Hallmarks of Bacterial Vaginosis
Abstract
1. Introduction
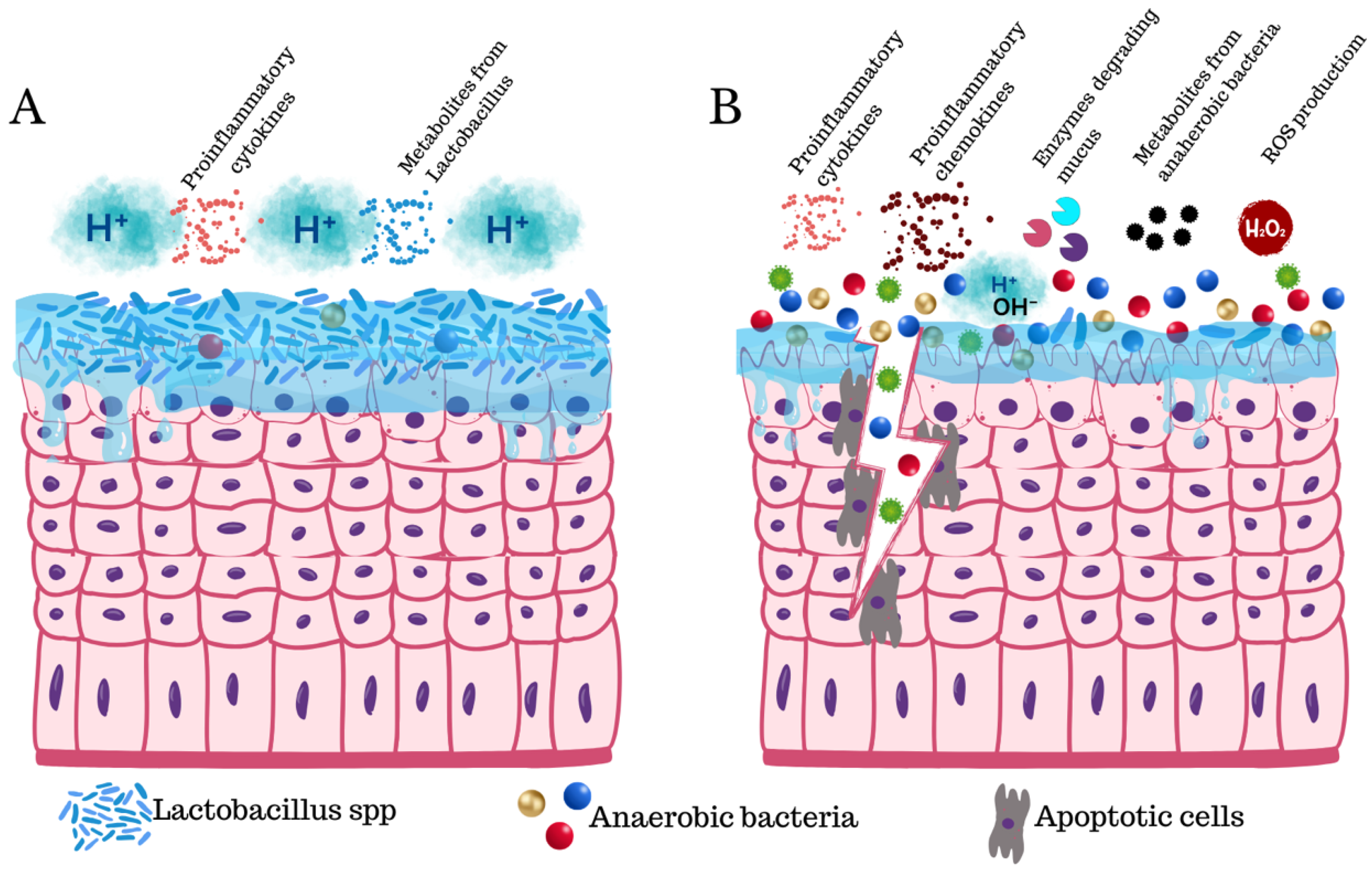
2. Dysbiosis and pH Basification
3. Inflammation
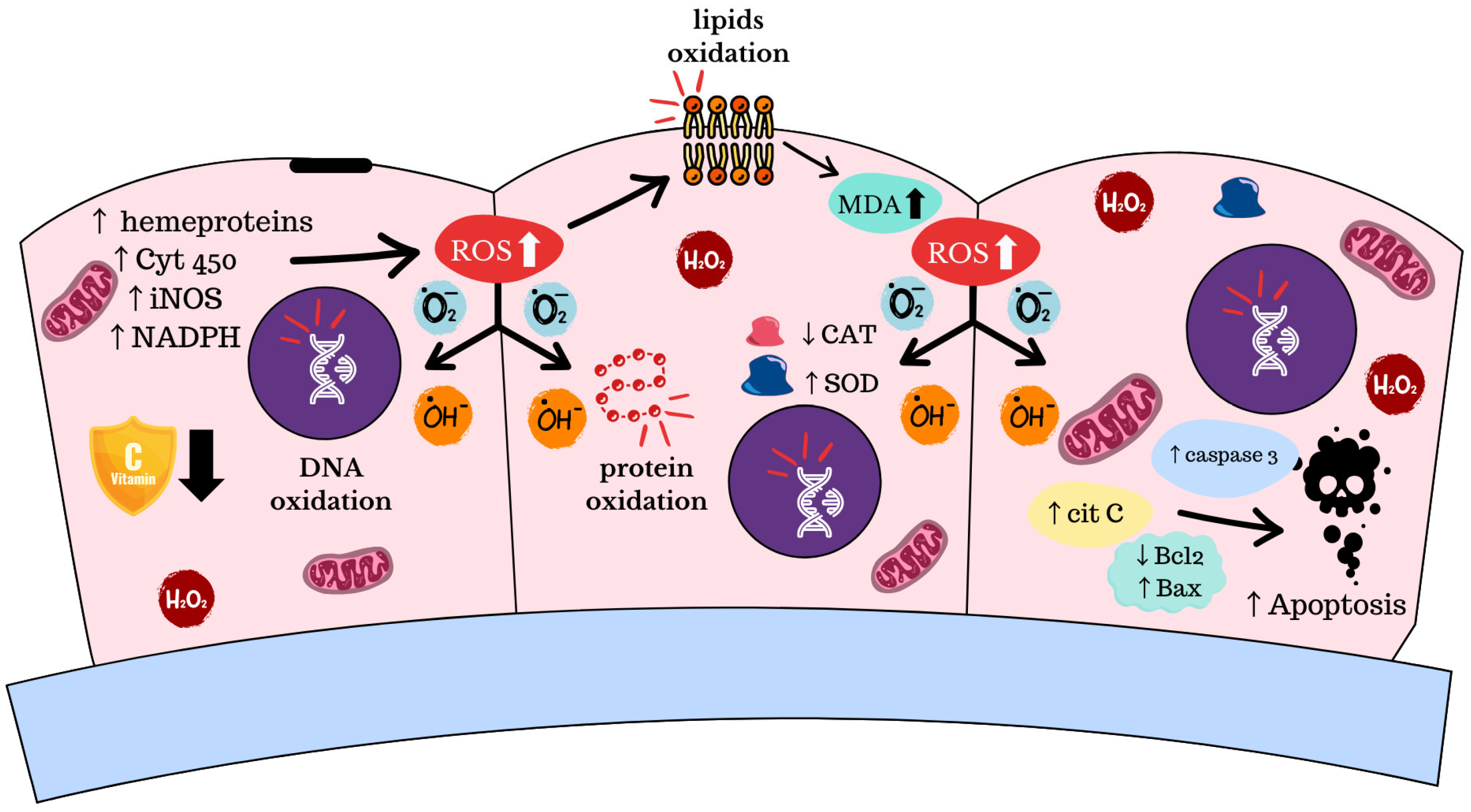
4. Oxidative Stress and Apoptosis
5. Genomic Instability and Pathway Activation
6. Metabolic Reconfiguration
7. Mucus Disruption
8. Epithelial Damage
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BV | Bacterial vaginosis |
| pH | Potential of hydrogen |
| STI | Sexually Transmitted Infections |
| FDA | Food and Drug Administration |
| qPCR | Quantitative Polymerase Chain Reaction |
| FISH | Fluorescent In Situ Hybridization |
| rRNA | Ribosomal Ribonucleic Acid |
| HPV | Human Papilloma Virus |
| ROS | Reactive Oxygen Species |
| WNT | Wingless Type 1 |
| IgA | Immunoglobulin A |
| PRR | Pattern Recognition Receptor |
| IL | Interleukin |
| MIP | Macrophage Inflammatory Protein |
| CCL-5 | Chemokine C–C motif ligand 5 |
| SLPI | Secretory Leukocyte Protease Inhibitor |
| TNF-α | Tumor necrosis factor alfa |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| NF-kB | Nuclear Factor-kB |
| NLRP3 | NOD-like Receptor family Pyrin domain-containing 3 |
| NOS | Reactive Nitrogen Species |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| iNOS | Inducible Nitric Oxide Synthesis |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| BCL-2 | B-Cell CLL/Lymphoma 2 |
| BAX | BCL2 Associated X |
| p53 | Protein 53 |
| pRB1 | Protein Retinoblastoma 1 |
| hTERT | Human Telomerase Reverse Transcriptase |
| FadA | Fusobacterium adhesin A |
| DnaK | Chaperone Protein DnaK |
| PARP1 | Poly (ADP-Ribose) Polymerase 1 |
| NAD | Nicotinamide Adenine Dinucleotide |
| MUC | Mucin |
References
- O’Hanlon, D.E.; Gajer, P.; Brotman, R.M.; Ravel, J. Asymptomatic Bacterial Vaginosis Is Associated With Depletion of Mature Superficial Cells Shed From the Vaginal Epithelium. Front. Cell. Infect. Microbiol. 2020, 10, 106. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. NPJ Biofilms Microb. 2021, 7, 88. [Google Scholar] [CrossRef]
- Kairys, N.; Carlson, K.; Garg, M. Bacterial Vaginosis; StatPearls: Treasure Island, FL, USA, 2004. [Google Scholar]
- Armstrong, E.; Kaul, R. Beyond bacterial vaginosis: Vaginal lactobacilli and HIV risk. Microbiome 2021, 9, 239. [Google Scholar] [CrossRef]
- Gillet, E.; Meys, J.F.; Verstraelen, H.; Bosire, C.; De Sutter, P.; Temmerman, M.; Broeck, D.V. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: A meta-analysis. BMC Infect. Dis. 2011, 11, 10. [Google Scholar] [CrossRef]
- Morris, M.; Nicoll, A.; Simms, I.; Wilson, J.; Catchpole, M. Bacterial vaginosis: A public health review. BJOG 2001, 108, 439–450. [Google Scholar]
- Sobel, J.D.; Vempati, Y.S. Bacterial Vaginosis and Vulvovaginal Candidiasis Pathophysiologic Interrelationship. Microorganisms 2024, 12, 108. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; De Seta, F.; Verstraelen, H.; Ventolini, G.; Lonnee-Hoffmann, R.; Vieira-Baptista, P. The Vaginal Microbiome: II. Vaginal Dysbiotic Conditions. J. Low. Genit. Tract. Dis. 2022, 26, 79–84. [Google Scholar] [CrossRef]
- Mojgantansaz Soheila, A.; Hamed, H.; Fazele Heydarian, M.; Homa, H. Vaginitis: Etiology and Role of Oxidative Stress, Inflammation and Antioxidants Therapy. Reprod. Med. Int. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Deese, J.; Pradhan, S.; Goetz, H.; Morrison, C. Contraceptive use and the risk of sexually transmitted infection: Systematic review and current perspectives. Open Access J. Contracept. 2018, 9, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Parvaiz, F.; Manzoor, S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb. Pathog. 2019, 127, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef]
- Jain, J.P.; Bristow, C.C.; Pines, H.A.; Harvey-Vera, A.; Rangel, G.; Staines, H.; Patterson, T.L.; Strathdee, S.A. Factors in the HIV risk environment associated with bacterial vaginosis among HIV-negative female sex workers who inject drugs in the Mexico-United States border region. BMC Public Health 2018, 18, 1032. [Google Scholar] [CrossRef]
- Eastment, M.C.; McClelland, R.S. Vaginal microbiota and susceptibility to HIV. AIDS 2018, 32, 687–698. [Google Scholar] [CrossRef]
- Canto-de Cetina, T.E.; Polanco-Reyes, L.E.; Fernández-González, V.; Cupul y Dzul, G. [Prevalence of bacterial vaginosis in a group of women at a family planning clinic]. Gac. Med. Mex. 2002, 138, 25–30. [Google Scholar]
- Pramanick, R.; Nathani, N.; Warke, H.; Mayadeo, N.; Aranha, C. Vaginal Dysbiotic Microbiome in Women With No Symptoms of Genital Infections. Front. Cell. Infect. Microbiol. 2021, 11, 760459. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Kalaivani, S. Prevalence of asymptomatic infections in sexually transmitted diseases attendees diagnosed with bacterial vaginosis, vaginal candidiasis, and trichomoniasis. Indian J. Sex. Transm. Dis. AIDS 2016, 37, 139–142. [Google Scholar]
- Khedkar, R.; Pajai, S. Bacterial Vaginosis: A Comprehensive Narrative on the Etiology, Clinical Features, and Management Approach. Cureus 2022, 14, e31314. [Google Scholar] [CrossRef]
- Cheu, R.K.; Mohammadi, A.; Schifanella, L.; Broedlow, C.; Driscoll, C.B.; Miller, C.J.; Reeves, R.K.; Yudin, M.H.; Hensley-McBain, T.; Kaul, R.; et al. Altered Innate Immunity and Damaged Epithelial Integrity in Vaginal Microbial Dysbiosis. Front. Reprod. Health 2022, 4, 876729. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res. Hum. Retroviruses 2019, 35, 219–228. [Google Scholar] [CrossRef]
- Gaydos, C.A.; Beqaj, S.; Schwebke, J.R.; Lebed, J.; Smith, B.; Davis, T.E.; Fife, K.H.; Nyirjesy, P.; Spurrell, T.; Furgerson, D.; et al. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obs. Gynecol. 2017, 130, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, S.; Moll, W.M.; Swidsinski, A. Bacterial Vaginosis-Vaginal Polymicrobial Biofilms and Dysbiosis. Dtsch. Arztebl. Int. 2023, 120, 347–354. [Google Scholar] [CrossRef]
- O’Brien, G. Bacterial vaginosis. Pediatr. Rev. 2008, 29, 209–211, discussion 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef]
- Parkin, D.M.; Hämmerl, L.; Ferlay, J.; Kantelhardt, E.J. Cancer in Africa 2018: The role of infections. Int. J. Cancer 2020, 146, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, J.D.; Łaniewski, P.; Herbst-Kralovetz, M.M. Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria. Commun. Biol. 2022, 5, 725. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, K.; Shoja, Z.; Jalilvand, S. The interplay between human papillomavirus and vaginal microbiota in cervical cancer development. Virol. J. 2023, 20, 73. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef]
- Plummer, E.L.; Vodstrcil, L.A.; Doyle, M.; Danielewski, J.A.; Murray, G.L.; Fehler, G.; Fairley, C.K.; Bulach, D.M.; Garland, S.M.; Chow, E.P.F.; et al. A Prospective, Open-Label Pilot Study of Concurrent Male Partner Treatment for Bacterial Vaginosis. mBio 2021, 12, e0232321. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 2021, 11, 672429. [Google Scholar] [CrossRef]
- Chandrashekhar, P.; Minooei, F.; Arreguin, W.; Masigol, M.; Steinbach-Rankins, J.M. Perspectives on Existing and Novel Alternative Intravaginal Probiotic Delivery Methods in the Context of Bacterial Vaginosis Infection. AAPS J. 2021, 23, 66. [Google Scholar] [CrossRef]
- Abbe, C.; Mitchell, C.M. Bacterial vaginosis: A review of approaches to treatment and prevention. Front. Reprod. Health 2023, 5, 1100029. [Google Scholar] [CrossRef]
- Patterson, J.L.; Girerd, P.H.; Karjane, N.W.; Jefferson, K.K. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am. J. Obs. Gynecol. 2007, 197, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J. Infect. Dis. 2016, 214 (Suppl. 1), S14–S20. [Google Scholar] [CrossRef]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2015, 6, 1528. [Google Scholar] [CrossRef] [PubMed]
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Nardis, C.; Mosca, L.; Mastromarino, P. Vaginal microbiota and viral sexually transmitted diseases. Ann. Ig. 2013, 25, 443–456. [Google Scholar]
- Mondal, A.S.; Sharma, R.; Trivedi, N. Bacterial vaginosis: A state of microbial dysbiosis. Med. Microecol. 2023, 16, 100082. [Google Scholar] [CrossRef]
- Villa, P.; Cipolla, C.; D’Ippolito, S.; Amar, I.D.; Shachor, M.; Ingravalle, F.; Scaldaferri, F.; Puca, P.; Di Simone, N.; Scambia, G. The interplay between immune system and microbiota in gynecological diseases: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5676–5690. [Google Scholar]
- Zhang, Y.; He, Z. Inflammatory mediators in bacterial vaginosis: The role of cytokines. APMIS 2024, 132, 245–255. [Google Scholar] [CrossRef]
- Aranha, C.; Goriwale, M.; Begum, S.; Gawade, S.; Bhor, V.; Patil, A.D.; Munne, K.; Bansal, V.; Tandon, D. Evaluation of cytokine profile in cervicovaginal lavage specimens of women having asymptomatic reproductive tract infections. J. Obs. Gynaecol. 2022, 42, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- McKeon, M.G.; Gallant, J.N.; Kim, Y.J.; Das, S.R. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers 2022, 14, 3120. [Google Scholar] [CrossRef]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. mBio 2013, 4, e00460-13. [Google Scholar] [CrossRef]
- Filler, S.G.; Pfunder, A.S.; Spellberg, B.J.; Spellberg, J.P.; Edwards, J.E., Jr. Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect. Immun. 1996, 64, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Shaio, M.F.; Lin, P.R.; Liu, J.Y.; Yang, K.D. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect. Immun. 1995, 63, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Wennerholm, U.B.; Holm, B.; Mattsby-Baltzer, I.; Nielsen, T.; Platz-Christensen, J.J.; Sundell, G.; Hagberg, H. Interleukin-1alpha, interleukin-6 and interleukin-8 in cervico/vaginal secretion for screening of preterm birth in twin gestation. Acta Obs. Gynecol. Scand. 1998, 77, 508–514. [Google Scholar]
- Akbari, E.; Milani, A.; Seyedinkhorasani, M.; Bolhassani, A. HPV co-infections with other pathogens in cancer development: A comprehensive review. J. Med. Virol. 2023, 95, e29236. [Google Scholar] [CrossRef]
- Shvartsman, E.; Hill, J.E.; Sandstrom, P.; MacDonald, K.S. Gardnerella Revisited: Species Heterogeneity, Virulence Factors, Mucosal Immune Responses, and Contributions to Bacterial Vaginosis. Infect. Immun. 2023, 91, e0039022. [Google Scholar] [CrossRef]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef]
- van Teijlingen, N.H.; Helgers, L.C.; Zijlstra-Willems, E.M.; van Hamme, J.L.; Ribeiro, C.M.S.; Strijbis, K.; Geijtenbeek, T.B.H. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J. Reprod. Immunol. 2020, 138, 103085. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Yin, T.; Chen, T. Gardnerella vaginalis induces NLRP3 inflammasome-mediated pyroptosis in macrophages and THP-1 monocytes. Exp. Ther. Med. 2021, 22, 1174. [Google Scholar] [CrossRef] [PubMed]
- Vick, E.J.; Park, H.S.; Huff, K.A.; Brooks, K.M.; Farone, A.L.; Farone, M.B. Gardnerella vaginalis triggers NLRP3 inflammasome recruitment in THP-1 monocytes. J. Reprod. Immunol. 2014, 106, 67–75. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T. Ascorbic Acid in human seminal plasma: Determination and its relationship to sperm quality. J. Clin. Biochem. Nutr. 2009, 45, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Ma, X.; Deng, J.; Cui, X.; Chen, Q.; Wang, W. Berberine exhibits antioxidative effects and reduces apoptosis of the vaginal epithelium in bacterial vaginosis. Exp. Ther. Med. 2019, 18, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Cindrova-Davies, T.; Johns, J.; Dunster, C.; Hempstock, J.; Kelly, F.J.; Burton, G.J. Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J. Clin. Endocrinol. Metab. 2004, 89, 1452–1458. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhang, H.; Xie, B. Analysis of the Oxidative Stress Status in Nonspecific Vaginitis and Its Role in Vaginal Epithelial Cells Apoptosis. Biomed. Res. Int. 2015, 2015, 795656. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Bogavac, M.; Lakic, N.; Simin, N.; Nikolic, A.; Sudji, J.; Bozin, B. Bacterial vaginosis and biomarkers of oxidative stress in amniotic fluid. J. Matern. Fetal Neonatal Med. 2012, 25, 1050–1054. [Google Scholar] [CrossRef]
- Roselletti, E.; Sabbatini, S.; Perito, S.; Mencacci, A.; Vecchiarelli, A.; Monari, C. Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci. Rep. 2020, 10, 1978. [Google Scholar] [CrossRef]
- Sanches, J.M.; Giraldo, P.C.; Amaral, R.; Eberlin, M.N.; Marques, L.A.; Migliorini, I.; Nakahira, M.; Bieleveld, M.J.M.; Discacciati, M.G. Vaginal lipidomics of women with vulvovaginal candidiasis and cytolytic vaginosis: A non-targeted LC-MS pilot study. PLoS ONE 2018, 13, e0202401. [Google Scholar] [CrossRef]
- Petersen, E.E.; Magnani, P. Efficacy and safety of vitamin C vaginal tablets in the treatment of non-specific vaginitis. A randomised, double blind, placebo-controlled study. Eur. J. Obs. Gynecol. Reprod. Biol. 2004, 117, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Darmon, E.; Leach, D.R. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 2014, 78, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Guidi, R.; Frisan, T. Do bacterial genotoxins contribute to chronic inflammation, genomic instability and tumor progression? FEBS J. 2011, 278, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Wu, Y.; Zhang, S.; Li, S.; Li, S.; Su, Y.; Zhang, L.; Li, Q.; Zou, H.; Zhang, X.; et al. Escherichia coli and HPV16 coinfection may contribute to the development of cervical cancer. Virulence 2024, 15, 2319962. [Google Scholar] [CrossRef]
- Benedetti, F.; Silvestri, G.; Saadat, S.; Denaro, F.; Latinovic, O.S.; Davis, H.; Williams, S.; Bryant, J.; Ippodrino, R.; Rathinam, C.V.; et al. Mycoplasma DnaK increases DNA copy number variants in vivo. Proc. Natl. Acad. Sci. USA 2023, 120, e2219897120. [Google Scholar] [CrossRef]
- Wolrath, H.; Forsum, U.; Larsson, P.G.; Borén, H. Analysis of bacterial vaginosis-related amines in vaginal fluid by gas chromatography and mass spectrometry. J. Clin. Microbiol. 2001, 39, 4026–4031. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Chen, R.; Li, M.; Zeng, Z.; Zhang, L.; Liao, Q.-P. The interplay between microbiota, metabolites, immunity during BV. Med. Microecol. 2022, 11, 100049. [Google Scholar] [CrossRef]
- Gill, B.; Schwecht, I.; Rahman, N.; Dhawan, T.; Verschoor, C.; Nazli, A.; Kaushic, C. Metabolic signature for a dysbiotic microbiome in the female genital tract: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2023, 90, e13781. [Google Scholar] [CrossRef]
- Witkin, S.S.; Ledger, W.J. Complexities of the uniquely human vagina. Sci. Transl. Med. 2012, 4, 132fs11. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L. Diagnostic microbiology of bacterial vaginosis. Am. J. Obs. Gynecol. 1993, 169 Pt 2, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Berard, A.R.; Brubaker, D.K.; Birse, K.; Lamont, A.; Mackelprang, R.D.; Noël-Romas, L.; Perner, M.; Hou, X.; Irungu, E.; Mugo, N.; et al. Vaginal epithelial dysfunction is mediated by the microbiome, metabolome, and mTOR signaling. Cell Rep. 2023, 42, 112474. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, K.; Czamara, K.; Bialecka, J.; Klimek, M.; Zawilinska, B.; Szostek, S.; Kaczor, A. HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution. Int. J. Mol. Sci. 2020, 21, 2667. [Google Scholar] [CrossRef]
- Dong, M.; Dong, Y.; Bai, J.; Li, H.; Ma, X.; Li, B.; Wang, C.; Li, H.; Qi, W.; Wang, Y.; et al. Interactions between microbiota and cervical epithelial, immune, and mucus barrier. Front. Cell. Infect. Microbiol. 2023, 13, 1124591. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Iwasaki, A. Unique features of antiviral immune system of the vaginal mucosa. Curr. Opin. Immunol. 2012, 24, 411–416. [Google Scholar] [CrossRef]
- Vagios, S.; Mitchell, C.M. Mutual Preservation: A Review of Interactions Between Cervicovaginal Mucus and Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 676114. [Google Scholar] [CrossRef]
- Yeaman, G.R.; Collins, J.E.; Fanger, M.W.; Wira, C.R.; Lydyard, P.M. CD8+ T cells in human uterine endometrial lymphoid aggregates: Evidence for accumulation of cells by trafficking. Immunology. 2001, 102, 434–440. [Google Scholar] [CrossRef]
- Lewis, W.G.; Robinson, L.S.; Gilbert, N.M.; Perry, J.C.; Lewis, A.L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 2013, 288, 12067–12079. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Choudhury, B.; Robinson, L.S.; Morrill, S.R.; Bouchibiti, Y.; Chilin-Fuentes, D.; Rosenthal, S.B.; Fisch, K.M.; Peipert, J.F.; Lebrilla, C.B.; et al. Resident microbes shape the vaginal epithelial glycan landscape. Sci. Transl. Med. 2023, 15, eabp9599. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Sun, S.; Zhou, J.; Liu, M. Virulence factors impair epithelial junctions during bacterial infection. J. Clin. Lab. Anal. 2021, 35, e23627. [Google Scholar] [CrossRef]
- Spinillo, A.; Zara, F.; Gardella, B.; Preti, E.; Gaia, G.; Maserati, R. Cervical intraepithelial neoplasia and cervicovaginal shedding of human immunodeficiency virus. Obs. Gynecol. 2006, 107 Pt 1, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Shishpal, P.; Kasarpalkar, N.; Singh, D.; Bhor, V.M. Characterization of Gardnerella vaginalis membrane vesicles reveals a role in inducing cytotoxicity in vaginal epithelial cells. Anaerobe 2020, 61, 102090. [Google Scholar] [CrossRef]
- Liu, R.; Armstrong, E.; Constable, S.; Buchanan, L.B.; Mohammadi, A.; Galiwango, R.M.; Huibner, S.; Perry, M.C.; Prodger, J.L.; Coburn, B.; et al. Soluble E-cadherin: A marker of genital epithelial disruption. Am. J. Reprod. Immunol. 2023, 89, e13674. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; García-Mejía, X.; Alcorta-Nuñez, F.; Solis-Coronado, O.; Escamilla, M.G.; Vidal-Gutiérrez, O.; Garza-Rodríguez, M.L. Hallmarks of Bacterial Vaginosis. Diagnostics 2025, 15, 1090. https://doi.org/10.3390/diagnostics15091090
Pérez-Ibave DC, Burciaga-Flores CH, García-Mejía X, Alcorta-Nuñez F, Solis-Coronado O, Escamilla MG, Vidal-Gutiérrez O, Garza-Rodríguez ML. Hallmarks of Bacterial Vaginosis. Diagnostics. 2025; 15(9):1090. https://doi.org/10.3390/diagnostics15091090
Chicago/Turabian StylePérez-Ibave, Diana Cristina, Carlos Horacio Burciaga-Flores, Ximena García-Mejía, Fernando Alcorta-Nuñez, Orlando Solis-Coronado, Moisés González Escamilla, Oscar Vidal-Gutiérrez, and María Lourdes Garza-Rodríguez. 2025. "Hallmarks of Bacterial Vaginosis" Diagnostics 15, no. 9: 1090. https://doi.org/10.3390/diagnostics15091090
APA StylePérez-Ibave, D. C., Burciaga-Flores, C. H., García-Mejía, X., Alcorta-Nuñez, F., Solis-Coronado, O., Escamilla, M. G., Vidal-Gutiérrez, O., & Garza-Rodríguez, M. L. (2025). Hallmarks of Bacterial Vaginosis. Diagnostics, 15(9), 1090. https://doi.org/10.3390/diagnostics15091090










