High-Quality Samples for Next-Generation Sequencing and PD-L1 Assessment in Non-Small Cell Lung Cancer: The Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic Testing and PD-L1 Analysis
2.3. Next-Generation Sequencing Study
2.3.1. Sample Preparation and Nucleic Acid Extraction
2.3.2. Sequencing and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| EBUS-TBNA | Endobronchial ultrasound-guided transbronchial needle aspiration |
| NGS | Next-generation sequencing |
| NCCN | National Comprehensive Cancer Network |
| CT | Computed tomography |
| PET-CT | Positron emission tomography-CT |
| SEAP-SEOM | Spanish Society of Pathology and Spanish Society of Medical Oncology |
| MCST | Molecular Committee for Solid Tumors |
| FFPE | Formalin-fixed, paraffin-embedded |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| CNV | Copy number variations |
References
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients with Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Servetto, A.; Di Maio, M.; Salomone, F.; Napolitano, F.; Paratore, C.; Di Costanzo, F.; Viscardi, G.; Santaniello, A.; Formisano, L.; Bianco, R. Analysis of phase III clinical trials in metastatic NSCLC to assess the correlation between QoL results and survival outcomes. BMC Med. 2023, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Shah, P.L.; Edmonds, L.; Lim, E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: Systematic review and meta-analysis. Thorax 2009, 64, 757–762. [Google Scholar] [CrossRef]
- McLean, A.E.B.; Barnes, D.J.; Troy, L.K. Diagnosing Lung Cancer: The Complexities of Obtaining a Tissue Diagnosis in the Era of Minimally Invasive and Personalised Medicine. J. Clin. Med. 2018, 7, 163. [Google Scholar] [CrossRef]
- Bilaçeroğlu, S. Molecular markers in lung cancer: Role of EBUS. Curr. Opin. Pulm. Med. 2017, 23, 247–253. [Google Scholar] [CrossRef]
- Diep, R.; MacDonald, M.; Cooper, R.; Grzegorczyk, A.; Rakocevic, R.; Chang, C.-F.; Uy, A.; Cowgill, N.; Nieva, J.J. Biopsy Method and Needle Size on Success of Next-Generation Sequencing in NSCLC: A Brief Report. JTO Clin. Res. Rep. 2023, 4, 100497. [Google Scholar] [CrossRef]
- Baratella, E.; Cernic, S.; Minelli, P.; Furlan, G.; Crimì, F.; Rocco, S.; Ruaro, B.; Cova, M.A. Accuracy of CT-Guided Core-Needle Biopsy in Diagnosis of Thoracic Lesions Suspicious for Primitive Malignancy of the Lung: A Five-Year Retrospective Analysis. Tomography 2022, 8, 2828–2838. [Google Scholar] [CrossRef]
- Steinfort, D.P.; Khor, Y.H.; Manser, R.L.; Irving, L.B. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: Systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 902–910. [Google Scholar] [CrossRef]
- Tone, M.; Inomata, M.; Awano, N.; Kuse, N.; Takada, K.; Minami, J.; Muto, Y.; Fujimoto, K.; Kumasaka, T.; Izumo, T. Comparison of adequacy between transbronchial lung cryobiopsy samples and endobronchial ultrasound-guided transbronchial needle aspiration samples for next-generation sequencing analysis. Thorac. Cancer 2021, 12, 251–258. [Google Scholar] [CrossRef]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Reck, M.; Carbone, D.P.; Garassino, M.; Barlesi, F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef]
- Hwang, I.; Choi, Y.-L.; Lee, H.; Hwang, S.; Lee, B.; Yang, H.; Chelakkot, C.; Han, J. Selection Strategies and Practical Application of BRAF V600E-Mutated Non-Small Cell Lung Carcinoma. Cancer Res. Treat. 2022, 54, 782–792. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef]
- Patel, S.A.; Weiss, J. Advances in the Treatment of Non-Small Cell Lung Cancer: Immunotherapy. Clin. Chest Med. 2020, 41, 237–247. [Google Scholar] [CrossRef]
- Isla, D.; Lozano, M.D.; Paz-Ares, L.; Salas, C.; de Castro, J.; Conde, E.; Felip, E.; Gómez-Román, J.; Garrido, P.; Enguita, A.B. New update to the guidelines on testing predictive biomarkers in non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2022, 25, 1252–1267. [Google Scholar] [CrossRef]
- Karadzovska-Kotevska, M.; Brunnström, H.; Kosieradzki, J.; Ek, L.; Estberg, C.; Staaf, J.; Barath, S.; Planck, M. Feasibility of EBUS-TBNA for histopathological and molecular diagnostics of NSCLC—A retrospective single-center experience. PLoS ONE 2022, 17, e0263342. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Aisner, D.L.; Wood, D.E.; Akerley, W.; Bauman, J.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; Dobelbower, M.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 5.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 807–821. [Google Scholar] [CrossRef]
- Gilbert, C.R.; Dust, C.; Argento, A.C.; Feller-Kopman, D.; Gonzalez, A.V.; Herth, F.; Iaccarino, J.M.; Illei, P.; O’Neil, K.; Pastis, N.; et al. Acquisition and Handling of Endobronchial Ultrasound Transbronchial Needle Samples. CHEST 2024, 167, 899–909. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Call, S.; Dooms, C.; Obiols, C.; Sánchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Respir. J. 2018, 51, 1800190. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Schil, P.V.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, P.; Clementsen, P.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.-M.; Herth, F.; Larghi, A.; Vasquez-Sequeiros, E.; Hassan, C.; et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015, 47, 545–559. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Thoracic-Tumours-2021 (accessed on 21 March 2023).
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA. Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gupta, A.; Omeogu, C.H.; Islam, J.Y.; Joshi, A.R.; Akinyemiju, T.F. Association of area-level socioeconomic status and non-small cell lung cancer stage by race/ethnicity and health care-level factors: Analysis of the National Cancer Database. Cancer 2022, 128, 3099–3108. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Uchimura, K.; Yanase, K.; Imabayashi, T.; Takeyasu, Y.; Furuse, H.; Tanaka, M.; Matsumoto, Y.; Sasada, S.; Tsuchida, T. The Impact of Core Tissues on Successful Next-Generation Sequencing Analysis of Specimens Obtained through Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Cancers 2021, 13, 5879. [Google Scholar] [CrossRef]
- Parente, P.; Carbonelli, C.; Biancofiore, G.; Sukthi, A.; Di Micco, C.M.; Vairo, M.; Fuso, P.; Taurchini, M.; Graziano, P. Handling and standardization of EBUS needle aspiration in NSCLC patients: The value of the cell block, a monoinstitutional experience. Thorac. Cancer 2022, 13, 2480–2488. [Google Scholar] [CrossRef]
- Mata, D.A.; Harries, L.; Williams, E.A.; Hiemenz, M.C.; Decker, B.; Tse, J.Y.; Janovitz, T.; Ferguson, D.C.; Speece, I.A.; Margolis, M.L.; et al. Method of Tissue Acquisition Affects Success of Comprehensive Genomic Profiling in Lung Cancer. Arch. Pathol. Lab. Med. 2023, 147, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Fernández Aceñero, M.J.; Díaz Del Arco, C.; Dinarés, C.; Labiano, T.; Tejerina, E.; Bernabé, M.J.; Forcen, E.; Saiz-Pardo, M.; Pérez, P.; Lozano, M.D. Overview and update on molecular testing in non-small cell lung carcinoma utilizing endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) samples. Diagn. Cytopathol. 2023, 51, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Piro, R.; Fontana, M.; Casalini, E.; Rossi, L.; Simeone, M.S.; Ghinassi, F.; Ruggiero, P.; Pollorsi, C.; Taddei, S.; Beghe’, B.; et al. Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA). Diagnostics 2023, 13, 1405. [Google Scholar] [CrossRef]
- Muthu, V.; Sehgal, I.; Dhooria, S.; Prasad, K.; Gupta, N.; Aggarwal, A.; Agarwal, R. Endobronchial ultrasound-guided transbronchial needle aspiration: Techniques and challenges. J. Cytol. 2019, 36, 65–70. [Google Scholar] [CrossRef]
- Agrawal, A.; Ghori, U.; Chaddha, U.; Murgu, S. Combined EBUS-IFB and EBUS-TBNA vs. EBUS-TBNA Alone for Intrathoracic Adenopathy: A Meta-Analysis. Ann. Thorac. Surg. 2022, 114, 340–348. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Semba, H.; Fukuoka, K.; Fujino, S.; et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: A nationwide survey by the Japan Society for Respiratory Endoscopy. Respir. Res. 2013, 14, 50. [Google Scholar] [CrossRef]
- Eapen, G.A.; Shah, A.M.; Lei, X.; Jimenez, C.A.; Morice, R.C.; Yarmus, L.; Filner, J.; Ray, C.; Michaud, G.; Greenhill, S.R.; et al. Complications, Consequences, and Practice Patterns of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: Results of the AQuIRE Registry. Chest 2013, 143, 1044–1053. [Google Scholar] [CrossRef]
- von Bartheld, M.B.; van Breda, A.; Annema, J.T. Complication rate of endosonography (endobronchial and endoscopic ultrasound): A systematic review. Respir. Int. Rev. Thorac. Dis. 2014, 87, 343–351. [Google Scholar] [CrossRef]
- Fielding, D.; Dalley, A.J.; Bashirzadeh, F.; Singh, M.; Nandakumar, L.; McCart Reed, A.E.; Black, D.; Kazakoff, S.; Pearson, J.V.; Nones, K.; et al. Diff-Quik Cytology Smears from Endobronchial Ultrasound Transbronchial Needle Aspiration Lymph Node Specimens as a Source of DNA for Next-Generation Sequencing Instead of Cell Blocks. Respiration 2019, 97, 525–539. [Google Scholar] [CrossRef]
- Turner, S.R.; Buonocore, D.; Desmeules, P.; Rekhtman, N.; Dogan, S.; Lin, O.; Arcila, M.E.; Jones, D.R.; Huang, J. Feasibility of endobronchial ultrasound transbronchial needle aspiration for massively parallel next-generation sequencing in thoracic cancer patients. Lung Cancer Amst. Neth. 2018, 119, 85–90. [Google Scholar] [CrossRef]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef] [PubMed]
- Righi, L.; Graziano, P.; Fornari, A.; Rossi, G.; Barbareschi, M.; Cavazza, A.; Pelosi, G.; Scagliotti, G.V.; Papotti, M. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology. Cancer 2011, 117, 3416–3423. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Mamotte, L.; Mesbah Ardakani, N.; Leslie, C.; Tesfai, Y.; Grieu-Iacopetta, F.; Izaac, K.; Singh, S.; Ardakani, R.; Thomas, M.; et al. Adequacy of cytology and small biopsy samples obtained with rapid onsite evaluation (ROSE) for predictive biomarker testing in non-small cell lung cancer. Pathology 2023, 55, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zheng, X.; Mao, X.; Zhao, R.; Ye, J.; Zhang, Y.; Sun, J. Next-Generation Sequencing for Genotyping of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Samples in Lung Cancer. Ann. Thorac. Surg. 2019, 108, 219–226. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, R.Y.; McGrath, C.M.; Andronov, M.; Haas, A.R.; Ma, K.C.; Lanfranco, A.R.; Hutchinson, C.T.; Morrissette, J.J.D.; DiBardino, D.M. The Performance of an Extended Next Generation Sequencing Panel Using Endobronchial Ultrasound-Guided Fine Needle Aspiration Samples in Non-Squamous Non-Small Cell Lung Cancer: A Pragmatic Study. Clin. Lung Cancer 2023, 24, e105–e112. [Google Scholar] [CrossRef]
- Yarmus, L.; Akulian, J.; Gilbert, C.; Feller-Kopman, D.; Lee, H.J.; Zarogoulidis, P.; Lechtzin, N.; Ali, S.Z.; Sathiyamoorthy, V. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann. Am. Thorac. Soc. 2013, 10, 636–643. [Google Scholar] [CrossRef]
- Martin-Deleon, R.; Teixido, C.; Lucena, C.M.; Martinez, D.; Fontana, A.; Reyes, R.; García, M.; Viñolas, N.; Vollmer, I.; Sanchez, M.; et al. EBUS-TBNA Cytological Samples for Comprehensive Molecular Testing in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2084. [Google Scholar] [CrossRef]
- Cho, J.; Choe, J.G.; Pahk, K.; Choi, S.; Kwon, H.R.; Eo, J.S.; Seo, H.J.; Kim, C.; Kim, S. Ratio of Mediastinal Lymph Node SUV to Primary Tumor SUV in 18F-FDG PET/CT for Nodal Staging in Non-Small-Cell Lung Cancer. Nucl. Med. Mol. Imaging 2017, 51, 140–146. [Google Scholar] [CrossRef]
- Mattes, M.D.; Ahsanuddin, S.; Apte, A.; Moshchinsky, A.B.; Rizk, N.P.; Foster, A.; Wu, A.J.; Ashamalla, H.; Deasy, J.O.; Weber, W.A.; et al. The Ratio of Lymph Node to Primary Tumor SUV on PET/CT Accurately Predicts Nodal Malignancy in Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2014, 90, S212–S213. [Google Scholar] [CrossRef]
- Stoy, S.P.; Segal, J.P.; Mueller, J.; Furtado, L.V.; Vokes, E.E.; Patel, J.D.; Murgu, S. Feasibility of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Cytology Specimens for Next Generation Sequencing in Non–small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, 230–238.e2. [Google Scholar] [CrossRef]
- Pepe, F.; De Luca, C.; Smeraglio, R.; Pisapia, P.; Sgariglia, R.; Nacchio, M.; Russo, M.; Serra, N.; Rocco, D.; Battiloro, C.; et al. Performance analysis of SiRe next-generation sequencing panel in diagnostic setting: Focus on NSCLC routine samples. J. Clin. Pathol. 2019, 72, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Dacic, S.; Ghofrani, M.; Illei, P.B.; Layfield, L.J.; Lee, C.; Michael, C.W.; Miller, R.A.; Mitchell, J.W.; Nikolic, B.; et al. Guideline From the College of American Pathologists in Collaboration with the American College of Chest Physicians, Association for Molecular Pathology, American Society of Cytopathology, American Thoracic Society, Pulmonary Pathology Society, Papanicolaou Society of Cytopathology, Society of Interventional Radiology, and Society of Thoracic Radiology. Arch. Pathol. Lab. Med. 2020, 144, 933–958. [Google Scholar] [CrossRef]
- Doxtader, E.E.; Cheng, Y.-W.; Zhang, Y. Molecular Testing of Non–Small Cell Lung Carcinoma Diagnosed by Endobronchial Ultrasound–Guided Transbronchial Fine-Needle Aspiration: The Cleveland Clinic Experience. Arch. Pathol. Lab. Med. 2019, 143, 670–676. [Google Scholar] [CrossRef]
- Nakajima, T.; Yasufuku, K.; Fujiwara, T.; Yoshino, I. Recent advances in endobronchial ultrasound-guided transbronchial needle aspiration. Respir. Investig. 2016, 54, 230–236. [Google Scholar] [CrossRef]
- Aljohaney, A.; Bakhsh, S.; Khayat, M. The Contribution of Cell Blocks in the Diagnosis of Mediastinal and Hilar Lymphadenopathy Samples From Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA). Cureus 2023, 15, e39673. [Google Scholar] [CrossRef]
- Sakata, S.; Otsubo, K.; Yoshida, H.; Ito, K.; Nakamura, A.; Teraoka, S.; Matsumoto, N.; Shiraishi, Y.; Haratani, K.; Tamiya, M.; et al. Real-world data on NGS using the Oncomine DxTT for detecting genetic alterations in non-small-cell lung cancer: WJOG13019L. Cancer Sci. 2022, 113, 221–228. [Google Scholar] [CrossRef]
- Froyen, G.; Geerdens, E.; Berden, S.; Cruys, B.; Maes, B. Diagnostic Validation of a Comprehensive Targeted Panel for Broad Mutational and Biomarker Analysis in Solid Tumors. Cancers 2022, 14, 2457. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Legras, A.; Barritault, M.; Tallet, A.; Fabre, E.; Guyard, A.; Rance, B.; Digan, W.; Pecuchet, N.; Giroux-Leprieur, E.; Julie, C.; et al. Validity of Targeted Next-Generation Sequencing in Routine Care for Identifying Clinically Relevant Molecular Profiles in Non–Small-Cell Lung Cancer: Results of a 2-Year Experience on 1343 Samples. J. Mol. Diagn. 2018, 20, 550–564. [Google Scholar] [CrossRef]
- Kage, H.; Kohsaka, S.; Shinozaki-Ushiku, A.; Hiraishi, Y.; Sato, J.; Nagayama, K.; Ushiku, T.; Takai, D.; Nakajima, J.; Miyagawa, K.; et al. Small lung tumor biopsy samples are feasible for high quality targeted next generation sequencing. Cancer Sci. 2019, 110, 2652–2657. [Google Scholar] [CrossRef]
- Botana-Rial, M.; Lojo-Rodríguez, I.; Leiro-Fernández, V.; Ramos-Hernández, C.; González-Montaos, A.; Pazos-Area, L.; Núñez-Delgado, M.; Fernández-Villar, A. Is the diagnostic yield of mediastinal lymph node cryobiopsy (cryoEBUS) better for diagnosing mediastinal node involvement compared to endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)? A systematic review. Respir. Med. 2023, 218, 107389. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, H.; Zhang, Q.; Herth, F.J.F.; Zhang, X. Comparison between Endobronchial Ultrasound-Guided Transbronchial Node Biopsy and Transbronchial Needle Aspiration: A Meta-Analysis. Respir. Int. Rev. Thorac. Dis. 2024, 103, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Matsumoto, S.; Nishino, K.; Honma, K.; Maeda, N.; Kuhara, H.; Tamiya, M.; Inoue, T.; Kawamura, T.; Kimura, T.; et al. Comparison of sampling methods for next generation sequencing for patients with lung cancer. Cancer Med. 2022, 11, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
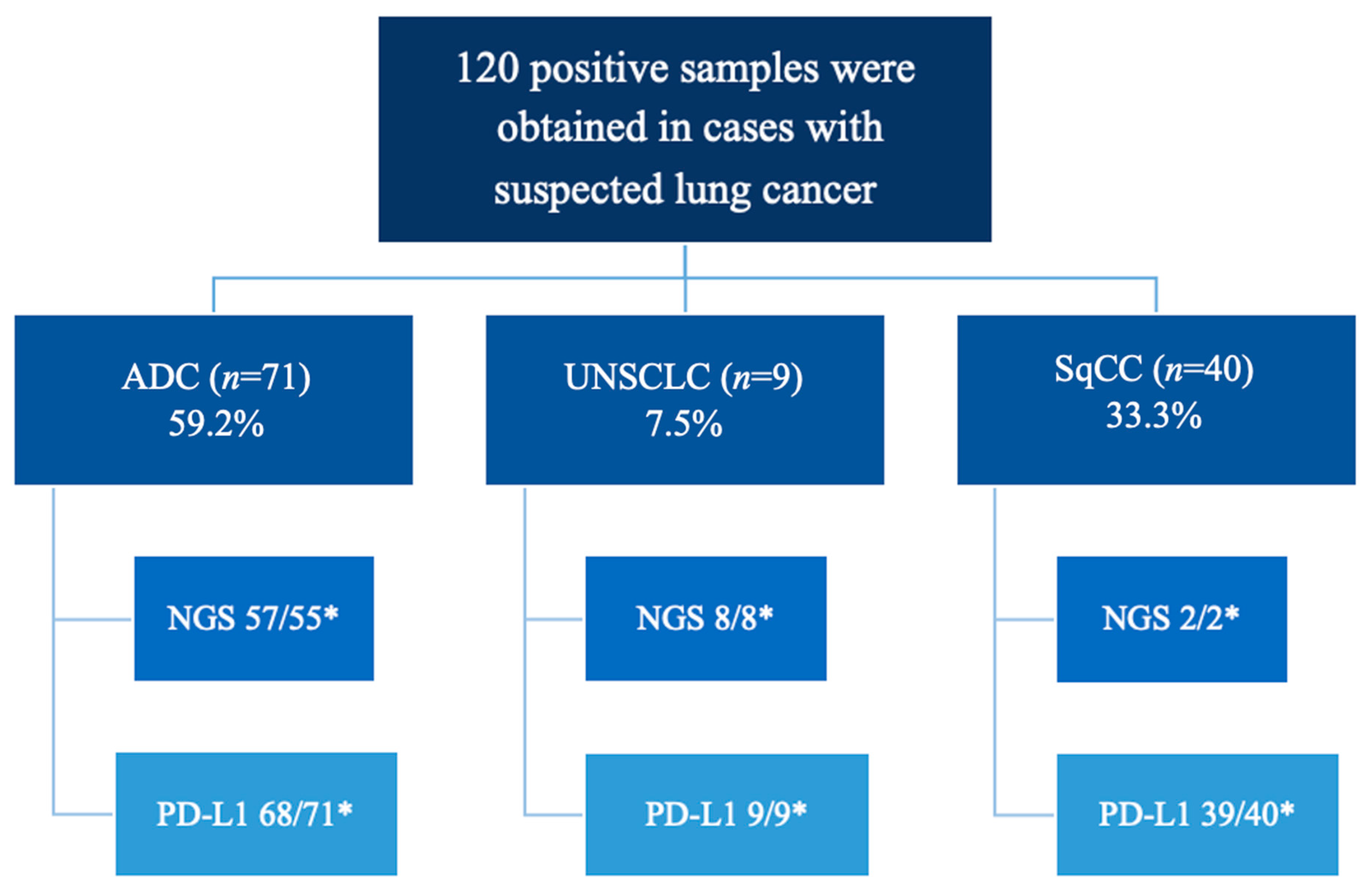
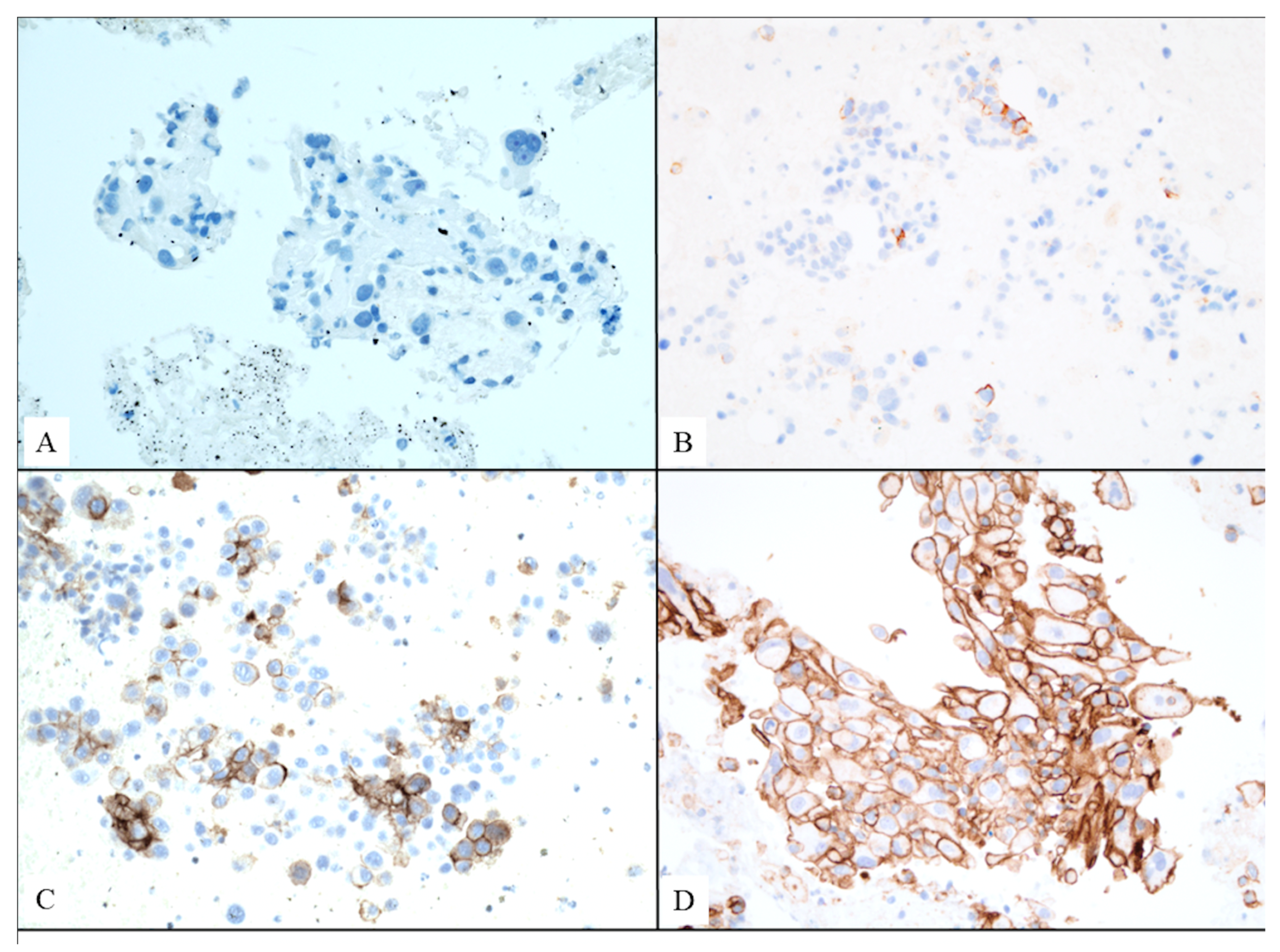
| Characteristics of Patients | N (%) or Median (Range) | |
|---|---|---|
| Age, years | ||
| 70.3 (45–88) | ||
| Gender | ||
| Male | 87 (72.5%) | |
| Female | 33 (27.5%) | |
| Smoking history | ||
| Never smoker | 15 (12.5%) | |
| Former smoker | 34 (28.3%) | |
| Current smoker | 71 (59.2%) | |
| Stage (based on 8th ed. of the AJCC) * | ||
| Ia | 1 (0.8%) | |
| Ib | 2 (1.7%) | |
| IIb | 4 (3.3%) | |
| IIIa | 20 (16.7%) | |
| IIIb | 24 (20.0%) | |
| IV | 69 (57.5%) | |
| PET-CT | ||
| Yes | 83 (69.2%) | |
| No | 37 (30.8%) | |
| PET SUVmax values | ||
| Primary tumor | 14.6 (2.9–58.9) | |
| Lymph node | 8.9 (3.4–23.5) | |
| Sample location | ||
| Primary tumor | 39 (32.5%) | |
| Lymph node | 81 (67.5%) | |
| Size by EBUS (mm) | ||
| Primary tumor | 26.7 (10–70) | |
| Lymph node | 13.4 (6.7–43.8) | |
| ROSE | ||
| Yes | 14 (11.7%) | |
| No | 106 (88.3%) | |
| Equipment * (n = 119) | ||
| 180 | 45 (37.5%) | |
| 190 | 74 (61.7%) | |
| Needle type * (n = 119) | ||
| 21G | 115 (95.8%) | |
| 22G | 4 (3.3%) | |
| Histological tumor type | ||
| Squamous cell carcinoma | 40 (33.3%) | |
| Adenocarcinoma | 71 (59.2%) | |
| Undifferentiated non-small cell carcinoma | 9 (7.5%) | |
| PD-L1 ** (n = 112) | ||
| 0% | 42 (37.5%) | |
| 1–49% | 46 (41.1%) | |
| 50% | 24 (21.4%) | |
| NGS quality parameters *** (n = 65) | ||
| Mapped reads DNA | 1,139,697.0 (12,550.0–5,693,196.0) | |
| Mean AQ 20 read lenght (bp) | 90.0 (69.0–98.0) | |
| Mean read length (bp) | 99.0 (60.0–105.0) | |
| Uniformity base coverage | 97.4 (73.6–100.0) | |
| Mapped reads RNA | 185,670.0 (17,515.0–2,075,290.0) | |
| Mean read length RNA | 91.0 (46.0–105.0) | |
| RNA expression control detected | 7.0 (3.0–7.0) | |
| Detection of treatment target by NGS *** (n = 65) | ||
| Yes | 28 (44.4%) | |
| No | 35 (55.6%) | |
| Mutation of TP53 *** (n = 65) | ||
| Yes | 23 (35.4%) | |
| No | 42 (64.6%) | |
| Deaths | ||
| Yes | 32 (26.7%) | |
| No | 88 (73.3%) | |
| NGS | PD-L1 | ||||||
|---|---|---|---|---|---|---|---|
| Candidates for NGS (n = 67) (Median and Range or n and %) | Sample Adequacy (n = 65) (Median and Range or n and %) | Significance (p) | Candidates for PD-L1 (n = 116) (Median and Range or n and %) | Sample Adequacy (n = 112) (Median and Range or n and %) | Significance (p) | ||
| Age, years (median, range) | |||||||
| 71 (45–88) | 70 (45–88) | 0.605 | 70 (53–84) | 71 (45–88) | 0.912 | ||
| Gender | |||||||
| Male | 42 (62.7%) | 41 (97.6%) | 0.706 | 84 (72.4%) | 81 (96.4%) | 0.919 | |
| Female | 25 (37.3%) | 24 (96.0%) | 32 (27.6%) | 31 (96.9%) | |||
| Smoking history | |||||||
| Never smoker | 15 (22.4%) | 14 (93.3%) | 15 (12.9%) | 14 (93.3%) | |||
| Former smoker | 16 (23.9%) | 16 (100.0%) | 0.549 | 33 (28.4%) | 33 (100.0%) | 0.346 | |
| Current smoker | 36 (53.7%) | 35 (97.2%) | 68 (58.7%) | 65 (95.6) | |||
| Stage (based on 8th ed. of the AJCC) | |||||||
| Ia | 1 (1.5%) | 1 (100.0%) | 1 (0.9%) | 1 (100.0%) | |||
| Ib | 1 (1.5%) | 1 (100.0%) | 2 (1.7%) | 2 (100.0%) | |||
| IIb | 2 (3.0%) | 2 (100.0%) | 0.849 | 4 (3.4%) | 3 (100.0%) | 0.560 | |
| IIIa | 12 (17.9%) | 12 (100.0%) | 19 (16.4%) | 18 (94.7%) | |||
| IIIb | 17 (25.4%) | 17 (100.0%) | 24 (20.7%) | 24 (100.0%) | |||
| IV | 34 (50.7%) | 32 (94.1%) | 66 (56.9%) | 64 (97.0%) | |||
| PET-CT | |||||||
| Yes | 40 (59.7%) | 39 (97.5%) | 0.776 | 79 (65.5%) | 76 (96.2%) | 0.800 | |
| No | 27 (40.3%) | 26 (96.3%) | 37 (34.5%) | 36 (97.1%) | |||
| PET SUVmax values | |||||||
| Primary tumor | 15.2 (4.7–37.0) | 15.2 (4.7–37.0) | 0.979 | 14.6 (2.9–58.9) | 14.7 (2.9–58.9) | 0.076 | |
| Lymph node | 9.3 (3.4–22.6) | 4.9 (3.4–22.6) | 9.3 (3.4–23.5) | 9.5 (3.4–23.5) | |||
| Sample location | |||||||
| Primary tumor | 17 (25.4%) | 17 (100.0%) | 0.402 | 39 (33.6%) | 38 (97.4%) | 0.743 | |
| Lymph node | 50 (74.6%) | 48 (96.0%) | 77 (66.4%) | 74 (96.1%) | |||
| Size by EBUS (mm) | |||||||
| Primary tumor | 29.9 (10.0–70.0) | 29.9 (10.0–70.0) | 0.473 | 26.7 (10.0–70.0) | 25.8 (10.0–70.0) | 0.000 | |
| Lymph node | 13.2 (6.9–40.0) | 16.2 (6.9–40.0) | 13.7 (6.7–43.8) | 14.5 (6.7–43.8) | |||
| ROSE | |||||||
| Yes | 10 (14.9%) | 10 (100.0%) | 0.548 | 14 (12.0%) | 12 (85.7%) | 0.045 | |
| No | 57 (85.1%) | 55 (96.5%) | 102 (88.0%) | 97 (95.1%) | |||
| Equipment (n = 119) | |||||||
| 180 | 21 (31.3%) | 21 (100.0%) | 0.332 | 43 (37.1%) | 43 (100.0%) | 0.202 | |
| 190 | 46 (68.7%) | 44 (95.7%) | 73 (62.9%) | 69 (94.5%) | |||
| Needle type (n = 119) | |||||||
| 21G | 64 (95.5%) | 62 (96.9%) | 0.756 | 112 (96.6%) | 108 (96.4%) | 0.840 | |
| 22G | 3 (4.5%) | 3 (100.0%) | 4 (3.4%) | 4 (100.0%) | |||
| Histological tumor type | |||||||
| Squamous cell carcinoma | 2 (3.0%) | 2 (100.0%) | 39 (33.6%) | 39 (100.0%) | |||
| Adenocarcinoma | 57 (85.1%) | 55 (96.5%) | 0.835 | 68 (58.6%) | 64 (94.1%) | 0.492 | |
| Undifferentiated non-small cell carcinoma | 8 (11.9%) | 8 (100.0%) | 9 (7.8%) | 9 (100.0%) | |||
| Collection Site | Total (n = 65) | Significance | |||
|---|---|---|---|---|---|
| Lesion (n = 17) | Lymph Node (n = 48) | (p) | |||
| Age, years (median, range) | |||||
| 69.0 (53–78) | 71.5 (45–88) | 70 (45–88) | 0.320 | ||
| Gender | |||||
| Male | 11 (65%) | 30 (63%) | 41 | 0.871 | |
| Female | 6 (35%) | 18 (37%) | 24 | ||
| Smoking history | |||||
| Never smoker | 4 (24%) | 10 (21%) | 14 | ||
| Former smoker | 4 (24%) | 12 (25%) | 16 | 0.972 | |
| Current smoker | 9 (52%) | 26 (54%) | 35 | ||
| Stage (based on 8th ed. of the AJCC) | |||||
| Ia | 0 (0%) | 1 (2%) | 1 | ||
| Ib | 0 (0%) | 1 (2%) | 1 | ||
| IIb | 0 (0%) | 2 (4%) | 2 | 0.049 | |
| IIIa | 4 (24%) | 8 (17%) | 12 | ||
| IIIb | 9 (52%) | 8 (17%) | 17 | ||
| IV | 4 (24%) | 28 (58%) | 32 | ||
| PET-CT | |||||
| Yes | 11 (65%) | 29 (58%) | 40 | 0.626 | |
| No | 6 (35%) | 21 (42%) | 27 | ||
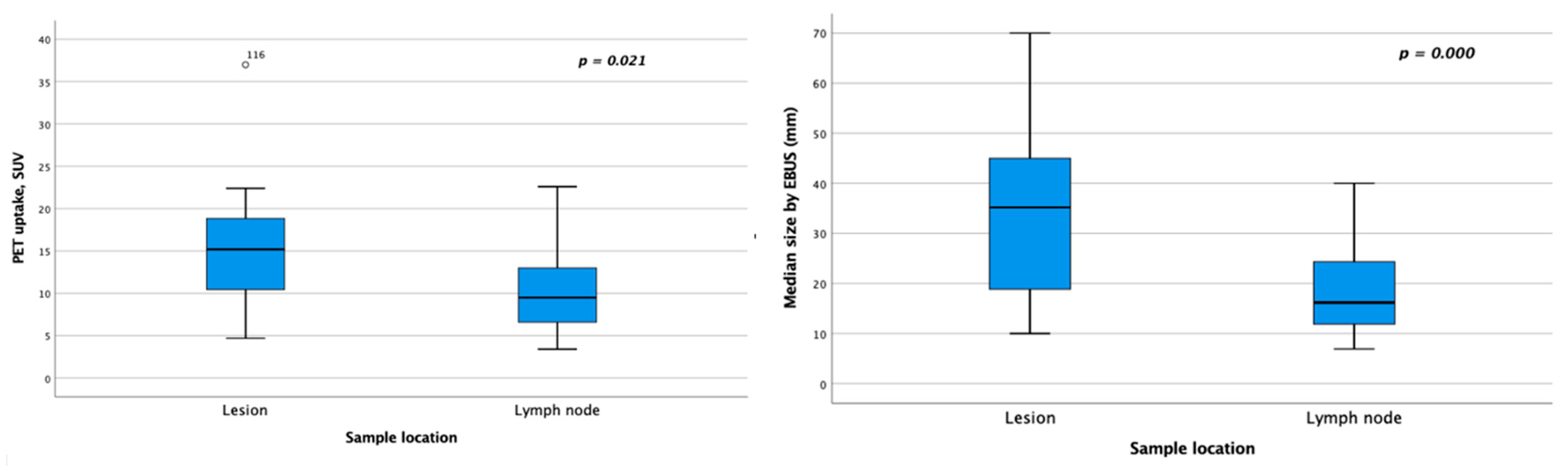
| PET SUVmax values (n = 38) | |||||
| 14.7 (2.9–58.9) | 8.9 (3.4–23.5) | 0.021 | |||
| Size by EBUS (mm) | |||||
| 26.7 (10.0–70.0) | 13.4 (6.7–43.8) | 0.000 | |||
| ROSE | |||||
| Yes | 3 (18%) | 7 (15%) | 10 | 0.764 | |
| No | 14 (82%) | 41 (85%) | 55 | ||
| Equipment | |||||
| 180 | 3 (18%) | 18 (38%) | 21 | 0.133 | |
| 190 | 14 (82%) | 30 (63%) | 44 | ||
| Needle type | |||||
| 21G | 16 (94%) | 46 (96%) | 62 | 0.772 | |
| 22G | 1 (6%) | 2 (4%) | 3 | ||
| Histological tumor type | |||||
| Squamous cell carcinoma | 0 (0%) | 2 (4%) | 2 | ||
| Adenocarcinoma | 14 (82%) | 41 (85%) | 55 | 0.534 | |
| Undifferentiated non-small cell carcinoma | 3 (18%) | 5 (11%) | 8 | ||
| NGS quality parameters | |||||
| Mapped reads DNA | 1,147,680.0 (147,321.0–1,538,582.0) | 1,139,602.5 (12,550.0–5,693,196.0) | 1,139,697.0 (12,550.0–5,693,196.0) | 0.941 | |
| Mean AQ 20 read lenght (bp) | 89.0 (69.0–95.0) | 90.0 (84.0–98.0) | 90.0 (69.0–98.0) | 0.863 | |
| Mean Read Length (bp) | 98.0 (60.0–102.0) | 99.0 (89.0–105.0) | 99.0 (60.0–105.0) | 0.775 | |
| Uniformity base coverage | 96.2 (91.6–98.9) | 97.5 (73.6–100.0) | 97.4 (73.6–100.0) | 0.624 | |
| Mapped reads RNA | 185,670.0 (75,667.0–364,685.0) | 182,873.5 (17,515.0–2,075,290.0) | 185,670.0 (17,515.0–2,075,290.0) | 0.941 | |
| Mean read length RNA (bp) | 89.0 (73.0–92.0) | 91.0 (46.0–105.0) | 91.0 (46.0–105.0) | 0.038 | |
| RNA Expression control detected | 7.0 (7.0–7.0) | 7.0 (3.0–7.0) | 7.0 (3.0–7.0) | not applicable | |
| Detection of treatment target by NGS | |||||
| Yes | 8 (47%) | 20 (44%) | 28 | 0.800 | |
| No | 9 (53%) | 26 (57%) | 35 | ||
| Mutation of TP53 | |||||
| Yes | 9 (53%) | 34 (71%) | 42 | 0.078 | |
| No | 8 (47%) | 14 (29%) | 23 | ||
| Collection Site | Total (n = 116) | Significance | |||
|---|---|---|---|---|---|
| Lesion (n = 38) | Lymph Node (n = 78) | (p) | |||
| Age, years (median, range) | |||||
| 71 (53–84) | 72 (45–88) | 71 (45–88) | 0.385 | ||
| Gender | |||||
| Male | 30 (79%) | 54 (69%) | 84 | 0.272 | |
| Female | 8 (21%) | 24 (31%) | 32 | ||
| Smoking history | |||||
| Never smoker | 4 (11%) | 10 (13%) | 14 | ||
| Former smoker | 13 (34%) | 21 (27%) | 34 | 0.711 | |
| Current smoker | 21 (55%) | 47 (60%) | 68 | ||
| Stage (based on 8th ed. of the AJCC) | |||||
| Ia | 0 (0%) | 1 (1%) | 1 | ||
| Ib | 0 (0%) | 2 (2%) | 1 | ||
| IIb | 0 (0%) | 3 (4%) | 2 | 0.130 | |
| IIIa | 6 (16%) | 13 (17%) | 12 | ||
| IIIb | 13 (34%) | 11 (14%) | 17 | ||
| IV | 19 (50%) | 48 (62%) | 32 | ||
| PET-CT | |||||
| Yes | 13 (34%) | 23 (29%) | 36 | 0.606 | |
| No | 25 (66%) | 55 (71%) | 80 | ||
| PET SUVmax values (n = 79) | |||||
| 14.1 (2.9–58.9) | 9.3 (3.4–23.5) | 9.9 (2.9–58.9) | 0.093 | ||
| Size by EBUS (mm) | |||||
| 26.3 (10.0–70.0) | 13.9 (6.7–43.8) | 17.2 (6.7–70.0) | 0.000 | ||
| ROSE | |||||
| Yes | 3 (8%) | 9 (12%) | 12 | 0.545 | |
| No | 35 (92%) | 69 (88%) | 104 | ||
| Equipment | |||||
| 180 | 13 (34%) | 32 (42%) | 21 | 0.133 | |
| 190 | 25 (66%) | 45 (58%) | 44 | ||
| Needle type | |||||
| 21G | 37 (97%) | 74 (95%) | 62 | 0.448 | |
| 22G | 1 (3%) | 3 (5%) | 3 | ||
| Histological tumor type | |||||
| Squamous cell carcinoma | 17 (45%) | 23 (29%) | 40 | ||
| Adenocarcinoma | 17 (45%) | 50 (64%) | 67 | 0.139 | |
| Undifferentiated non-small cell carcinoma | 4 (10%) | 5 (7%) | 9 | ||
| Result of immunohistochemistry for PDL1 | |||||
| Negative (0, <1%) | 11 (29%) | 31 (45%) | 42 | 0.180 | |
| Positive (1%) | 27 (71%) | 43 (55%) | 70 | ||
| PDL1 expressors (n = 70) | |||||
| Low expresión (1–49%) | 20 (74%) | 26 (60%) | 46 | 0.243 | |
| High expresión (50%) | 7 (26%) | 17 (40%) | 24 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez González, M.; Montero, J.C.; Sayagués, J.M.; Sánchez, T.C.; Ruiz, J.R.; Iglesias Heras, M.; Rivas Marcos, M.B.; Abad, M.; Cordovilla Pérez, R. High-Quality Samples for Next-Generation Sequencing and PD-L1 Assessment in Non-Small Cell Lung Cancer: The Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Diagnostics 2025, 15, 1064. https://doi.org/10.3390/diagnostics15091064
Rodríguez González M, Montero JC, Sayagués JM, Sánchez TC, Ruiz JR, Iglesias Heras M, Rivas Marcos MB, Abad M, Cordovilla Pérez R. High-Quality Samples for Next-Generation Sequencing and PD-L1 Assessment in Non-Small Cell Lung Cancer: The Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Diagnostics. 2025; 15(9):1064. https://doi.org/10.3390/diagnostics15091064
Chicago/Turabian StyleRodríguez González, Marta, Juan Carlos Montero, José María Sayagués, Tamara Clavero Sánchez, Jonnathan Roldán Ruiz, Miguel Iglesias Heras, María Belén Rivas Marcos, Mar Abad, and Rosa Cordovilla Pérez. 2025. "High-Quality Samples for Next-Generation Sequencing and PD-L1 Assessment in Non-Small Cell Lung Cancer: The Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration" Diagnostics 15, no. 9: 1064. https://doi.org/10.3390/diagnostics15091064
APA StyleRodríguez González, M., Montero, J. C., Sayagués, J. M., Sánchez, T. C., Ruiz, J. R., Iglesias Heras, M., Rivas Marcos, M. B., Abad, M., & Cordovilla Pérez, R. (2025). High-Quality Samples for Next-Generation Sequencing and PD-L1 Assessment in Non-Small Cell Lung Cancer: The Role of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Diagnostics, 15(9), 1064. https://doi.org/10.3390/diagnostics15091064





