Contemporary Perspectives on Congestion in Heart Failure: Bridging Classic Signs with Evolving Diagnostic and Therapeutic Strategies
Abstract
:1. Introduction
2. Pathophysiology of Congestion
2.1. Regional Distribution
2.1.1. Pulmonary
2.1.2. Systemic
2.2. Fluid Compartment
2.2.1. Intravascular Congestion
2.2.2. Tissue Congestion
2.3. Third-Space
2.4. Subclinical Congestion
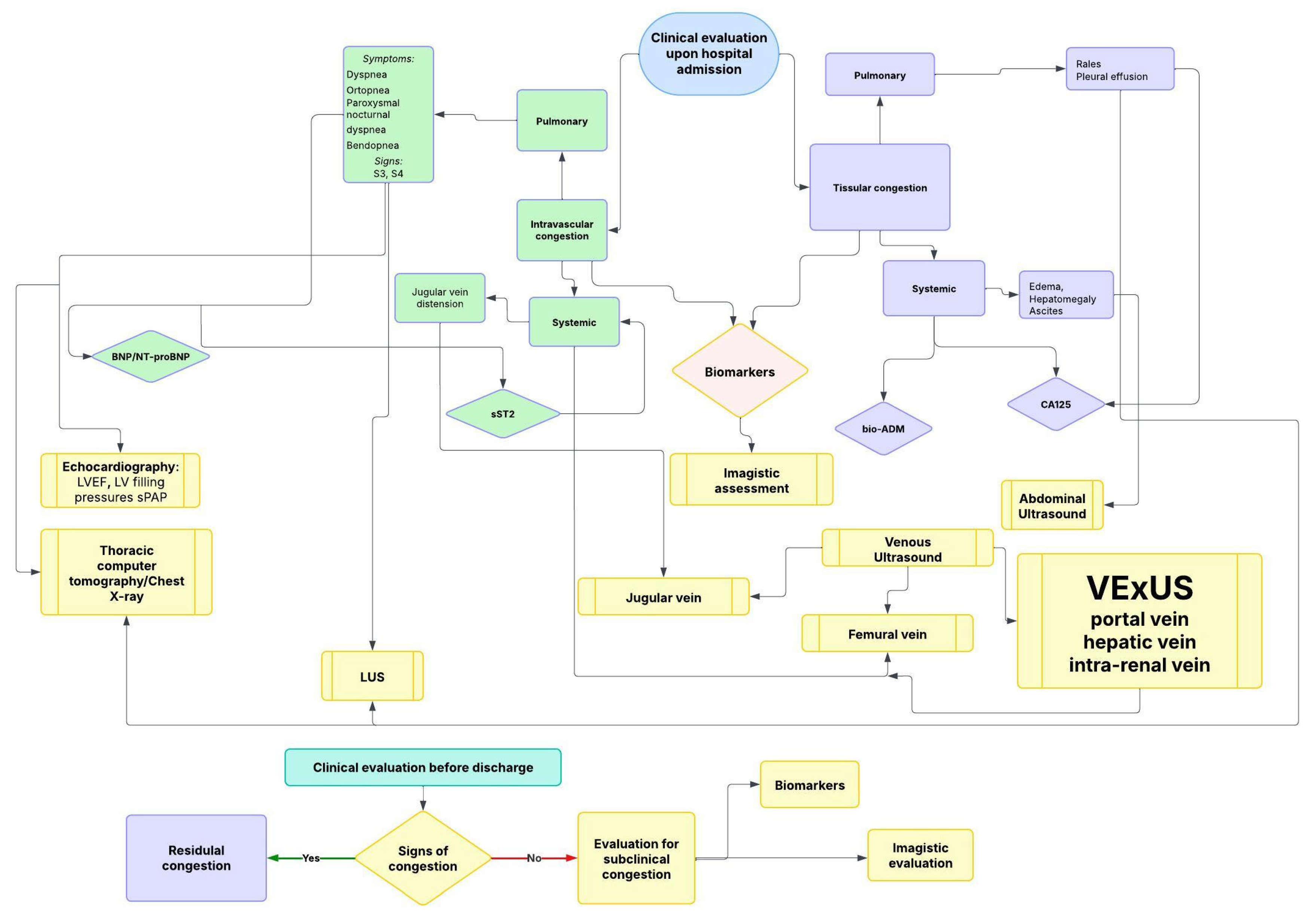
3. Clinical Assessment of Congestion Status
3.1. Symptoms
3.2. Clinical Evaluation of ADHF
3.3. Quantification of Congestion in HF Based on Clinical Parameters
| Clinical Congestion | EVEREST | OPTIMIZE-HF [20] | PROTECT [12,21] | DOSE-HF [22] | LUCAS [4] | Rohde |
|---|---|---|---|---|---|---|
| Dyspnea | 0 p—none; 1 p—seldom; 2 p—frequent; 3 p—continuous | 0 p—none; 2 p—on exertion; 3 p—at rest | Not included | Not included | Not included | Not included |
| Orthopnea | 0 p—none; 1 p—seldom; 2 p—frequent; 3 p—continuous | 0 p—none; 2 p—yes | 0 p—none; 1 p—2 pillows; 2 p—3 pillows; 3 p—>30° | 0 p—<2 pillows; 2 p—≥2 pillows; | 1 p—any respiratory distress associated with lying down or perceived need to use > 1 pillow to avoid respiratory distress | Graded form 0 to 4 0 p—no more than 1 pillow needed; 4 p—at least 1 night spent sleeping in a sitting position |
| Fatigue | 0 p—absent; 1 p—slight; 3 p—moderate; 4 p—continuous | 0 p—none; 2 p—yes | Not included | Not included | Not included | Not included |
| JVD (cm H2O) | 0 p ≤ 6 cm H2O; 1 p 6–9 cm H2O; 2 p 10–15 cm H2O; 3 p ≥ 15 cm H2O | 0 p—<6; 1 p—6–9; 2 p—10–15; 3 p—>15 | 0 p—<6; 1 p—6–9; 2 p—10–15; 3 p—>15 | Not included | 1 p—≥10 cm H2O | Graded from 0 to 4 0 p—jugular veins not visible 4 p—crests visible at the earlobe with the patient at 30–40°. |
| Rales | 0 p—none; 1 p—bases; 2 p—up to <50%; 3 p > 50% | 0 p—none; 1 p—<1/3; 2 p > 1/3 | Not included | Not included | Not included | 0 p—none; 1 p—<25; 2 p—25 to 50%; 3 p > 50%; 4 p—entire lung |
| Edema | 0 p—absent; 1 p—slight; 2 p—moderate; 3 p—marked | 0—absent; 1 p—slight; 2 p—moderate; 3—marked | 0 p—absent; 1 p—slight; 2 p—moderate; 3 p—marked | 0 p—trace; 1 p—moderate; 2 p—severe | 1 p = Yes | 0 p—none; 1–4 p—according to the indentation at the ankle |
| Other | Not included | Not included | Not included | Not included | 1 p—diuretics increased over the past week; 1 p ≥ 1 kg increase since the last visit | 1 p—3rd heart sound |
| Strengths | Easy to apply, frequently used in trials | Simple, applicable at discharge | Well-structured, JVD-based | Good stratification for diuretic therapy trials | Includes clinical trajectory, weight | Detailed severity assessment (0–4 scale) |
| Dependence on operator skill | Low (subjective scoring) | Low | Low | Low | Medium (JVD eval.) | Medium |
| Pulmonary vs. Systemic Congestion | Both—slightly favors systemic | Both | Favors systemic | Favors systemic | Both | Both |
| Points | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Signs and Symptoms | ||||
| Ambrosy Score [13] | ||||
| Dyspnea | Absent | Minimal | Frequent | Continuous |
| Orthopnea | Absent | Minimal | Frequent | Continuous |
| Asthenia | Absent | Minimal | Frequent | Continuous |
| Jugular vein distension (cm H2O) | <6 | 6–9 | 10–15 | >15 |
| Pulmonary crackles | Absent | In base | <50% | >50% |
| Leg edema | Absent | Mild | Moderate | Pronounced |
| Rubio Score [12] | ||||
| Orthopnea | Absent | 1-Pillow | 2-Pillow | >30 |
| Edema | Absent | Mild | Moderate | Pronounced |
| Jugular vein distension (cm H2O) | <6 | 6–10 | >10 | - |
4. Biomarkers as Adjuncts to Clinical Assessment in Congestion
4.1. Natriuretic Peptides
4.1.1. Natriuretic Peptides and Congestion
4.1.2. Variation in Natriuretic Peptides and Decongestion Monitoring
4.1.3. Prognostic Role of Natriuretic Peptides in Ambulatory HF
4.1.4. Limitations in Interpretation of Natriuretic Peptide Variation in HF
4.2. Hemoconcentration
Plasma Volume Variation
4.3. Other Biomarkers
4.3.1. Biologically Active Adrenomedullin
4.3.2. Soluble CD146
4.3.3. Soluble ST2
4.4. Carbohydrate Antigen 125
5. The Role of Imaging in Evaluating Congestion
5.1. Intravascular Pulmonary Congestion
5.1.1. Lung Ultrasound (LUS)
Pleural Effusion
5.1.2. Thoracic Computer Tomography and Chest Radiography
5.1.3. Echocardiography
Patterns of Congestion Across HFpEF and HFrEF
5.2. Intravascular Systemic Congestion
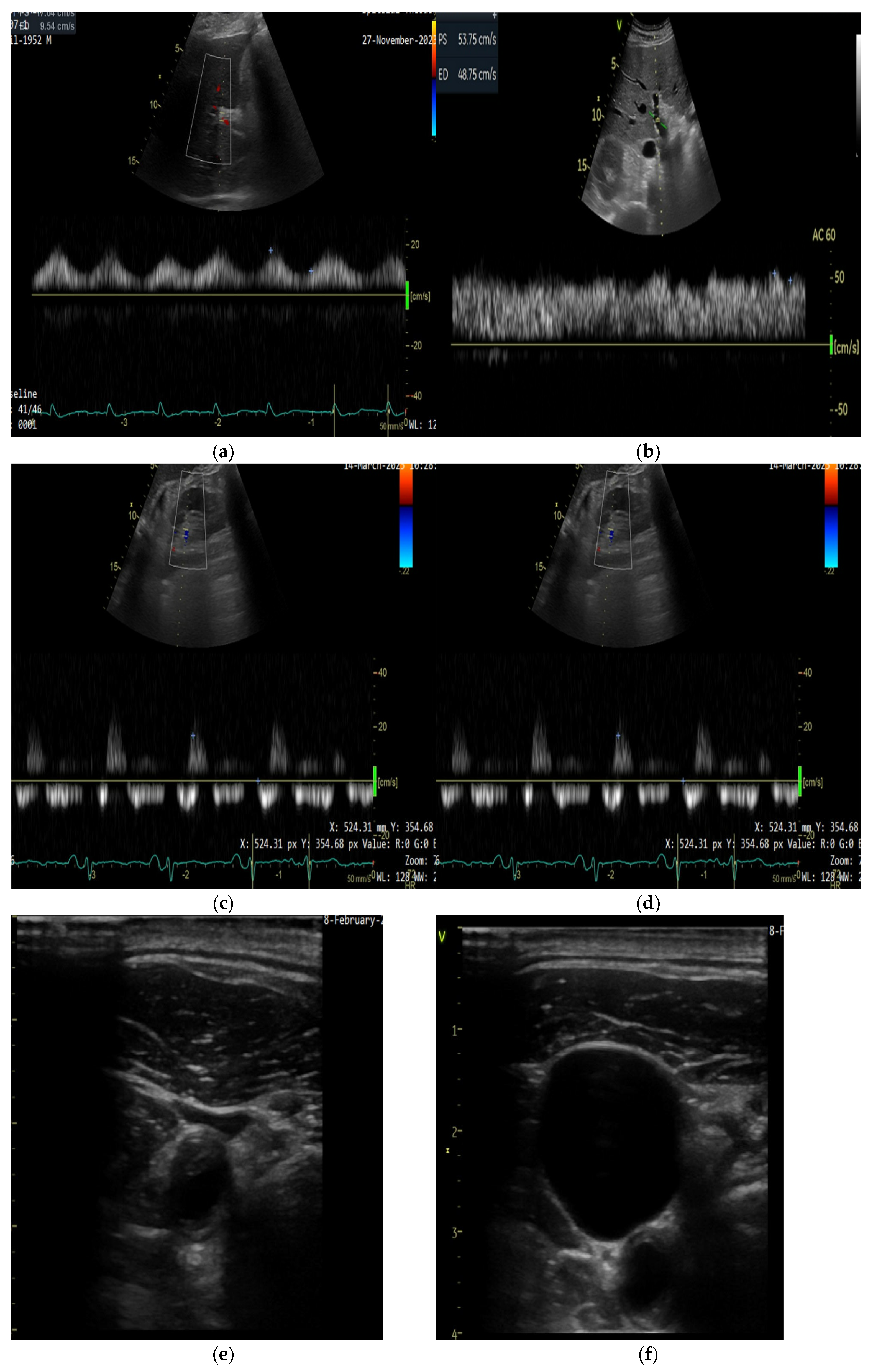
5.2.1. Ultrasound Assessment of Systemic Venous Congestion: Role of VExUS
- VExUS 0: IVC diameter < 20 mm; no additional Doppler evaluation is necessary.
- VExUS 1: IVC diameter ≥ 20 mm, with normal or mildly abnormal Doppler waveforms in the hepatic, portal, and renal veins.
- VExUS 2: IVC diameter ≥ 20 mm, with one severely abnormal Doppler waveform among the three veins assessed.
- VExUS 3: IVC diameter ≥ 20 mm, with two or more severely abnormal Doppler waveforms [72].
5.2.2. Technical Aspects of Doppler Evaluation
5.2.3. Jugular Vein Ultrasound
5.2.4. Femural Vein
5.3. Tissue Systemic Congestion
5.3.1. Peripheral Edema
5.3.2. Ascites
6. Emerging Technologies for Congestion Monitoring in HF
6.1. Remote Dielectric Sensing (ReDS)
6.2. HeartLogic™
6.3. CardioMEMS
7. Integrated Multimodal Assessment and Clinical Application
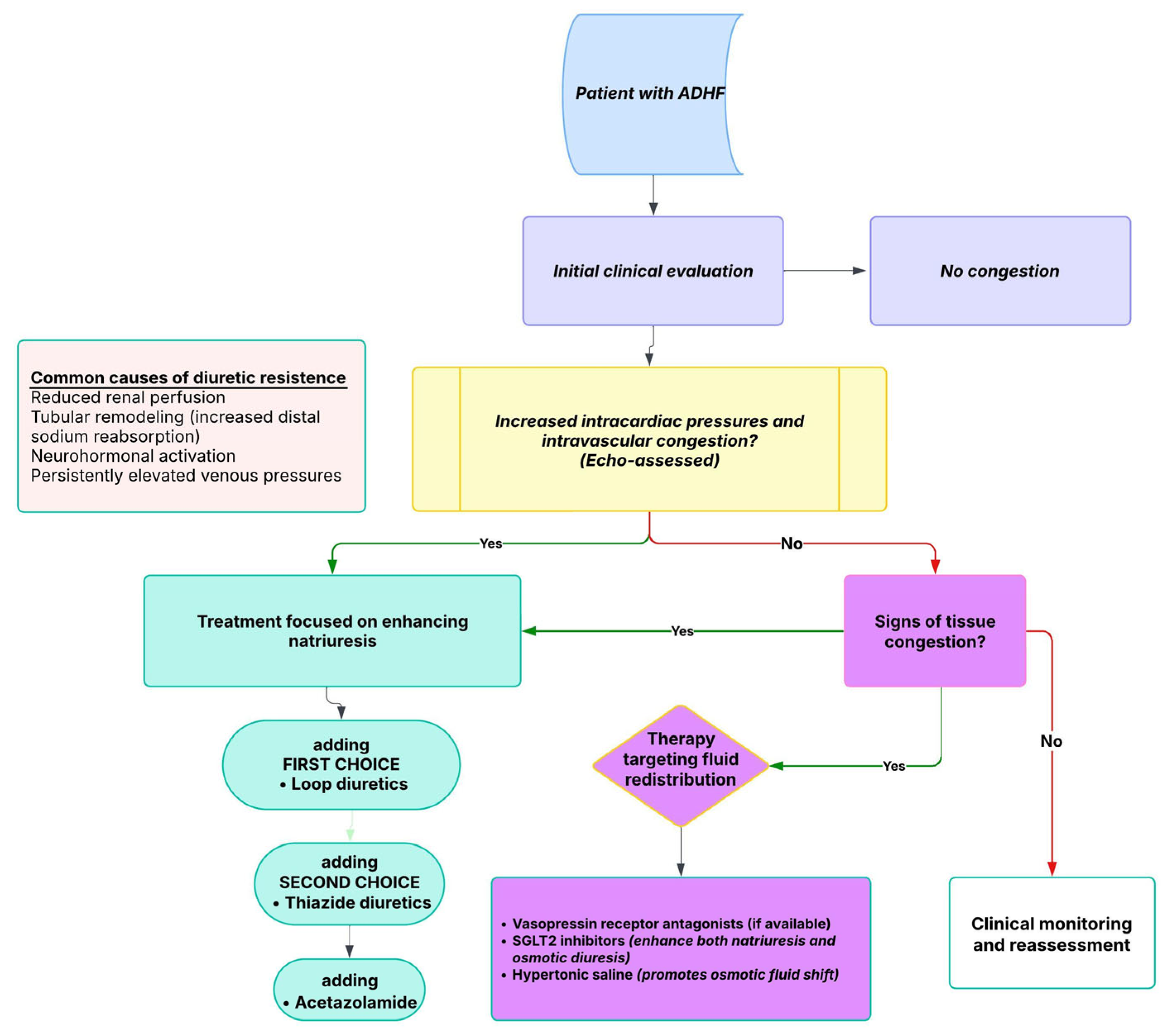
8. Intravascular vs. Tissue Congestion: Implications for Diuretic Strategy and Resistance
Diuretic Resistance in HF
9. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, J. A Hystorical Review of Heart Failure. Scott. Med. J. 1993, 38, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Schafer, A.I. (Eds.) Quick Reference (QR) Video Access. In Goldman’s Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–7. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Seronde, M.-F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766. [Google Scholar] [CrossRef]
- Boorsma, E.M.; ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Soloveva, A.; Fudim, M. A Contemporary Picture of Congestion in Heart Failure: From Dropsy Impression to Multifaceted Reality. J. Cardiovasc. Transl. Res. 2020, 13, 507–508. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Mullens, W. Current Approach to Decongestive Therapy in Acute Heart Failure. Curr. Heart Fail. Rep. 2015, 12, 367–378. [Google Scholar] [CrossRef]
- Zucker, I.H.; Schultz, H.D.; Li, Y.-F.; Wang, Y.; Wang, W.; Patel, K.P. The origin of sympathetic outflow in heart failure: The roles of angiotensin II and nitric oxide. Prog. Biophys. Mol. Biol. 2004, 84, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S.; Warner, D.S.; Warner, M.A. Venous Function and Central Venous Pressure. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef]
- Pérez Calvo, J.I.; Rubio Gracia, J.; Laorden, C.J.; Morales Rull, J.L. Revista Clínica Española Residual congestion and clinical intuition in decompensated heart failure. Rev. Clínica Española (Engl. Ed.) 2019, 219, 327–331. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Demissei, B.G.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Thibodeau, J.T.; Drazner, M.H. The Role of the Clinical Examination in Patients With Heart Failure. JACC Heart Fail. 2018, 6, 543–551. [Google Scholar] [CrossRef] [PubMed]
- McGee, S. Congestive Heart Failure. In Evidence-Based Physical Diagnosis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–418.e3. [Google Scholar] [CrossRef]
- Albert, N.; Trochelman, K.; Li, J.; Lin, S. Signs and Symptoms of Heart Failure: Are You Asking the Right Questions? Am. J. Crit. Care 2010, 19, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Tajik, A.J. The Valsalva Maneuver and Response Revisited. Mayo Clin. Proc. 1986, 61, 211–217. [Google Scholar] [CrossRef]
- Zema, M.J.; Restivo, B.; Sos, T.; Sniderman, K.W.; Kline, S. Left ventricular dysfunction—Bedside Valsalva manoeuvre. Heart 1980, 44, 560–569. [Google Scholar] [CrossRef]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart FailureThe EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Cooper, L.B.; Lippmann, S.J.; DiBello, J.R.; Gorsh, B.; Curtis, L.H.; Sikirica, V.; Hernandez, A.F.; Sprecher, D.L.; Laskey, W.K.; Saini, R.; et al. The Burden of Congestion in Patients Hospitalized With Acute Decompensated Heart Failure. Am. J. Cardiol. 2019, 124, 545–553. [Google Scholar] [CrossRef]
- Pandhi, P.; ter Maaten, J.M.; Emmens, J.E.; Struck, J.; Bergmann, A.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur. J. Heart Fail. 2020, 22, 683–691. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure. Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Yasue, H.; Okumura, K.; Ogawa, H.; Jougasaki, M.; Mukoyama, M.; Nakao, K.; Imura, H. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993, 87, 464–469. [Google Scholar] [CrossRef]
- Francis, G.S.; Felker, G.M.; Tang, W.W. A Test in Context. J. Am. Coll. Cardiol. 2016, 67, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, M.; Ruocco, G.; Ibrahim, A.; Lucani, B.; Franci, B.; Nuti, R.; Palazzuoli, A. Different trajectories and significance of B-type natriuretic peptide, congestion and acute kidney injury in patients with heart failure. Intern. Emerg. Med. 2017, 12, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Miñana, G.; Santas, E.; Núñez, E.; de la Espriella, R.; Valero, E.; Bodí, V.; Chorro, F.J.; Fernández-Cisnal, A.; D’Ascoli, G.; et al. CA125 outperforms NT-proBNP in acute heart failure with severe tricuspid regurgitation. Int. J. Cardiol. 2020, 308, 54–59. [Google Scholar] [CrossRef]
- Stienen, S.; Salah, K.; Moons, A.H.; Bakx, A.L.; van Pol, P.; Kortz, R.A.M.; Ferreira, J.P.; Marques, I.; Schroeder-Tanka, J.M.; Keijer, J.T.; et al. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure. Circulation 2018, 137, 1671–1683. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef]
- Bruins, S.; Fokkema, M.R.; Römer, J.W.P.; DeJongste, M.J.L.; van der Dijs, F.P.L.; van den Ouweland, J.M.W.; Muskiet, F.A. High Intraindividual Variation of B-Type Natriuretic Peptide (BNP) and Amino-Terminal proBNP in Patients with Stable Chronic Heart Failure. Clin. Chem. 2004, 50, 2052–2058. [Google Scholar] [CrossRef]
- Testani, J.M.; Chen, J.; McCauley, B.D.; Kimmel, S.E.; Shannon, R.P. Potential Effects of Aggressive Decongestion During the Treatment of Decompensated Heart Failure on Renal Function and Survival. Circulation 2010, 122, 265–272. [Google Scholar] [CrossRef]
- Boyle, A.; Sobotka, P.A. Redefining the Therapeutic Objective in Decompensated Heart Failure: Hemoconcentration as a Surrogate for Plasma Refill Rate. J. Card. Fail. 2006, 12, 247–249. [Google Scholar] [CrossRef]
- ter Maaten, J.M.; Valente, M.A.; Damman, K.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’connor, C.M.; Teerlink, J.R.; Ponikowski, P.; Bloomfield, D.M.; et al. Combining Diuretic Response and Hemoconcentration to Predict Rehospitalization After Admission for Acute Heart Failure. Circ. Heart Fail. 2016, 9, e002845. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.; Postmus, D.; Ponikowski, P.; Cleland, J.G.; O’Connor, C.M.; Cotter, G.; Metra, M.; Davison, B.A.; Givertz, M.M.; Mansoor, G.A.; et al. The Predictive Value of Short-Term Changes in Hemoglobin Concentration in Patients Presenting with Acute Decompensated Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 1973–1981. [Google Scholar] [CrossRef]
- Davila, C.; Reyentovich, A.; Katz, S.D. Clinical Correlates of Hemoconcentration During Hospitalization for Acute Decompensated Heart Failure. J. Card. Fail. 2011, 17, 1018–1022. [Google Scholar] [CrossRef]
- Grigore, A.-M.; Grigore, M.; Balahura, A.-M.; Uscoiu, G.; Verde, I.; Nicolae, C.; Bădilă, E.; Ilieșiu, A.-M. The Role of the Estimated Plasma Volume Variation in Assessing Decongestion in Patients with Acute Decompensated Heart Failure. Biomedicines 2025, 13, 88. [Google Scholar] [CrossRef]
- Hudson, S.R.; Chan, D.; Ng, L.L. Change in plasma volume and prognosis in acute decompensated heart failure: An observational cohort study. J. R. Soc. Med. 2016, 109, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Girerd, N.; Duarte, K.; Chouihed, T.; Chikamori, T.; Pitt, B.; Zannad, F.; Rossignol, P. Estimated plasma volume status in heart failure: Clinical implications and future directions. Clin. Res. Cardiol. 2021, 110, 1159–1172. [Google Scholar] [CrossRef]
- Swolinsky, J.S.; Tuvshinbat, E.; Leistner, D.M.; Edelmann, F.; Knebel, F.; Nerger, N.P.; Lemke, C.; Roehle, R.; Haase, M.; Costanzo, M.R.; et al. Discordance between estimated and measured changes in plasma volume among patients with acute heart failure. ESC Heart Fail. 2022, 9, 66–76. [Google Scholar] [CrossRef]
- Sprenger, K.B.G.; Huber, K.; Kratz, W.; Henze, E. Nomograms for the prediction of patient’s plasma volume in plasma exchange therapy from height, weight, and hematocrit. J. Clin. Apher. 1987, 3, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Buffaloe, G.W.; Heineken, F.G. Plasma volume nomograms for use in therapeutic plasma exchange. Transfusion 1983, 23, 355–357. [Google Scholar] [CrossRef]
- Ansari Ramandi, M.M.; Hendriks, P.M.; Voors, A.A.; van den Bosch, A.E.; van Melle, J.P. Bioactive adrenomedullin as a marker of congestion and disease progression in patients with a systemic right ventricle. Int. J. Cardiol. 2024, 408, 132107. [Google Scholar] [CrossRef]
- Koyama, T.; Ochoa-Callejero, L.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Iinuma, N.; Arai, T.; Yoshizawa, T.; Iesato, Y.; Lei, Y.; et al. Vascular Endothelial Adrenomedullin-RAMP2 System Is Essential for Vascular Integrity and Organ Homeostasis. Circulation 2013, 127, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Kremer, D.; Geven, C.; ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Tolppanen, H.; Rivas-Lasarte, M.; Lassus, J.; Sans-Roselló, J.; Hartmann, O.; Lindholm, M.; Arrigo, M.; Tarvasmäki, T.; Köber, L.; Thiele, H.; et al. Adrenomedullin: A marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann. Intensiv. Care 2017, 7, 6. [Google Scholar] [CrossRef]
- Arrigo, M.; Parenica, J.; Ganovska, E.; Pavlusova, M.; Mebazaa, A. Plasma bio-adrenomedullin is a marker of acute heart failure severity in patients with acute coronary syndrome. IJC Heart Vasc. 2019, 22, 174–176. [Google Scholar] [CrossRef]
- Arrigo, M.; Truong, Q.A.; Onat, D.; Szymonifka, J.; Gayat, E.; Tolppanen, H.; Sadoune, M.; Demmer, R.T.; Wong, K.Y.; Launay, J.M.; et al. Soluble CD146 Is a Novel Marker of Systemic Congestion in Heart Failure Patients: An Experimental Mechanistic and Transcardiac Clinical Study. Clin. Chem. 2017, 63, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.L.; Arrigo, M.; Placido, R.; Akiyama, E.; Girerd, N.; Zannad, F.; Manivet, P.; Rossignol, P.; Badoz, M.; Sadoune, M.; et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur. J. Heart Fail. 2018, 20, 738–747. [Google Scholar] [CrossRef]
- Bergers, G.; Reikerstorfer, A.; Braselmann, S.; Graninger, P.; Busslinger, M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994, 13, 1176–1188. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Bayés-Genís, A.; Núñez, J.; Lupón, J. Soluble ST2 for Prognosis and Monitoring in Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 2389–2392. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Bartunek, J.; Delrue, L.; Van Durme, F.; Muller, O.; Casselman, F.; De Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Nonmyocardial Production of ST2 Protein in Human Hypertrophy and Failure Is Related to Diastolic Load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.A.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Gröger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell. Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Pérez-Martínez, M.T.; Asensio-Lopez, M.C.; Sanchez-Más, J.; García-García, M.E.; Martinez, C.M.; Lencina, M.; Jara, R.; Januzzi, J.L.; Lax, A. Pulmonary Production of Soluble ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005488. [Google Scholar] [CrossRef] [PubMed]
- Zilinski, J.L.; Shah, R.V.; Gaggin, H.K.; Gantzer, M.L.; Wang, T.J.; Januzzi, J.L. Measurement of multiple biomarkers in advanced stage heart failure patients treated with pulmonary artery catheter guided therapy. Crit. Care 2012, 16, R135. [Google Scholar] [CrossRef]
- De La Espriella, R.; Bayés-Genis, A.; Revuelta-López, E.; Miñana, G.; Santas, E.; Llàcer, P.; García-Blas, S.; Fernández-Cisnal, A.; Bonanad, C.; Ventura, S.; et al. Soluble ST2 and Diuretic Efficiency in Acute Heart Failure and Concomitant Renal Dysfunction. J. Card. Fail. 2021, 27, 427–434. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Figal, D.A.P.; de Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017, 5, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Zamora, E.; de Antonio, M.; Galán, A.; Vila, J.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Lupón, J. Soluble ST2 Serum Concentration and Renal Function in Heart Failure. J. Card. Fail. 2013, 19, 768–775. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Lupu, S.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Stan, A.; Scurtu, A.-C.; Aniței, D.; Harpa, M.; Brînzaniuc, K.; et al. Natriuretic peptides and soluble ST2 improves echocardiographic diagnosis of elevated left ventricular filling pressures. Sci. Rep. 2024, 14, 22171. [Google Scholar] [CrossRef]
- de la Espriella-Juan, R.; Núñez, E.; Sanchis, J.; Bayés-Genis, A.; Núñez, J. Carbohydrate Antigen-125 in Heart Failure. JACC Heart Fail. 2018, 6, 441–442. [Google Scholar] [CrossRef]
- Kouris, N.T.; Zacharos, I.D.; Kontogianni, D.D.; Goranitou, G.S.; Sifaki, M.D.; Grassos, H.E.; Kalkandi, E.M.; Babalis, D.K. The significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur. J. Heart Fail. 2005, 7, 199–203. [Google Scholar] [CrossRef]
- DurakNalbantic, A.; Resic, N.; Kulic, M.; Pecar, E.; Zvizdic, F.; Dzubur, A.; Dilic, M.; Gojak, R.; Sokolovic, S.; Hodzic, E.; et al. Serum Level of Tumor Marker Carbohydrate Antigen-CA125 in Heart Failure. Med. Arch. 2013, 67, 241–244. [Google Scholar] [CrossRef]
- Núñez, J.; Miñana, G.; Núñez, E.; Chorro, F.J.; Bodí, V.; Sanchis, J. Clinical utility of antigen carbohydrate 125 in heart failure. Heart Fail. Rev. 2014, 19, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Scholler, N.; Urban, N. CA125 in Ovarian Cancer. Biomark. Med. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Lloyd, K.O.; Yin, B.W.T. Synthesis and Secretion of the Ovarian Cancer Antigen CA 125 by the Human Cancer Cell Line NIH:OVCAR-3. Tumor Biol. 2001, 22, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; ter Maaten, J.M.; Miñana, G.; Barallat, J.; Cserkóová, A.; Bodi, V.; Fernández-Cisnal, A.; Núñez, E.; et al. Clinical Role of CA125 in Worsening Heart Failure. JACC Heart Fail. 2020, 8, 386–397. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- Núñez-Marín, G.; de la Espriella, R.; Santas, E.; Lorenzo, M.; Miñana, G.; Núñez, E.; Bodí, V.; González, M.; Górriz, J.L.; Bonanad, C.; et al. CA125 but not NT-proBNP predicts the presence of a congestive intrarenal venous flow in patients with acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Takano, M.; Kita, T.; Kudoh, K.; Sasaki, N.; Kato, M.; Watanabe, A.; Miyamoto, M.; Goto, T.; Furuya, K. Normal serum CA125 half-life and normal serum nadir CA125 level in patients with ovarian cancers. Eur. J. Gynaecol. Oncol. 2012, 33, 269–273. [Google Scholar]
- Stein, J.H.; Neumann, A.; Marcus, R.H. Comparison of Estimates of Right Atrial Pressure by Physical Examination and Echocardiography in Patients With Congestive Heart Failure and Reasons for Discrepancies. Am. J. Cardiol. 1997, 80, 1615–1618. [Google Scholar] [CrossRef]
- Anastasiou, V.; Peteinidou, E.; Moysidis, D.V.; Daios, S.; Gogos, C.; Liatsos, A.C.; Didagelos, M.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; et al. Multiorgan Congestion Assessment by Venous Excess Ultrasound Score in Acute Heart Failure. J. Am. Soc. Echocardiogr. 2024, 37, 923–933. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Beltrami, M.; Girerd, N.; Maw, A.; Ruocco, G.; Platz, E. The assessment, interpretation and implementation of lung ultrasound examinations in Heart Failure: Current evidence and gaps in knowledge. Eur. J. Intern. Med. 2024, 130, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Pellikka, P.A. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 2016, 37, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L. Lung ultrasound: A new tool for the cardiologist. Cardiovasc. Ultrasound 2011, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef]
- Coiro, S.; Porot, G.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Tritto, I.; Huttin, O.; Lemoine, S.; Sadoul, N.; Donal, E.; et al. Prognostic value of pulmonary congestion assessed by lung ultrasound imaging during heart failure hospitalisation: A two-centre cohort study. Sci. Rep. 2016, 6, 39426. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Alvarez-Garcia, J.; Fernández-Martínez, J.; Maestro, A.; López-López, L.; Solé-González, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvià, P.; et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Pang, P.S.; Russell, F.M.; Ehrman, R.; Ferre, R.; Gargani, L.; Levy, P.D.; Noble, V.; Lane, K.A.; Li, X.; Collins, S.P. Lung Ultrasound–Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF): A Randomized Controlled Pilot Trial. JACC Heart Fail. 2021, 9, 638–648. [Google Scholar] [CrossRef]
- Lindner, M.; Thomas, R.; Claggett, B.; Lewis, E.F.; Groarke, J.; Merz, A.A.; Silverman, M.B.; Swamy, V.; Rivero, J.; Hohenstein, C.; et al. Quantification of pleural effusions on thoracic ultrasound in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 513–521. [Google Scholar] [CrossRef]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Packer, M. Abnormalities of diastolic function as a potential cause of exercise intolerance in chronic heart failure. Circulation 1990, 81, III78–III86. [Google Scholar] [PubMed]
- Ilieșiu, A.M.; Hodorogea, A.S.; Balahura, A.-M.; Bădilă, E. Non-Invasive Assessment of Congestion by Cardiovascular and Pulmonary Ultrasound and Biomarkers in Heart Failure. Diagnostics 2022, 12, 962. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Ferre, R.M.; Chioncel, O.; Pang, P.S.; Lang, R.M.; Gheorghiade, M.; Collins, S.P. Acute heart failure: The role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur. J. Heart Fail. 2015, 17, 1223–1227. [Google Scholar] [CrossRef]
- Mullens, W.; Borowski, A.G.; Curtin, R.J.; Thomas, J.D.; Tang, W.H. Tissue Doppler Imaging in the Estimation of Intracardiac Filling Pressure in Decompensated Patients With Advanced Systolic Heart Failure. Circulation 2009, 119, 62–70. [Google Scholar] [CrossRef]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; O’meara, E.; Desai, A.S.; Heitner, J.F.; Li, G.; Fang, J.; et al. Cardiac Structure and Function and Prognosis in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 740–751. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Balaney, B.; Medvedofsky, D.; Mediratta, A.; Singh, A.; Ciszek, B.; Kruse, E.; Shah, A.P.; Addetia, K.; Lang, R.M.; Mor-Avi, V. Invasive Validation of the Echocardiographic Assessment of Left Ventricular Filling Pressures Using the 2016 Diastolic Guidelines: Head-to-Head Comparison with the 2009 Guidelines. J. Am. Soc. Echocardiogr. 2018, 31, 79–88. [Google Scholar] [CrossRef]
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler estimation of left ventricular filling pressure: Results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 961–968. [Google Scholar] [CrossRef]
- Ha, J.-W.; Andersen, O.S.; Smiseth, O.A. Diastolic Stress Test. JACC Cardiovasc. Imaging 2020, 13, 272–282. [Google Scholar] [CrossRef]
- Pastore, M.C.; Mandoli, G.E.; Stefanini, A.; Ghionzoli, N.; Carrucola, C.; De Carli, G.; Lisi, M.; Cavigli, L.; D’Ascenzi, F.; Focardi, M.; et al. Prediction of congestive state in acute and chronic heart failure: The association between NT-proBNP and left atrial strain and its prognostic value. Int. J. Cardiol. 2023, 371, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Alkassas, A.; Alaarag, A. Tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure ratio as a predictor of in-hospital mortality for acute heart failure. BMC Cardiovasc. Disord. 2022, 22, 414. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Kapłon-Cieślicka, A.; Benson, L.; Chioncel, O.; Crespo-Leiro, M.G.; Coats, A.J.; Anker, S.D.; Filippatos, G.; Ruschitzka, F.; Hage, C.; Drożdż, J.; et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction—insights from the ESC-HFA EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2022, 24, 335–350. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Bhatt, A.S.; Gallup, D.; Anstrom, K.J.; Butler, J.; DeVore, A.D.; Felker, G.M.; Fudim, M.; Greene, S.J.; Hernandez, A.F.; et al. Trajectory of Congestion Metrics by Ejection Fraction in Patients With Acute Heart Failure (from the Heart Failure Network). Am. J. Cardiol. 2017, 120, 98–105. [Google Scholar] [CrossRef]
- Nagao, K.; Kato, T.; Yaku, H.; Morimoto, T.; Aida, K.; Maruichi, S.K.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; et al. Differential Prognostic Impact of Clinical Congestion between Preserved versus Reduced Ejection Fraction in Patients Hospitalized for Acute Decompensated Heart Failure: Findings from the Japanese Kyoto Congestive Heart Failure Registry. J. Card. Fail. 2024. [Google Scholar] [CrossRef]
- Chayapinun, V.; Koratala, A.; Assavapokee, T. Seeing beneath the surface: Harnessing point-of-care ultrasound for internal jugular vein evaluation. World J. Cardiol. 2024, 16, 73–79. [Google Scholar] [CrossRef]
- Longino, A.; Martin, K.; Leyba, K.; Siegel, G.; Gill, E.; Douglas, I.S.; Burke, J. Correlation between the VExUS score and right atrial pressure: A pilot prospective observational study. Crit. Care 2023, 27, 205. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Grigore, M.; Grigore, A.-M.; Ilieșiu, A.-M. Portal Vein Pulsatility: A Valuable Approach for Monitoring Venous Congestion and Prognostic Evaluation in Acute Decompensated Heart Failure. Diagnostics 2024, 14, 2029. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Gill, E.A.; Douglas, I.J.; Longino, A.A. Evaluation of a modified venous excess ultrasound (VExUS) protocol for estimation of venous congestion: A cohort study. Ultrasound J. 2025, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Assavapokee, T.; Thadanipon, K. Examination of the Neck Veins. N. Engl. J. Med. 2020, 383, e132. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Platz, E.; Dauw, J.; ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.; McMurray, J.J.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef]
- Pellicori, P.; Kallvikbacka-Bennett, A.; Dierckx, R.; Zhang, J.; Putzu, P.; Cuthbert, J.; Boyalla, V.; Shoaib, A.; Clark, A.L.; Cleland, J.G.F. Prognostic significance of ultrasound-assessed jugular vein distensibility in heart failure. Heart 2015, 101, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Schnatz, R.G.; Romeo, J.D.; Pacella, J.J. Bedside Ultrasound Assessment of Jugular Venous Compliance as a Potential Point-of-Care Method to Predict Acute Decompensated Heart Failure 30-Day Readmission. J. Am. Heart Assoc. 2018, 7, e008184. [Google Scholar] [CrossRef]
- Wang, L.; Harrison, J.; Dranow, E.; Aliyev, N.; Khor, L. Accuracy of Ultrasound Jugular Venous Pressure Height in Predicting Central Venous Congestion. Ann. Intern. Med. 2022, 175, 344–351. [Google Scholar] [CrossRef]
- Andrei, S.; Bahr, P.-A.; Alissant, M.; Saccu, M.; Nguyen, M.; Popescu, B.A.; Bouhemad, B.; Guinot, P.-G. Pulsatile Femoral Vein Doppler Pattern is a Parameter of Venous Congestion in ICU Patients. J. Cardiothorac. Vasc. Anesthesia 2024, 38, 1361–1368. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Rola, P.; Denault, A.; Vikneswaran, G.; Spiegel, R. Femoral vein pulsatility: A simple tool for venous congestion assessment. Ultrasound J. 2023, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Kobayashi, M.; Tsuchiya, S.; Ohno, N.; Dai, M.; Matsumoto, M.; Ogai, K.; Sato, A.; Sawazaki, T.; Miyati, T.; et al. Objective assessment of leg edema using ultrasonography with a gel pad. PLoS ONE 2017, 12, e0182042. [Google Scholar] [CrossRef] [PubMed]
- Getnet, W.; Kebede, T.; Atinafu, A.; Sultan, A. The Value of Ultrasound in Characterizing and Determining the Etiology of Ascites. Ethiop. J. Health Sci. 1970, 29, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, J.; Lala, A.; Rivas-Lasarte, M.; De Rueda, C.; Brunjes, D.; Lozano-Jimenez, S.; Garcia-Sebastian, C.; Mitter, S.; Remior, P.; Bravo, M.J.-B.; et al. Remote Dielectric Sensing Before and After Discharge in Patients With ADHF. JACC Heart Fail. 2024, 12, 695–706. [Google Scholar] [CrossRef] [PubMed]
- van der Lande, A.C.M.H.; Feijen, M.; Egorova, A.D.; Beles, M.; van Bockstal, K.; Phagu, A.A.S.; Schalij, M.J.; Heggermont, W.A.; Beeres, S.L. CIED-based remote monitoring in heart failure using the HeartLogic™ algorithm: Which patients benefit most? Int. J. Cardiol. 2024, 415, 132421. [Google Scholar] [CrossRef] [PubMed]
- Radhoe, S.P.; Brugts, J.J. CardioMEMS™: A Tool for Remote Hemodynamic Monitoring of Chronic Heart Failure Patients. Futur. Cardiol. 2022, 18, 173–183. [Google Scholar] [CrossRef]
- de la Espriella, R.; Santas, E.; Reiriz, I.Z.; Górriz, J.L.; Marcos, M.C.; Núñez, J. Quantification and Treatment of Congestion in Heart Failure: A Clinical and Pathophysiological Overview. Nefrologia 2022, 42, 145–162. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Swoboda, K.; Gajewski, P.; Jankowska, E.A.; Banasiak, W.; et al. Distinct clinical phenotypes of congestion in acute heart failure: Characteristics, treatment response, and outcomes. ESC Heart Fail. 2020, 7, 3830–3840. [Google Scholar] [CrossRef]
- Veeraveedu, P.T.; Watanabe, K.; Ma, M.; Palaniyandi, S.S.; Yamaguchi, K.; Kodama, M.; Aizawa, Y. Effects of V2-receptor antagonist tolvaptan and the loop diuretic furosemide in rats with heart failure. Biochem. Pharmacol. 2008, 75, 1322–1330. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Smith, W.B.; Boerrigter, G.; Ouyang, J.; Zimmer, C.A.; Orlandi, C.; Burnett, J.J.C. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am. J. Physiol. Physiol. 2006, 290, F273–F278. [Google Scholar] [CrossRef]
- Udelson, J.E.; Bilsker, M.; Hauptman, P.J.; Sequeira, R.; Thomas, I.; O’brien, T.; Zimmer, C.; Orlandi, C.; Konstam, M.A. A Multicenter, Randomized, Double-blind, Placebo-controlled Study of Tolvaptan Monotherapy Compared to Furosemide and the Combination of Tolvaptan and Furosemide in Patients With Heart Failure and Systolic Dysfunction. J. Card. Fail. 2011, 17, 973–981. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Paterna, S.; Di Gaudio, F.; La Rocca, V.; Balistreri, F.; Greco, M.; Torres, D.; Lupo, U.; Rizzo, G.; di Pasquale, P.; Indelicato, S.; et al. Hypertonic Saline in Conjunction with High-Dose Furosemide Improves Dose–Response Curves in Worsening Refractory Congestive Heart Failure. Adv. Ther. 2015, 32, 971–982. [Google Scholar] [CrossRef]
- Gandhi, S.; Mosleh, W.; Myers, R.B.H. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, L.; Niu, H.; Ma, X.; Yang, J.; Yuan, C.; Mu, G.; Zhang, J. Impact of Compound Hypertonic Saline Solution on Decompensated Heart Failure. Int. Heart J. 2017, 58, 601–607. [Google Scholar] [CrossRef]
- Thibodeau, J.T.; Turer, A.T.; Gualano, S.K.; Ayers, C.R.; Velez-Martinez, M.; Mishkin, J.D.; Patel, P.C.; Mammen, P.P.; Markham, D.W.; Levine, B.D.; et al. Characterization of a Novel Symptom of Advanced Heart Failure: Bendopnea. JACC Heart Fail. 2014, 2, 24–31. [Google Scholar] [CrossRef]
| Regional Distribution | Pulmonary | Systemic | |
|---|---|---|---|
| Compartment | |||
| Intravascular | Symptoms Dyspnea Orthopnea Paroxysmal nocturnal dyspnea Bendopnea Dry cough | Symptoms Abdominal symptoms Loss of appetite Abdominal discomfort | |
| Signs S3 and/or S4 (gallop rhythm) | Signs Jugulary vein distension (elevated JVP) | ||
| Extravascular (Tissular) and Third-space | Signs Inspiratory crackles at lung bases Pleural effusion (bilateral or right-sided) | Signs Ankle and/or sacral edema Hepatomegaly Ascites (sometimes) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore, M.; Nicolae, C.; Grigore, A.-M.; Balahura, A.-M.; Păun, N.; Uscoiu, G.; Verde, I.; Ilieșiu, A.-M. Contemporary Perspectives on Congestion in Heart Failure: Bridging Classic Signs with Evolving Diagnostic and Therapeutic Strategies. Diagnostics 2025, 15, 1083. https://doi.org/10.3390/diagnostics15091083
Grigore M, Nicolae C, Grigore A-M, Balahura A-M, Păun N, Uscoiu G, Verde I, Ilieșiu A-M. Contemporary Perspectives on Congestion in Heart Failure: Bridging Classic Signs with Evolving Diagnostic and Therapeutic Strategies. Diagnostics. 2025; 15(9):1083. https://doi.org/10.3390/diagnostics15091083
Chicago/Turabian StyleGrigore, Mihai, Camelia Nicolae, Andreea-Maria Grigore, Ana-Maria Balahura, Nicolae Păun, Gabriela Uscoiu, Ioana Verde, and Adriana-Mihaela Ilieșiu. 2025. "Contemporary Perspectives on Congestion in Heart Failure: Bridging Classic Signs with Evolving Diagnostic and Therapeutic Strategies" Diagnostics 15, no. 9: 1083. https://doi.org/10.3390/diagnostics15091083
APA StyleGrigore, M., Nicolae, C., Grigore, A.-M., Balahura, A.-M., Păun, N., Uscoiu, G., Verde, I., & Ilieșiu, A.-M. (2025). Contemporary Perspectives on Congestion in Heart Failure: Bridging Classic Signs with Evolving Diagnostic and Therapeutic Strategies. Diagnostics, 15(9), 1083. https://doi.org/10.3390/diagnostics15091083







