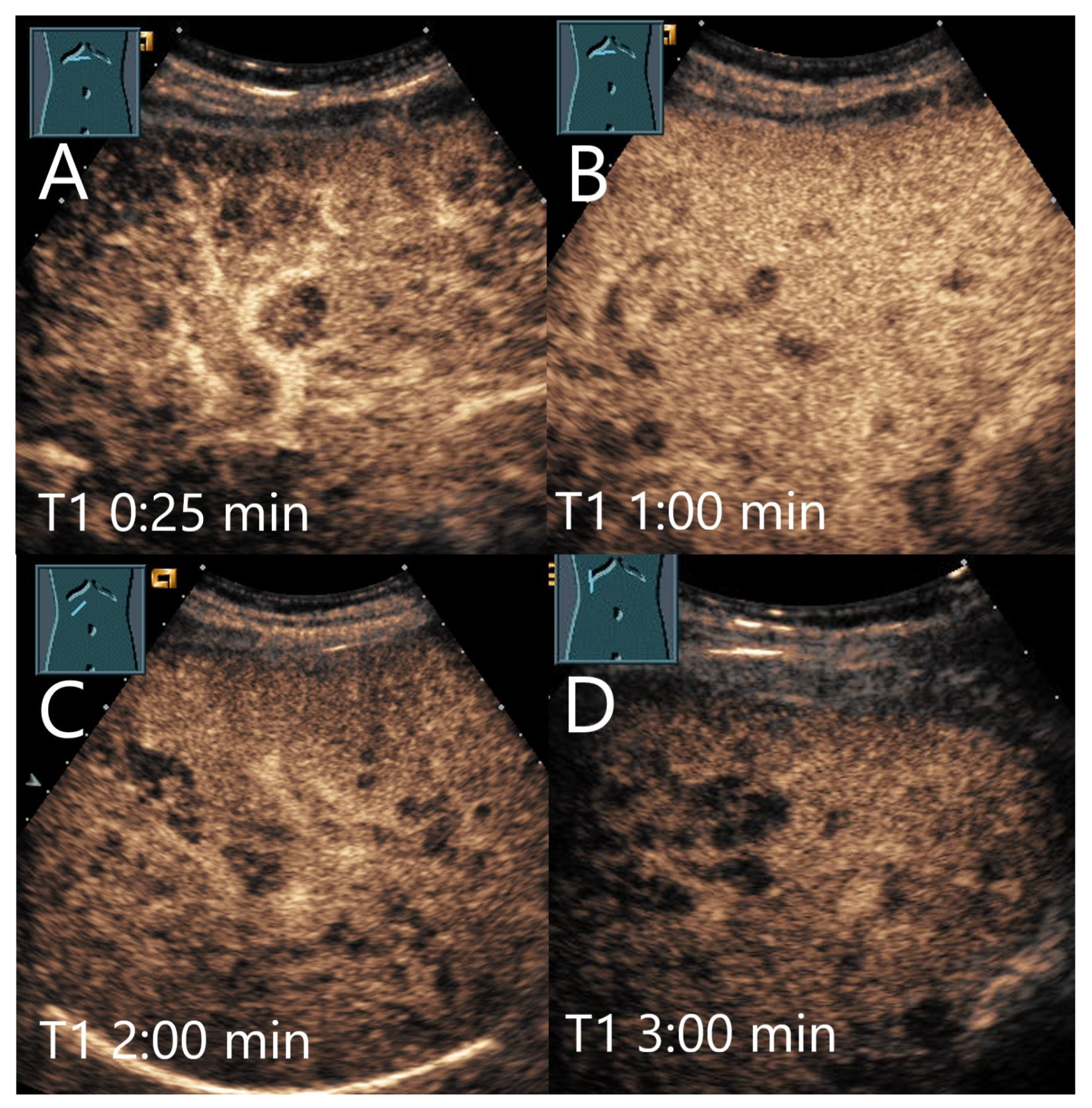
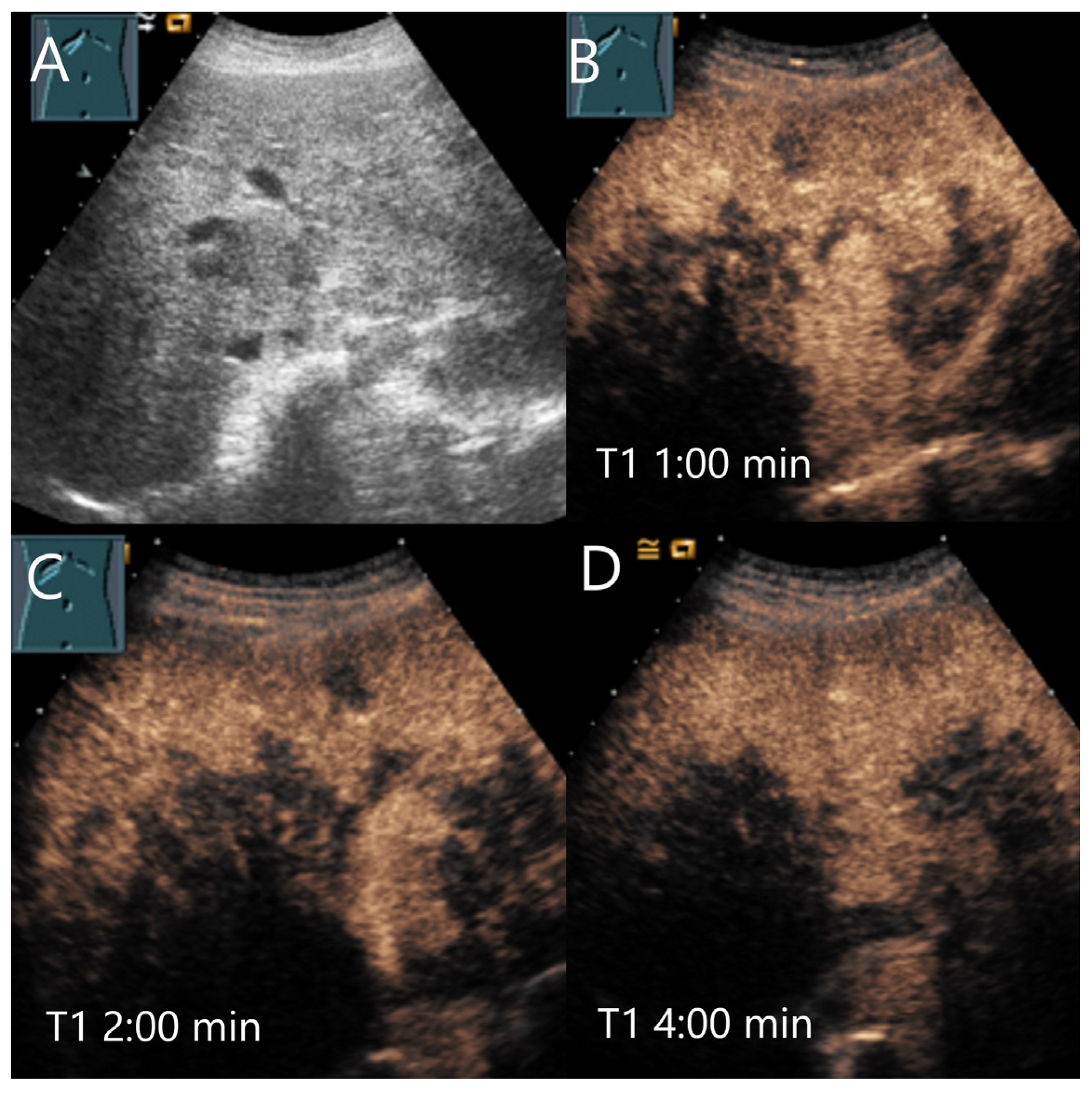
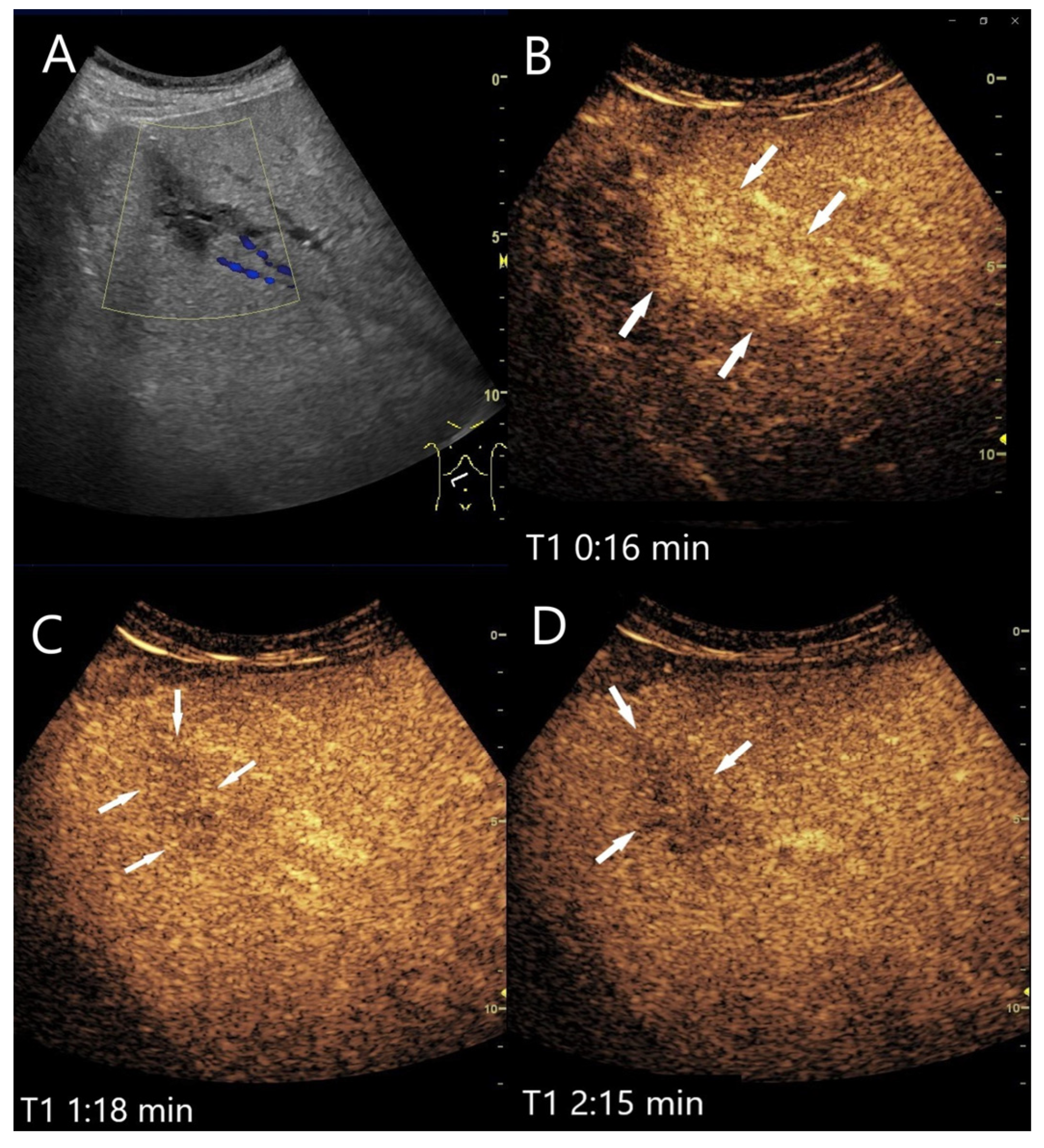


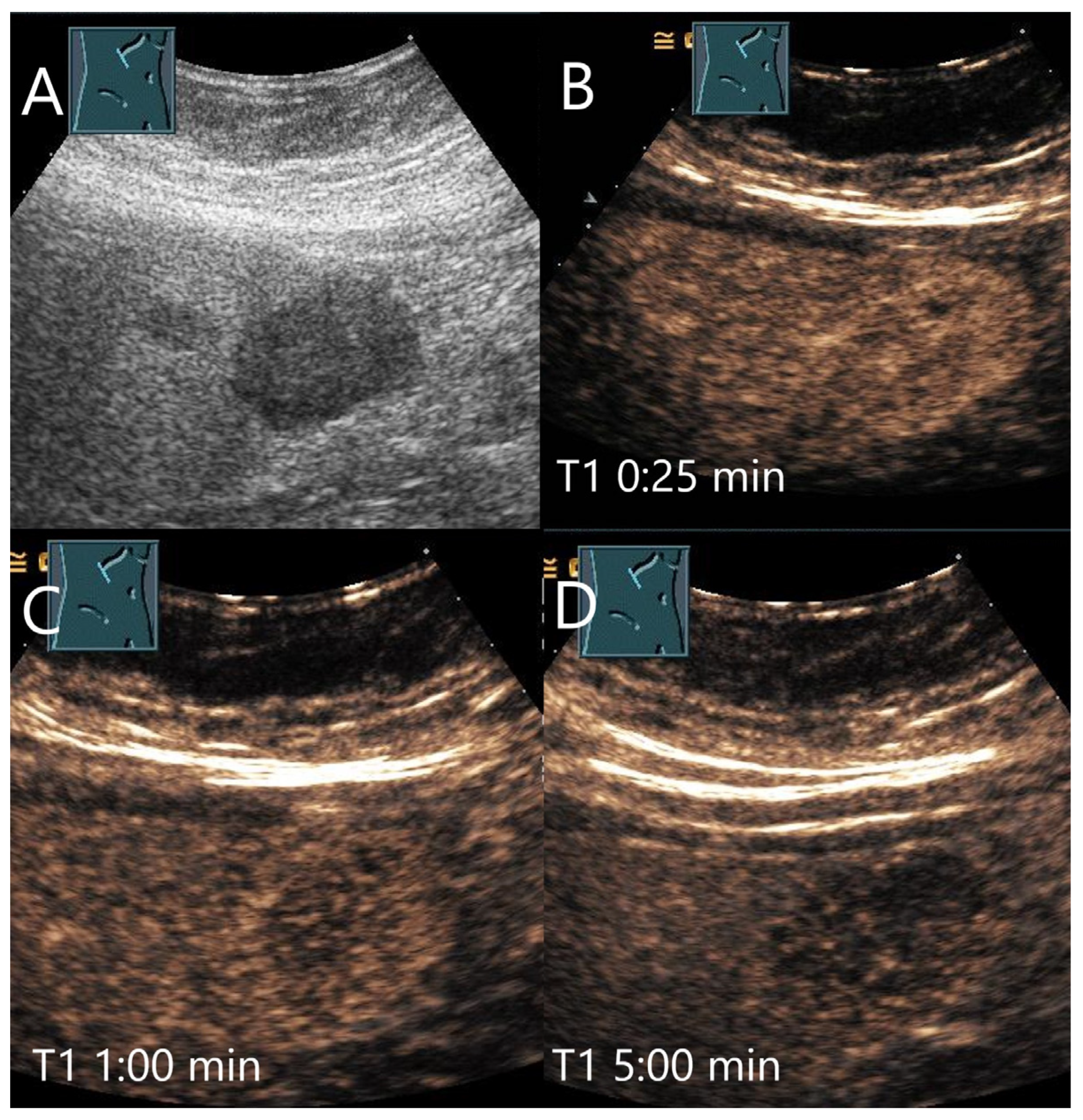
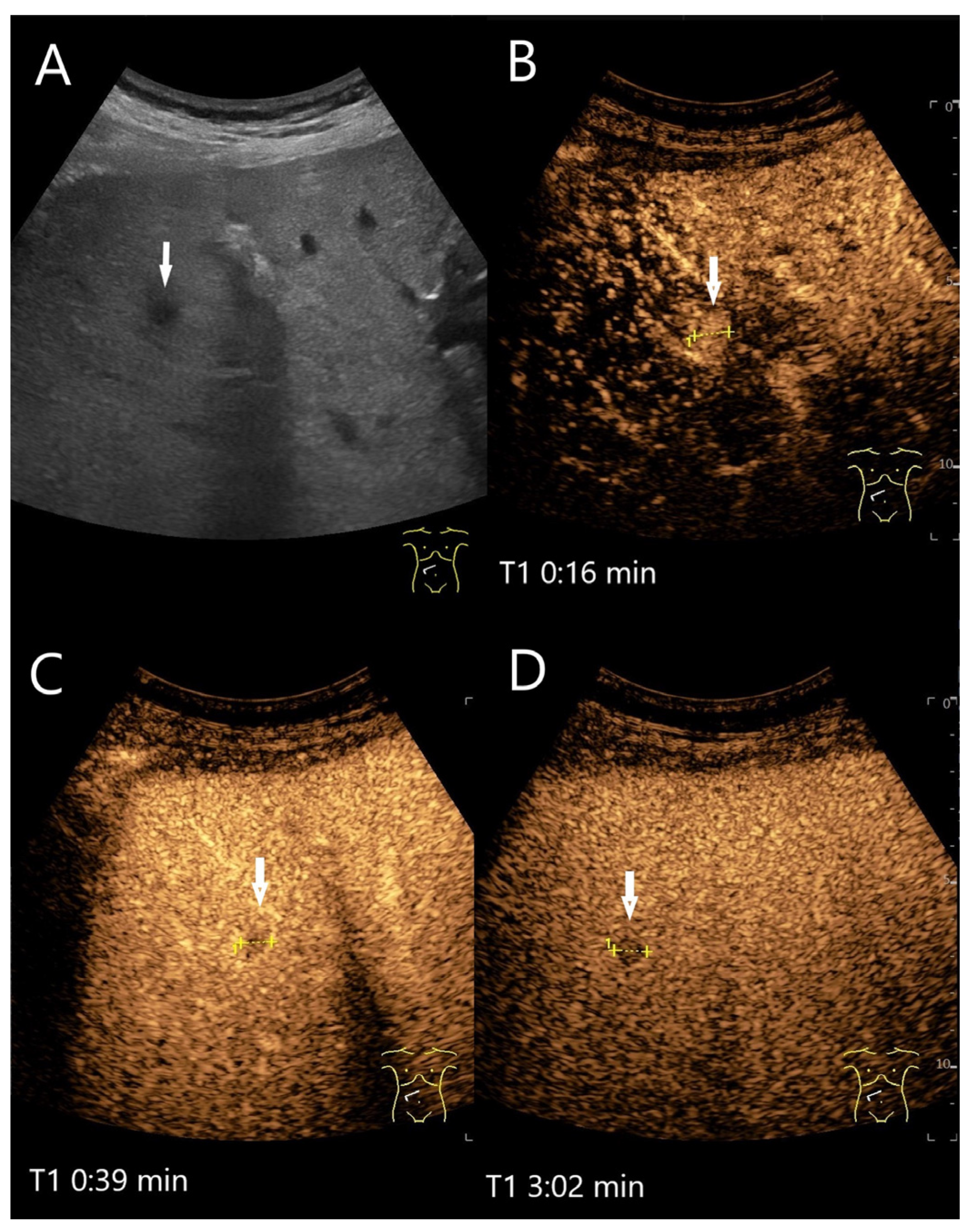

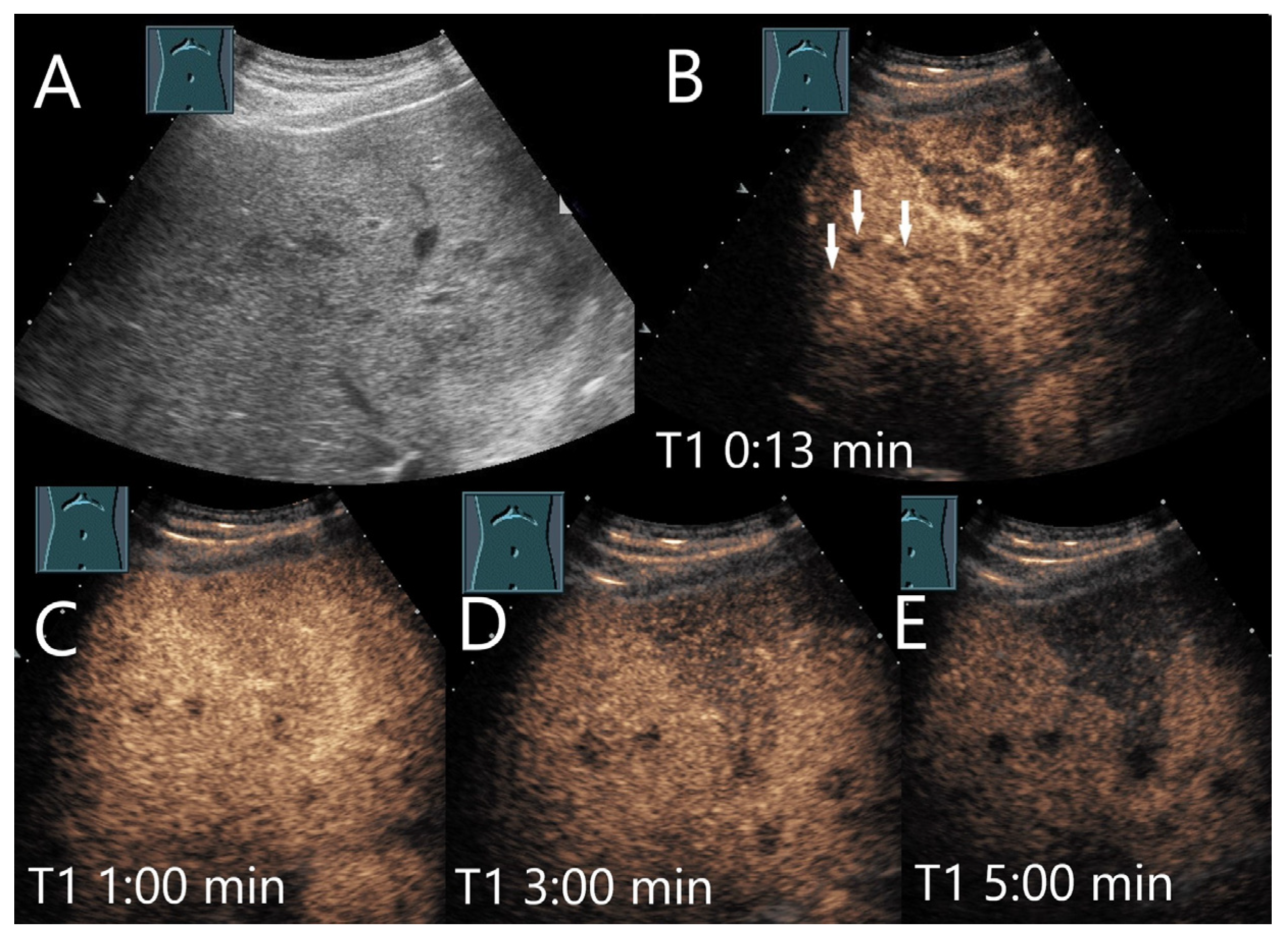
| Phase | Start | End |
|---|---|---|
| Arterial (AP) | 10–20 s | 25–45 s |
| Portal venous (PVP) | 20–45 s | ≤120 s |
| Late (LP) | >120 s | Up to 4–8 min, depending on the presence of the UCA bubbles. With continuous sonication, the bubbles are destroyed prematurely. |
| Study | FLL (n) | LP Washout | Comments |
|---|---|---|---|
| Ding 2005 [52] | Benign lesions n = 51 | n = 11/51 (22%) | |
| Liver abscess n = 5 | n = 3/5 (60%) | ||
| IPT n = 3 | n = 3/3 (100%) | ||
| Liu 2008 [65] | Inflammatory lesions n = 53 | ||
| Pyogenic abscesses n = 32 (n = 31 with hyper- or isoenhancement in the AP) | n = 25/31 (80.6%) | In 54.8%, hypoenhancement started in PVP. | |
| Infected granulomas n = 15 | n = 15/15 (100%) | In 100%, hypoenhancement started in PVP. | |
| IPT n = 6 | n = 6/6 (100%) | In 100%, hypoenhancement started in PVP. | |
| Bhayana 2010 [4] | Hypervascular benign FLL n = 74 (overall n = 146 FLL) | 36% of all benign FLL | Washout occurred in 36% of benign and 97% of malignant FLL. The onset of washout after injection was defined as <30 s/after 30–75 s/75–180 s/<180 s. |
| Inflammatory lesions n = 5 | n = 5/5 (100%) | Mostly (80%) < 30 s. | |
| Popescu 2025 [67] | Liver abscesses n = 41 | n = 22/41 (53.6%) | Hypoenhancement in LP according to the marginal rim. |
| Guo 2020 [66] | Inflammatory lesions n = 44 | n = 37/44 (84%) | Start of hypoenhancement in PVP n = 30/44 (68%). |
| Francica 2020 [68] | Liver abscesses n = 44 | n = 30/38 (79%) | Data refer to peripheral hyperenhancing rim in AP. |
| n = 10/20 (50%) | The data refer to hyperenhanced septa. |
| Lesion | Characteristics on B-Mode and CDI | Typical Characteristics on CEUS | PVP and LP | Explanation of Washout in LP |
|---|---|---|---|---|
| FNH | Isoechoic, hypoechoic, sometimes hypoechoic rim. | Wheel spoke pattern, central artery, rarely peripheral artery and wheel. spoke pattern. Centrifugal filling | Hyperenhancement to isoenhanced, central scare. | Fibrosis and vascular obliteration. |
| Hemangioma | Hyperechoic, beyond liver veins, hypoechoic in steatosis and with shunts. | Peripheral globular enhancement, centripetal filling. Rapid homogeneous filling in shunt hemangiomas. | Hyperenhancement and isoenhancement. | Permanent video loops with destruction of the UCA bubbles and slow refill. Fibrosis. |
| HCA | Hypo-/iso-/hyperechoic HNF1a HCA (steatotic HCA) are frequently hyperechoic. | centripetal or mixed/diffuse filling. | Iso- or late slight hypoenhancement. Hyperenhancement in some I-HCA. | Absence of portal and central veins. |
| Abscesses | Hypo-/anechoic. | Hyperenhancement in phlegmonous stage, transient hyperenhancement in surrounding parenchyma. Non-enhancement of necrotic parts. Honeycomb sign. | Early washout. | Formation of thromboses of the small hepatic and portal veins, as well as pylephlebitis of the small portalvenous vessels. |
| Tuberculosis | Miliary or macronodular lesions or the serohepatic form with thickened liver capsule and subcapsular lesions. | Hyperenhancement in granulomatous inflammation, hyperenhanced rim, and central hypoenhancement in caseous necrosis. Enhanced septa. | Hypoenhancement in the PVV. | Destruction of the hepatic sinusoids with inflammatory granulation. |
| IPT | Mostly hypoechoic, irregular shapes. | Arterial hyperenhancement, Homogenous, heterogenous, rim like. | Early washout. | Obliterative phlebitis is due to an inflammatory infiltration of the vessel walls and lumina and thrombosis, varying degrees of fibrosis. |
| Cholangiocellular adenoma | Small hypoechoic lesions, well circumscript. | Hyper- or isoenhancement. | Marked washout in the LP. | No liver tissue. |
| Peliosis | Heterogeneously hypoechoic, well-defined margins, irregular shapes | Often hyperenhanced in the AP | Hypoenhancement in PVP or LP | Post sinusoidal outflow obstruction. |
| HAML | Variable appearance, hyper- and hypoechoic separation specific is the strong hyperechoic appearance with attenuation. | Homogeneous or inhomogeneous hyperenhancement in AP, no or mild washout, hyperenhancement is described. Partial washout and non-washout in hyperechoic-hypoechoic separation. | Hypoenhancement not before 60 s or late after 120 s, mostly slight hypoenhancement, isoenhancement, and hyperenhancement is described. | |
| PEComa/ HEAML | Variable appearance, peripheral vessels in CDI in PEComas. | Arterial hyperenhancement. Variable appearance in PVP and LP. | No marked washout. | Dilated and distorted vascular networks, a direct outflow of arterial blood into the hepatic vein branch “causing a short circuit in the hepatic artery-portal vein” is suggested. |
| Extramedullary hematopoiesis | Hepatosplenomegaly, hypoechoic, hyperechoic, isoechoic lesions. | No information. | In our case, hypoenhancement in the LP. | Non-hepatic tissue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, K.; Görg, C.; Krix, M.; Jenssen, C.; Dong, Y.; Cui, X.-W.; Dietrich, C.F. Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions—A Review on Its Frequency and Possible Causes. Diagnostics 2025, 15, 998. https://doi.org/10.3390/diagnostics15080998
Möller K, Görg C, Krix M, Jenssen C, Dong Y, Cui X-W, Dietrich CF. Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions—A Review on Its Frequency and Possible Causes. Diagnostics. 2025; 15(8):998. https://doi.org/10.3390/diagnostics15080998
Chicago/Turabian StyleMöller, Kathleen, Christian Görg, Martin Krix, Christian Jenssen, Yi Dong, Xin-Wu Cui, and Christoph F. Dietrich. 2025. "Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions—A Review on Its Frequency and Possible Causes" Diagnostics 15, no. 8: 998. https://doi.org/10.3390/diagnostics15080998
APA StyleMöller, K., Görg, C., Krix, M., Jenssen, C., Dong, Y., Cui, X.-W., & Dietrich, C. F. (2025). Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions—A Review on Its Frequency and Possible Causes. Diagnostics, 15(8), 998. https://doi.org/10.3390/diagnostics15080998








