The Effectiveness of Deep Learning in the Differential Diagnosis of Hemorrhagic Transformation and Contrast Accumulation After Endovascular Thrombectomy in Acute Ischemic Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Set Characteristics
2.1.1. Patient Selection
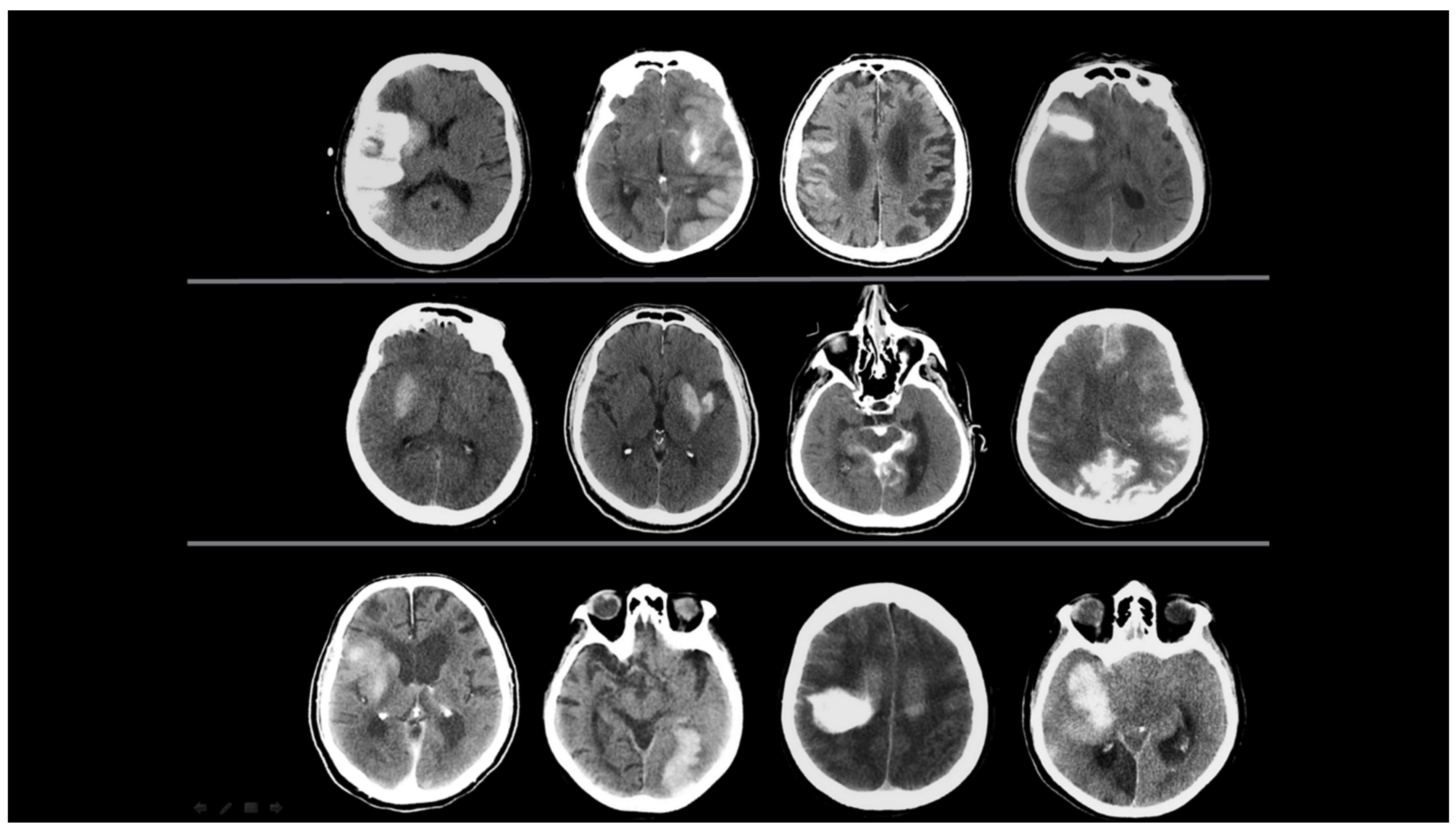
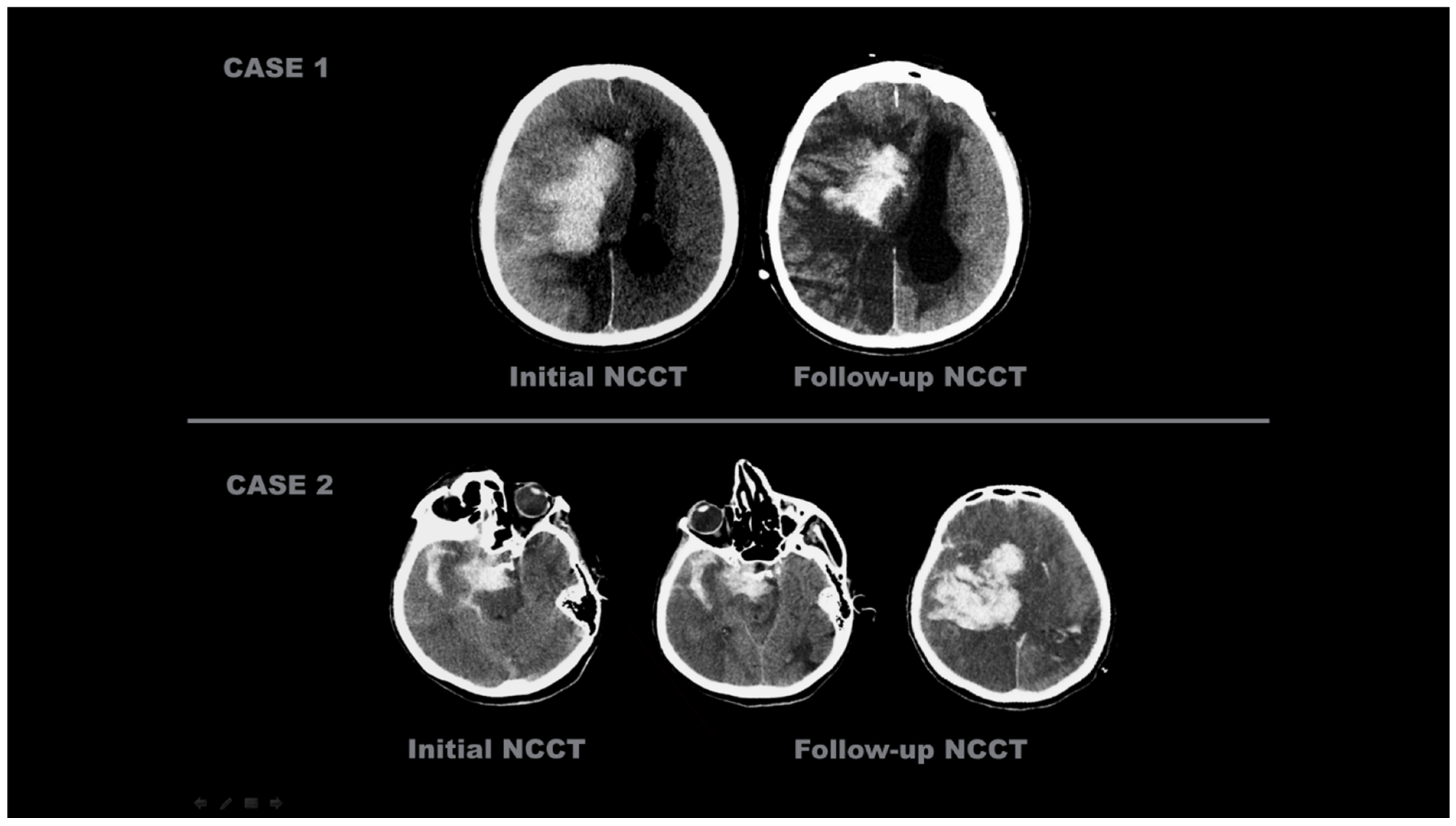
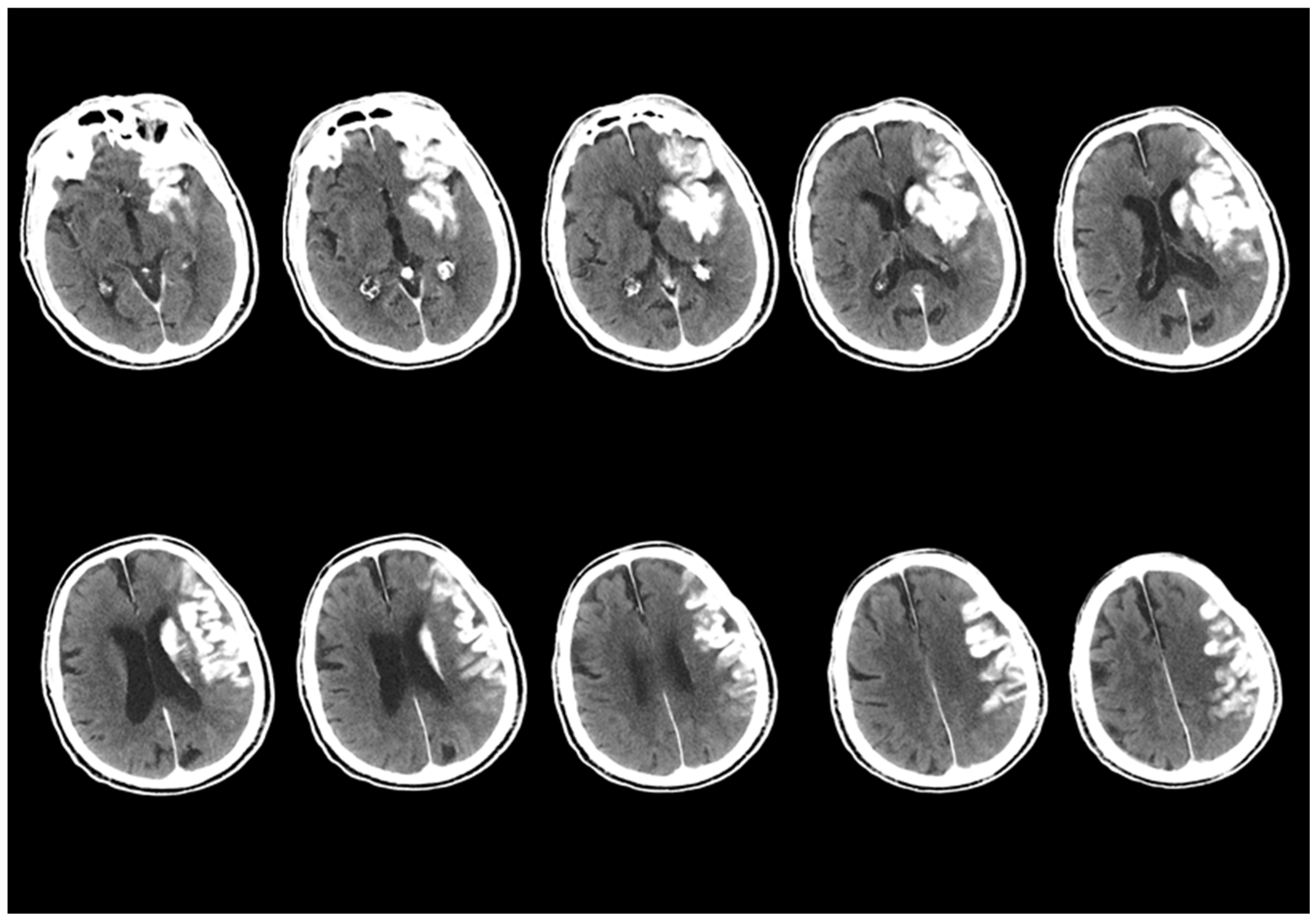
- New emerging densities were evaluated as hematoma developing after 24 h.
- The presence of a hematoma was identified; however, cases with a hyperdense size that did not diminish according to the first screening of NCCT but decreased by more than 50% were evaluated as hematoma and CA together.
- Sequential imaging, images where the decreasing component of the hyperdense area was greater than the increasing component, were evaluated as CA and HT together.
2.1.2. Image Acquisition
2.1.3. Imaging Evaluation
- Contrast Accumulation: The first scan found in the NCCT and the follow-up scan in the NCCT met all of the following conditions;
- Sequential follow-up scan showed hyperdensity that completely disappeared without leaving a hematoma area.
- Sequential follow-up scans show no newly developed hyperdense area.
- In sequential follow-up scans, hyperdensity that gradually decreases and completely disappears in NCCT images after 72 h.
- Hemorrhagic Transformation: The initial scan shows NCCT, and the follow-up scan shows NCCT meeting any of the following conditions.
- Hyperdensity that does not decrease or remains constant.
- Increase in hyperdensity size.
2.2. Dataset Features
2.2.1. Image Preprocessing
2.2.2. Labeling and Data Augmentation
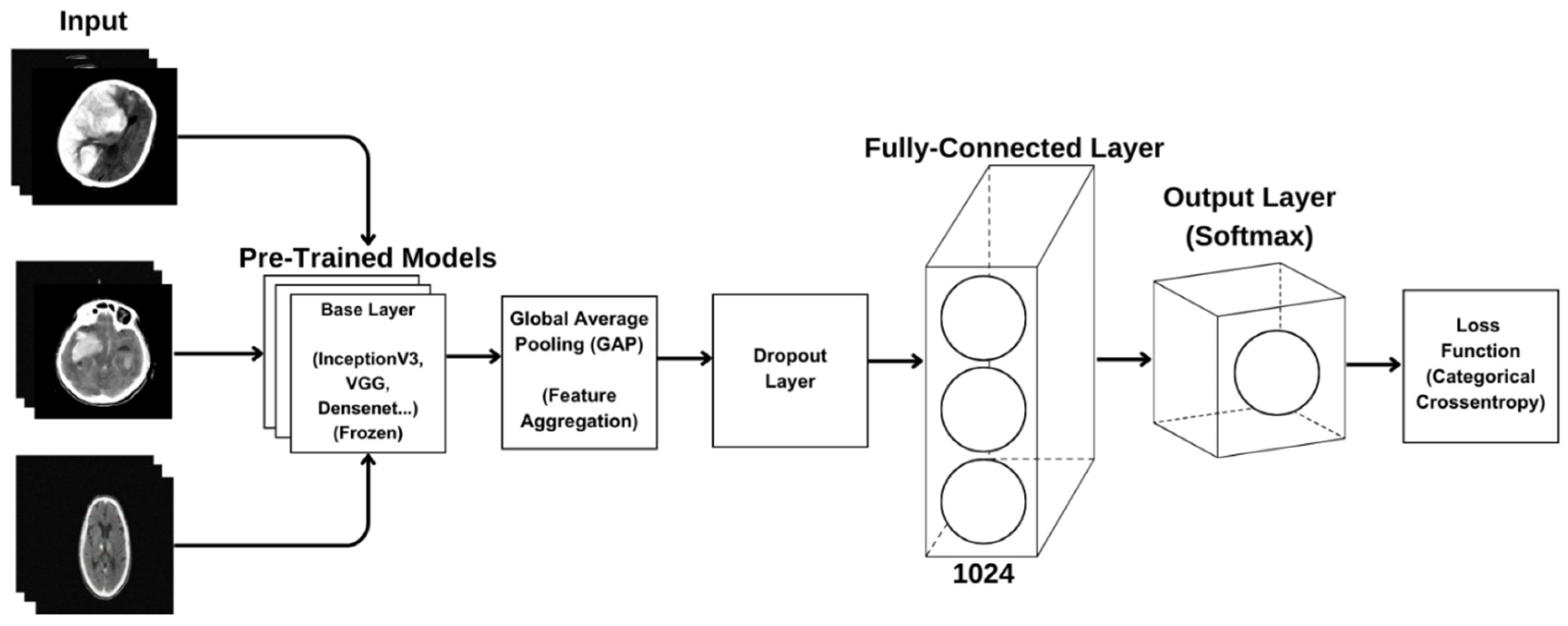
2.2.3. Transfer Learning and Used Models
2.2.4. Model Architecture
2.2.5. Model Training
2.2.6. CNN Model Analyses
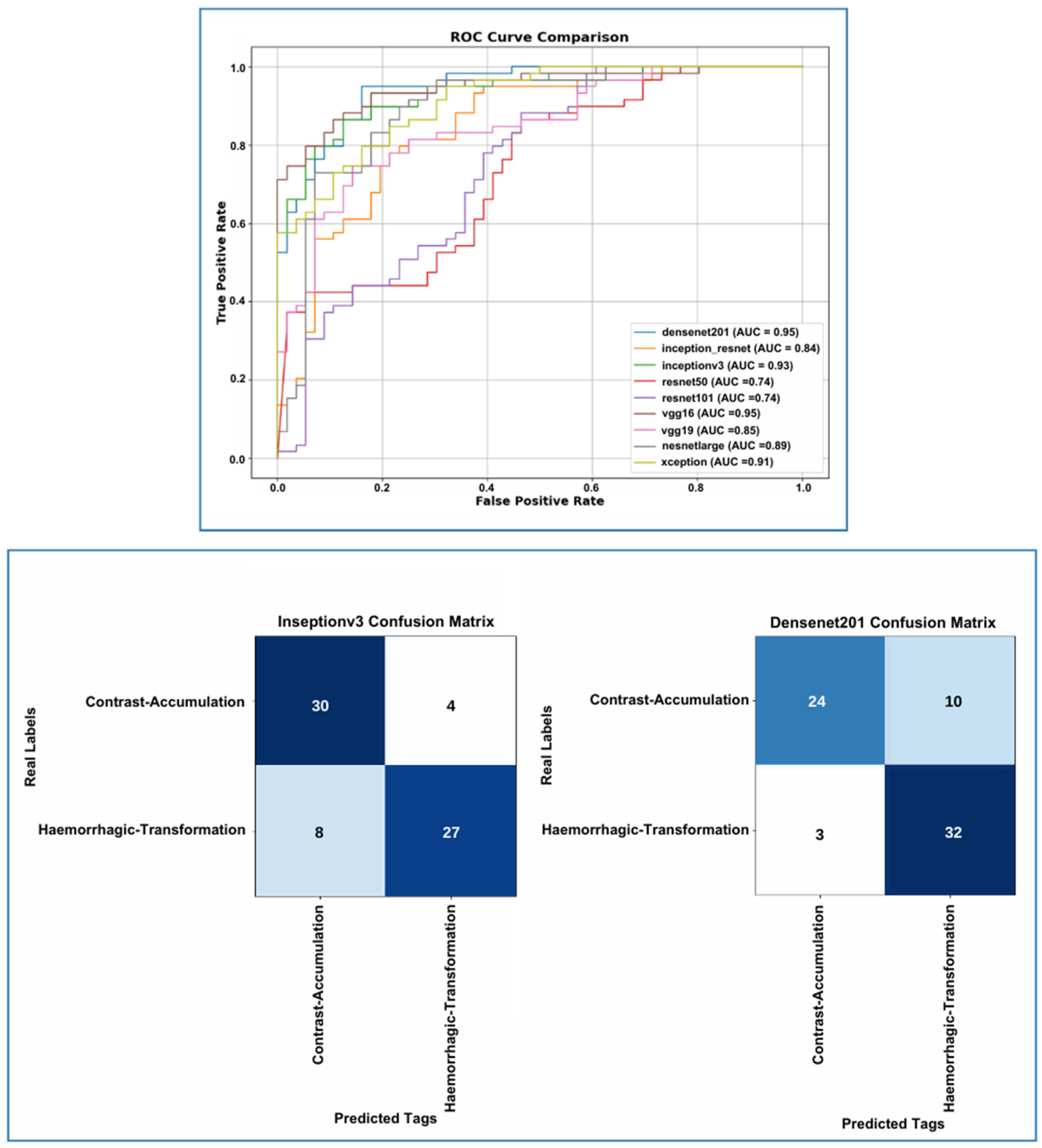
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCCT | Non-Contrast Computed Tomography (NCCT) |
| AIS | Acute Ischemic Stroke (AIS) |
| EVT | Endovascular Thrombectomy (EVT) |
| CNN | Convolutional Neural Network (CNN) |
| IV-tPA | Intravenous Tissue Plasminogen Activator (IV-tPA) |
| FDA | Food and Drug Administration (FDA) |
| LVO | Large Vessel Occlusion (LVO) |
| BBB | Blood–Brain Barrier (BBB) |
| AI | Artificial Intelligence (AI) |
| MT | Mechanical Thrombectomy (MT) |
| ASPECT | Alberta stroke program early CT score (ASPECTS) |
| NIHSS | National Institutes of Health Stroke Scale (NIHSS) |
| ICA | Internal Carotid Artery (ICA) |
| MCA | Middle Cerebral Artery (MCA) |
| mTICI | modified thrombolysis in cerebral infarction (mTICI) |
| PIL | Python Imaging Library (PIL) |
| GAP | Global Average Pooling (GAP) |
| ReLU | Rectified Linear Unit (ReLU) |
| ROC | Receiver Operating Characteristic (ROC) |
| TPR | True Positive Rate (TPR) |
| TP | True Positive (TP) |
| FPR | False Positive Rate (FPR) |
| FP | False Positive (FP) |
| AUC | Area Under the Curve (AUC) |
| CA | Contrast Accumulation (CA) |
| HT | Hemorrhagic Transformation (HT) |
References
- Chen, Z.; Zhang, Y.; Su, Y.; Sun, Y.; He, Y.; Chen, H. Contrast Extravasation is Predictive of Poor Clinical Outcomes in Patients Undergoing Endovascular Therapy for Acute Ischemic Stroke in the Anterior Circulation. J. Stroke Cerebrovasc. Dis. 2020, 29, 104494. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.C.; Ma, D.C.; Li, W.; Qiu, J.; Sun, X.H.; Zhao, Y.G.; Liu, X.; Zhao, Z.A.; Liu, L.; Nguyen, T.N.; et al. Contrast Enhancement by Location and Volume is Associated with Long-Term Outcome after Thrombectomy in Acute Ischemic Stroke. Sci. Rep. 2022, 12, 16998. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Abdalkader, M.; Fischer, U.; Qiu, Z.; Nagel, S.; Chen, H.S.; Miao, Z.; Khatri, P. Endovascular Management of Acute Stroke. Lancet 2024, 404, 1265–1278. [Google Scholar] [CrossRef]
- Meyers, P.M.; Schumacher, H.C.; Higashida, R.T.; Barnwell, S.L.; Creager, M.A.; Gupta, R.; McDougall, C.G.; Pandey, D.K.; Sacks, D.; Wechsler, L.R. Indications for the Performance of Intracranial Endovascular Neurointerventional Procedures: A Scientific Statement from the American Heart Association. Circulation 2009, 119, 2235–2249. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Silva, G.S.; Nogueira, R.G. Endovascular Treatment of Acute Ischemic Stroke. Continuum 2020, 26, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Román, L.S.; Menon, B.K.; Blasco, J.; Hernández-Pérez, M.; Dávalos, A.; Majoie, C.B.L.M.; Campbell, B.C.V.; Guillemin, F.; Lingsma, H.; Anxionnat, R.; et al. Imaging Features and Safety and Efficacy of Endovascular Stroke Treatment: A Meta-Analysis of Individual Patient-Level Data. Lancet Neurol. 2018, 17, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.A. Mechanical Thrombectomy for Acute Ischemic Stroke. Continuum 2023, 29, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Whitney, E.; Khan, Y.R.; Alastra, A.; Schiraldi, M.; Siddiqi, J. Contrast Extravasation Post Thrombectomy in Patients With Acute Cerebral Stroke: A Review and Recommendations for Future Studies. Cureus 2020, 12, e10616. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Iseda, T.; Yoneyama, T.; Wakisaka, S. Early CT Signs in Patients with Acute Middle Cerebral Artery Occlusion: Incidence of Contrast Staining and Haemorrhagic Transformations after Intra-Arterial Reperfusion Therapy. Clin. Radiol. 2006, 61, 156–162. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, K.Y.; Lee, W.J.; Byun, J.S.; Kim, J.K.; Park, M.S.; Ahn, S.W.; Shin, H.W. The Cortical Contrast Accumulation from Brain Computed Tomography after Endovascular Treatment Predicts Symptomatic Hemorrhage. Eur. J. Neurol. 2015, 22, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Yedavalli, V.; Sammet, S. Contrast Extravasation versus Hemorrhage after Thrombectomy in Patients with Acute Stroke. J. Neuroimaging 2017, 27, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Saver, J.L.; Adams, H.P.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Tomsick, T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am. J. Neuroradiol. 2007, 28, 382–384. [Google Scholar] [PubMed] [PubMed Central]
- Parrilla, G.; García-Villalba, B.; Espinosa de Rueda, M.; Zamarro, J.; Carrión, E.; Hernández-Fernández, F.; Martín, J.; Hernández-Clares, R.; Morales, A.; Moreno, A. Hemorrhage/Contrast Staining Areas after Mechanical Intra-Arterial Thrombectomy in Acute Ischemic Stroke: Imaging Findings and Clinical Significance. AJNR Am. J. Neuroradiol. 2012, 33, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Payabvash, S.; Qureshi, M.H.; Khan, S.M.; Khan, M.; Majidi, S.; Pawar, S.; Qureshi, A.I. Differentiating Intraparenchymal Hemorrhage from Contrast Extravasation on Post-Procedural Noncontrast CT Scan in Acute Ischemic Stroke Patients Undergoing Endovascular Treatment. Neuroradiology 2014, 56, 737–744. [Google Scholar] [CrossRef]
- Fukazawa, S.; Waki, R.; Hidaka, A.; Kishimoto, R.; Kimura, K.; Tamakawa, N.; Teramachi, H.; Shimizu, K. Angiographic A-V Shunt during Interventional Thrombolysis for Acute Cerebral Embolism: A New Predictive Sign for Hemorrhagic Complication. Interv. Neuroradiol. 1997, 3, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Yokogami, K.; Nakano, S.; Ohta, H.; Goya, T.; Wakisaka, S. Prediction of Hemorrhagic Complications after Thrombolytic Therapy for Middle Cerebral Artery Occlusion: Value of Pre- and Post-Therapeutic Computed Tomographic Findings and Angiographic Occlusive Site. Neurosurgery 1996, 39, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Lummel, N.; Schulte-Altedorneburg, G.; Bernau, C.; Pfefferkorn, T.; Patzig, M.; Janssen, H.; Opherk, C.; Brückmann, H.; Linn, J. Hyperattenuated Intracerebral Lesions after Mechanical Recanalization in Acute Stroke. AJNR Am. J. Neuroradiol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Gong, C.; Wang, Y.; Yuan, J.; Zhang, J.; Jiang, S.; Xu, T.; Chen, Y. The Association of the Spatial Location of Contrast Extravasation with Symptomatic Intracranial Hemorrhage after Endovascular Therapy in Acute Ischemic Stroke Patients. Curr. Neurovasc. Res. 2023, 20, 354–361. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Yuan, J.; Chen, Y.; Luo, H. Contrast Extravasation and Outcome of Endovascular Therapy in Acute Ischaemic Stroke: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e044917. [Google Scholar] [CrossRef]
- Hussain, M.; Purrucker, J.; Ringleb, P.; Schönenberger, S. Akuttherapie des ischämischen Schlaganfalls. Med. Klin. Intensivmed. Notfmed. 2025, 120, 120–128. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Y.; Chen, Z.; He, Y.; Zhang, Y.; Chen, H. Contrast Extravasation After Endovascular Treatment in Posterior Circulation Stroke. World Neurosurg. 2019, 130, e583–e587. [Google Scholar] [CrossRef]
- An, H.; Zhao, W.; Wang, J.; Wright, J.C.; Elmadhoun, O.; Wu, D.; Shang, S.; Wu, C.; Li, C.; Wu, L.; et al. Contrast Staining may be Associated with Intracerebral Hemorrhage but Not Functional Outcome in Acute Ischemic Stroke Patients Treated with Endovascular Thrombectomy. Aging Dis. 2019, 10, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Ahn, S.Y.; Rhee, J.J.; Lee, J.W.; Hur, J.W.; Lee, H.K. Extent of Contrast Enhancement on Non-Enhanced Computed Tomography after Intra-Arterial Thrombectomy for Acute Infarction on Anterior Circulation: As a Predictive Value for Malignant Brain Edema. J. Korean Neurosurg. Soc. 2015, 58, 321–327. [Google Scholar] [CrossRef]
- You, S.H.; Kim, B.; Kim, B.K.; Suh, S.I. MR Imaging for Differentiating Contrast Staining from Hemorrhagic Transformation after Endovascular Thrombectomy in Acute Ischemic Stroke: Phantom and Patient Study. AJNR Am. J. Neuroradiol. 2018, 39, 2313–2319. [Google Scholar] [CrossRef]
- Yang, S.J.; Lu, Y.H.; Huang, Y.C.; Chan, L.; Ting, W.Y. Immediate CT Change after Thrombectomy Predicting Symptomatic Hemorrhagic Transformation. J. Chin. Med. Assoc. 2023, 86, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Song, H.; Cui, S.; Xiong, H.; Long, B.; Li, Y. Deep Learning of Noncontrast CT for Fast Prediction of Hemorrhagic Transformation of Acute Ischemic Stroke: A Multicenter Study. Eur. Radiol. Exp. 2025, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.B.; Zoga, A.C. Artificial Intelligence in Radiology: Current Technology and Future Directions. Semin. Musculoskelet. Radiol. 2018, 22, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.S.; Judge, C.; Bollard, S.M.; Clifford, S.M.; Healy, G.M.; Aziz, A.; Mathur, P.; Islam, S.; Yeom, K.W.; Lawlor, A.; et al. Radiology Artificial Intelligence: A Systematic Review and Evaluation of Methods (RAISE). Eur. Radiol. 2022, 32, 7998–8007. [Google Scholar] [CrossRef]
- Gorenstein, L.; Soffer, S.; Apter, S.; Konen, E.; Klang, E. AI in Radiology: Is It the Time for Randomized Controlled Trials? Eur. Radiol. 2023, 33, 4223–4225. [Google Scholar] [CrossRef]
- Tijssen, M.P.; Hofman, P.A.; Stadler, A.A.; van Zwam, W.H.; de Graaf, R.A.; van Oostenbrugge, R.J.; Klomp, D.W.J.; Postma, A.A. The Role of Dual-Energy CT in Differentiating Between Brain Haemorrhage and Contrast Medium After Mechanical Revascularisation in Acute Ischaemic Stroke. Eur. Radiol. 2014, 24, 834–840. [Google Scholar] [CrossRef]


| Image Size | Batch Size | Base Model | GAP | Dense | Output Layer | Optimizer | Loss Function | Epoch |
|---|---|---|---|---|---|---|---|---|
| (640,640) | 32 | DenseNet201 InceptionResNet InceptionV3 NASNetLarge ResNet50 ResNet101 VGG16 VGG19 Xception | GlobalAvere Pooling2D | 512 Neurons ReLU activation Dropout(0.5) | Softmax | Adam | Categorical Crossentropy | 40 |
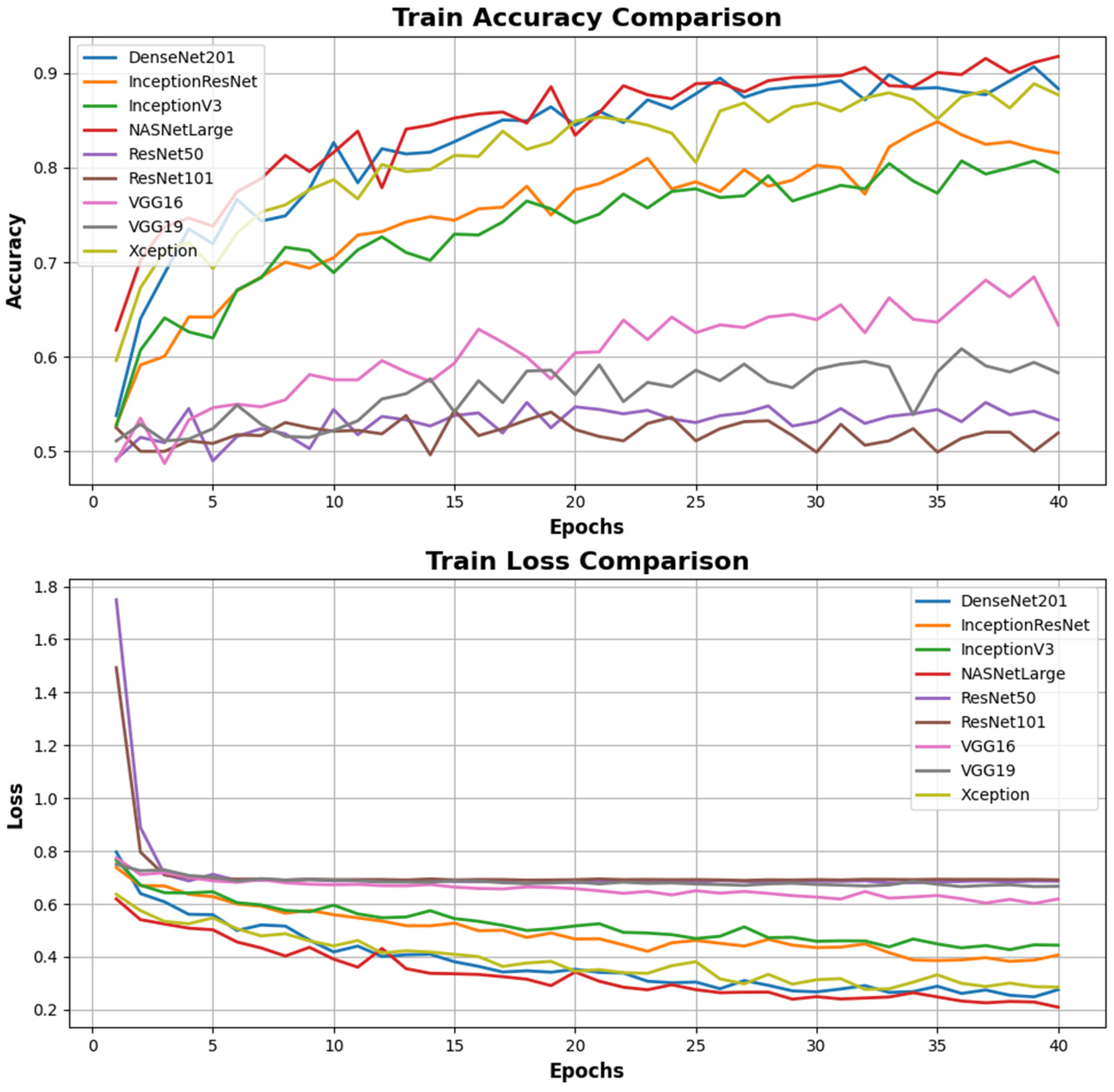
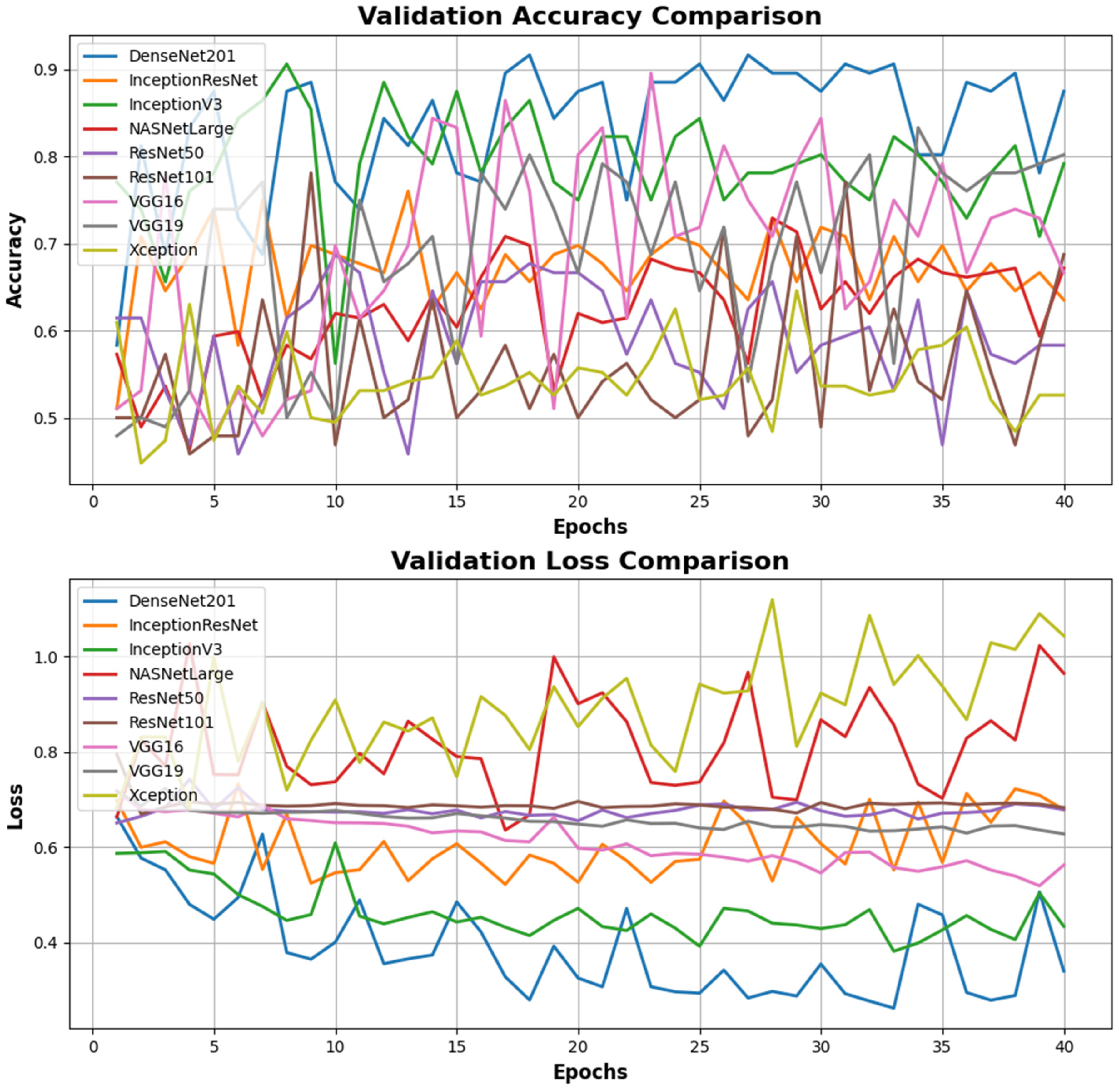
| Models | Accuracy (0.7–0.9+) | Loss (0–0.5) | Valiation Accuracy (0.7–0.9) | Validation Loss (0–0.5) | F1 Score (0.7–1) | Precision (0.7–1) | Recall (0.7–1) |
|---|---|---|---|---|---|---|---|
| DenseNet201 | 0.883 | 0.275 | 0.875 | 0.339 | 0.79 | 0.80 | 0.79 |
| InceptionResNet | 0.815 | 0.406 | 0.635 | 0.6 | 0.81 | 0.83 | 0.81 |
| InceptionV3 | 0.795 | 0.444 | 0.791 | 0.43 | 0.80 | 0.80 | 0.80 |
| NASNetLarge | 0.917 | 0.209 | 0.671 | 0.965 | 0.92 | 0.93 | 0.92 |
| ResNet50 | 0.533 | 0.686 | 0.583 | 0.67 | 0.52 | 0.65 | 0.58 |
| ResNet101 | 0.519 | 0.691 | 0.687 | 0.682 | 0.57 | 0.57 | 0.57 |
| VGG16 | 0.633 | 0.619 | 0.666 | 0.562 | 0.69 | 0.77 | 0.71 |
| VGG19 | 0.583 | 0.666 | 0.802 | 0.62 | 0.54 | 0.64 | 0.58 |
| Xception | 0.877 | 0.285 | 0.526 | 0.93 | 0.87 | 0.87 | 0.87 |
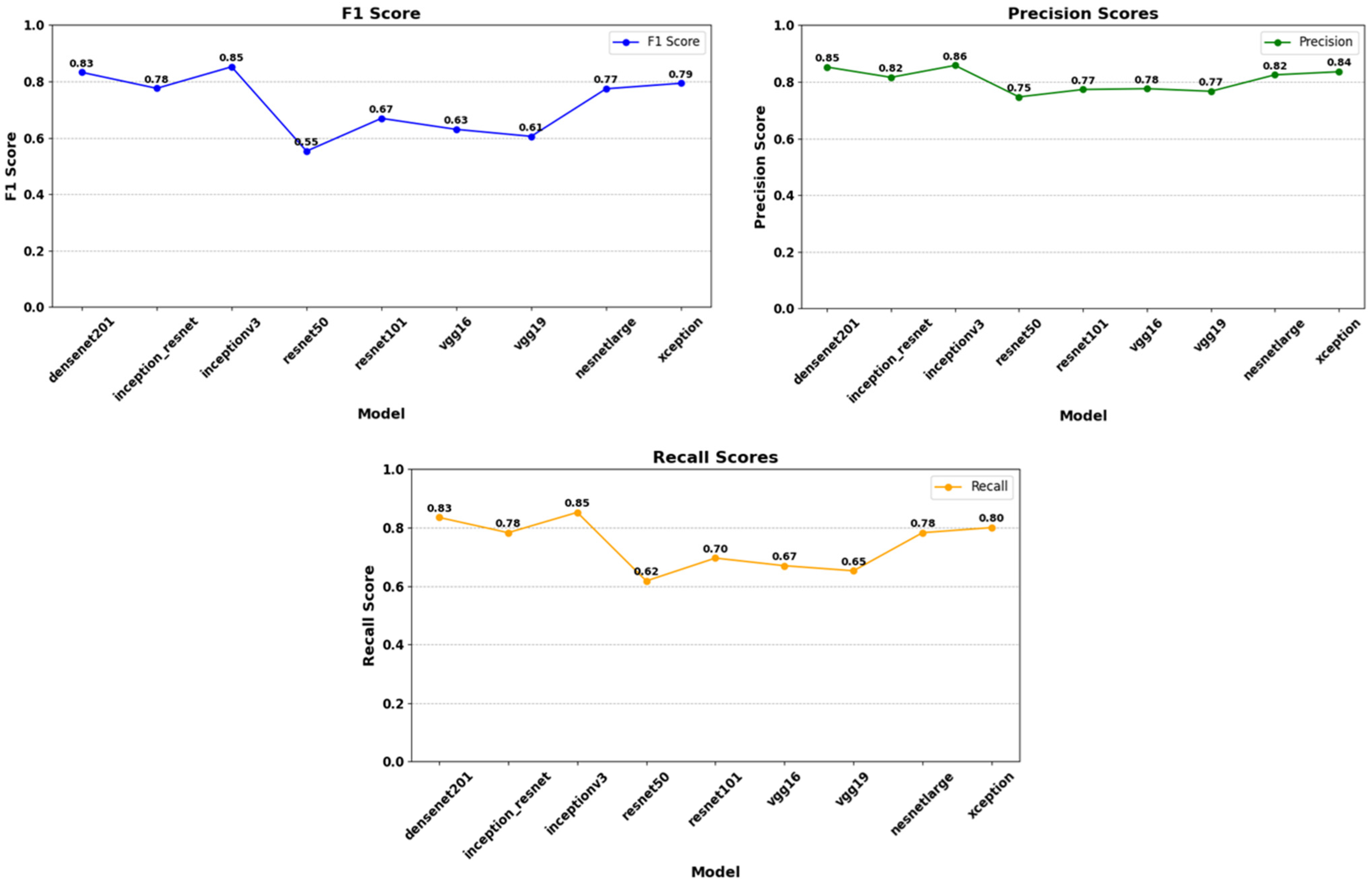
| Models | Accuracy (0.7–0.9) | Loss (0–0.5) | F1 Score (0.7–1) | Precision (0.7–1) | Recall (0.7–1) |
|---|---|---|---|---|---|
| DenseNet201 | 0.853 | 0.253 | 0.83 | 0.85 | 0.83 |
| InceptionResNet | 0.792 | 0.366 | 0.78 | 0.82 | 0.78 |
| InceptionV3 | 0.832 | 0.321 | 0.85 | 0.86 | 0.85 |
| NASNetLarge | 0.823 | 0.214 | 0.77 | 0.82 | 0.78 |
| ResNet50 | 0.543 | 0.645 | 0.55 | 0.75 | 0.62 |
| ResNet101 | 0.554 | 0.706 | 0.67 | 0.77 | 0.70 |
| VGG16 | 0.612 | 0.617 | 0.63 | 0.78 | 0.67 |
| VGG19 | 0.659 | 0.683 | 0.61 | 0.77 | 0.65 |
| Xception | 0.827 | 0.219 | 0.79 | 0.84 | 0.80 |
| Model | Confidence İnterval (95%) | p-Value |
|---|---|---|
| InceptionV3 | (0.720, 0.898) | 0.388 |
| DenseNet201 | (0.704, 0.886) | 0.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyazal, M.; Solak, M.; Tören, M.; Asan, B.; Kaba, E.; Çeliker, F.B. The Effectiveness of Deep Learning in the Differential Diagnosis of Hemorrhagic Transformation and Contrast Accumulation After Endovascular Thrombectomy in Acute Ischemic Stroke Patients. Diagnostics 2025, 15, 1080. https://doi.org/10.3390/diagnostics15091080
Beyazal M, Solak M, Tören M, Asan B, Kaba E, Çeliker FB. The Effectiveness of Deep Learning in the Differential Diagnosis of Hemorrhagic Transformation and Contrast Accumulation After Endovascular Thrombectomy in Acute Ischemic Stroke Patients. Diagnostics. 2025; 15(9):1080. https://doi.org/10.3390/diagnostics15091080
Chicago/Turabian StyleBeyazal, Mehmet, Merve Solak, Murat Tören, Berkutay Asan, Esat Kaba, and Fatma Beyazal Çeliker. 2025. "The Effectiveness of Deep Learning in the Differential Diagnosis of Hemorrhagic Transformation and Contrast Accumulation After Endovascular Thrombectomy in Acute Ischemic Stroke Patients" Diagnostics 15, no. 9: 1080. https://doi.org/10.3390/diagnostics15091080
APA StyleBeyazal, M., Solak, M., Tören, M., Asan, B., Kaba, E., & Çeliker, F. B. (2025). The Effectiveness of Deep Learning in the Differential Diagnosis of Hemorrhagic Transformation and Contrast Accumulation After Endovascular Thrombectomy in Acute Ischemic Stroke Patients. Diagnostics, 15(9), 1080. https://doi.org/10.3390/diagnostics15091080






