Utility of Rapid Molecular Assays for Detecting Multidrug-Resistant Mycobacterium tuberculosis in Extrapulmonary Samples
Abstract
:1. Introduction
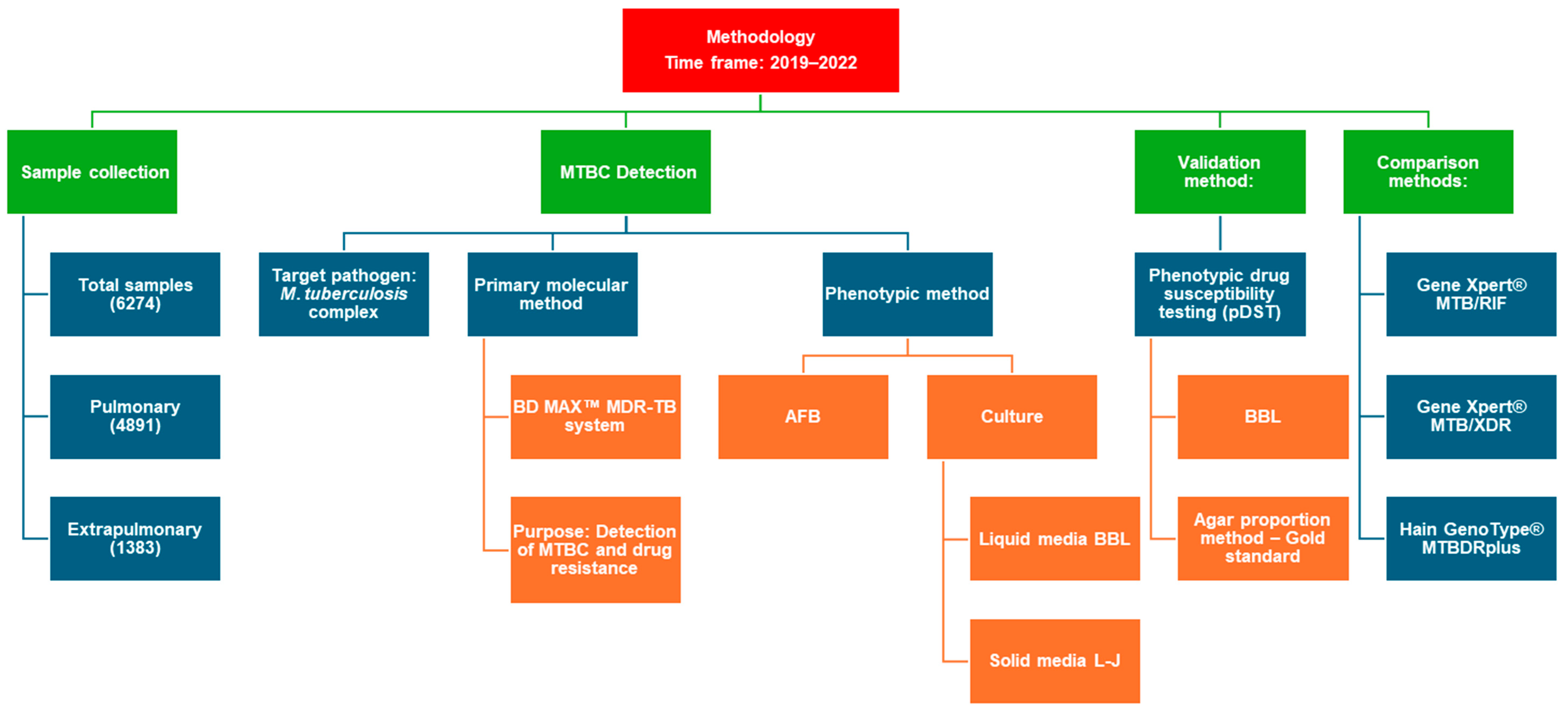
2. Materials and Methods
2.1. Study Samples
2.2. Phenotypic Drug Susceptibility Test
2.2.1. Susceptibility Testing for Anti-TB Agents Using Liquid Media
2.2.2. Susceptibility Testing for Anti-TB Agents Using Solid Media
2.3. Rapid Molecular Detection of M. tuberculosis and Isoniazid and Rifampicin Resistance
2.3.1. BD MAX MDR-TB Assay
2.3.2. GenoType MTBDR Plus
2.3.3. GeneXpert MTB/RIF
2.3.4. GeneXpert MTB/XDR
2.4. Quality Control
2.5. Statistics and Data Analysis
2.6. Limitations
2.7. Ethics Statement
3. Results
3.1. Detection of M. tuberculosis in Pulmonary and Extrapulmonary Samples
3.1.1. Phenotypic Assays
3.1.2. Molecular Assay for the Detection of M. tuberculosis
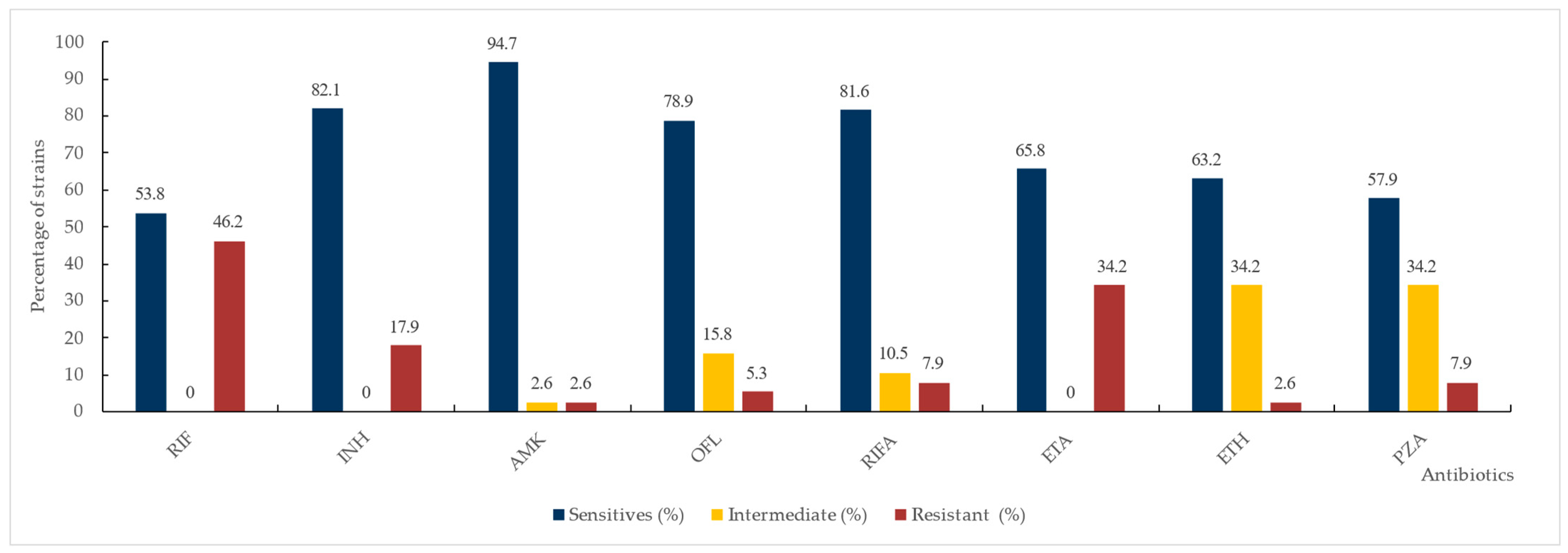
3.2. Susceptibility Testing
3.2.1. Phenotypic Drug Susceptibility Testing
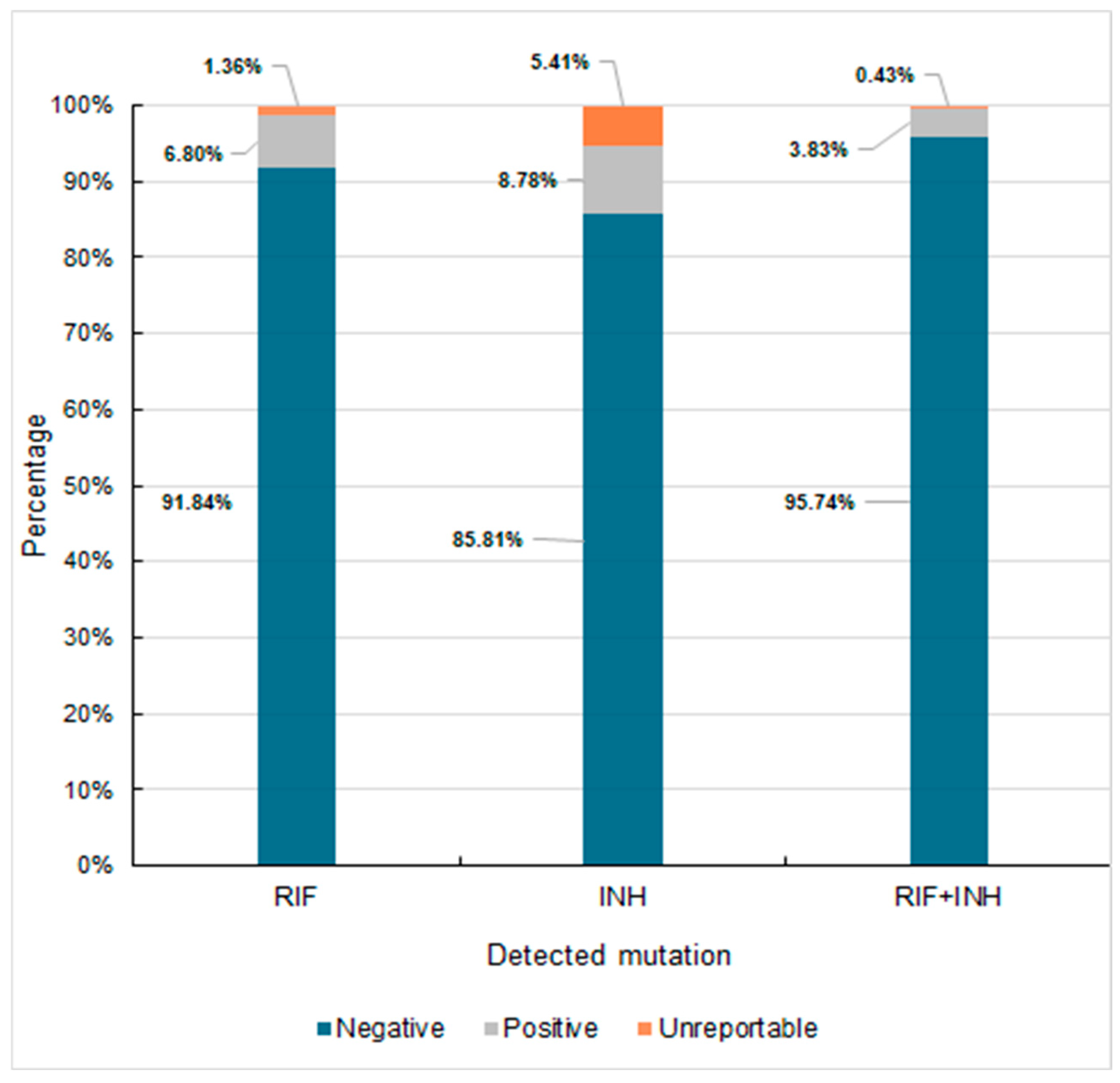
3.2.2. Susceptibility Testing Using Molecular Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFB | Acid-fast bacilli smear microscopy |
| AN | Amikacin |
| DST | drug susceptibility testing |
| EMB | Ethambutol |
| ETH | Ethionamide |
| INH | Isoniazid |
| LJ | Löwenstein–Jensen |
| MDR/RR-TB | Multidrug-resistant or rifampicin-resistant tuberculosis |
| MDR-TB | Multidrug-resistant tuberculosis |
| MIC | Minimum inhibitory concentration |
| MTBC | Mycobacterium tuberculosis complex |
| NAAT | Nucleic acid amplification test |
| OFX | Ofloxacin |
| PZA | Pyrazinamide |
| RIF | Rifampicin |
| RIFA | Rifabutin |
| RR-TB | Rifampicin-resistant tuberculosis |
| STM | Streptomycin |
| TB | Tuberculosis |
Appendix A
References
- WHO Bacterial Priority Pathogens List. 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023.
- CLSI. Reference Method for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes; Approved Standard—Third Edition. CLSI Document M24; Gail, L., Ed.; Woods, MD Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Liebenberg, D.; Gordhan, B.G.; Kana, B.D. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front. Cell Infect. Microbiol. 2022, 23, 943545. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017, 8, e00812-17. [Google Scholar] [CrossRef] [PubMed]
- Beutler, M.; Plesnik, S.; Mihalic, M.; Olbrich, L.; Heinrich, N.; Schumacher, S.; Lindner, M.; Koch, I.; Grasse, W.; Metzger-Boddien, C.; et al. A pre-clinical validation plan to evaluate analytical sensitivities of molecular diagnostics such as BD MAX MDR-TB, Xpert MTB/Rif Ultra and FluoroType MTB. PLoS ONE 2020, 7, e0227215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, D.; Qiu, B.; Martinez, L.; Ji, Y.; Song, H.; Li, Z.; Wang, J. Drug resistance gene mutations and treatment outcomes in MDR-TB: A prospective study in Eastern China. PLoS Negl. Trop. Dis. 2021, 15, e0009068. [Google Scholar] [CrossRef]
- Manual for Selection of Molecular WHO-Recommended Rapid Diagnostic Tests for Detection of Tuberculosis and Drug-Resistant Tuberculosis; World Health Organization: Geneva, Switzerland, 2022.
- WHO. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789241514842 (accessed on 23 October 2024).
- Technical Report on Critical Concentrations for Drug Susceptibility Testing of Isoniazid and the Rifamycins (Rifampicin, Rifabutin and Rifapentine); World Health Organization: Geneva, Switzerland, 2021.
- BD MAX MDR-TB Assay Package Insert; BD Life Sciences: Sparks, MD, USA, 2020; Available online: https://emea.bd.com/advancing-diagnostics/assays/respiratory-infections/bd-max-mdr-tb/# (accessed on 5 December 2024).
- GenoType MTBDRplus VER 2.0 Instructions for Use. Available online: https://www.hain-lifescience.de/en/products/microbiology/mycobacteria/tuberculosis/genotype-mtbdrplus.html (accessed on 5 December 2024).
- Xpert MTB/RIF Package Insert. Available online: https://www.cepheid.com/Package%20Insert%20Files/Xpert-MTB-RIF-ENGLISH-Package-Insert-301-1404-Rev-G.pdf (accessed on 5 December 2024).
- Xpert MTB/XDR Package Insert. Available online: https://www.cepheid.com/Package%20Insert%20Files/Xpert%20MTB-XDR%20ENGLISH%20Package%20Insert%20302-3514%20Rev%20C.pdf (accessed on 5 December 2024).
- Salari, N.; Kanjoori, A.H.; Hosseinian-Far, A.; Hasheminezhad, R.; Mansouri, K.; Mohammadi, M. Global prevalence of drug-resistant tuberculosis: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 57. [Google Scholar] [CrossRef]
- Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020; World Health Organization: Geneva, Switzerland, 2021.
- Armstrong, D.; Fisher, S.; Totten, M.; Parrish, N. An analytic feasibility study of the BD MAX MDR-TB assay for testing of non-sputum specimens for detection of the Mycobacterium tuberculosis complex (MTBC) and isoniazid (INH) and rifampin (RIF) resistance. Diagn. Microbiol. Infect. Dis. 2023, 106, 115925. [Google Scholar] [CrossRef]
- Behera, D.; Sahu, S.; Behera, S.; Panda, B.K. Utility of GeneXpert in Diagnosis of Extrapulmonary Tuberculosis. Int. J. Med. Res. Rev. 2022, 10, 422–428. [Google Scholar]
- Joean, O.; Thiele, T.; Schütz, K.; Schwerk, N.; Sedlacek, L.; Kalsdorf, B.; Baumann, U.; Stoll, M. Multidrug-resistant Mycobacterium tuberculosis: A report of cosmopolitan microbial migration and an analysis of best management practices. BMC Infect Dis. 2020, 17, 678. [Google Scholar] [CrossRef]
- Vogensen, V.B.; Anthony, R.M.; Kerstjens, H.A.M.; Tiberi, S.; de Steenwinkel, J.E.M.; Akkerman, O.W. The case for expanding worldwide access to point of care molecular drug susceptibility testing for isoniazid. Clin. Microbiol. Infect. 2022, 28, 1047–1049. [Google Scholar] [CrossRef]
- Sanchini, A.; Lanni, A.; Giannoni, F.; Mustazzolu, A. Exploring diagnostic methods for drug-resistant tuberculosis: A comprehensive overview. Tuberculosis 2024, 148, 102522. [Google Scholar] [CrossRef]
- Norbis, L.; Alagna, R.; Tortoli, E.; Codecasa, L.R.; Migliori, G.B.; Cirillo, D.M. Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert. Rev. Anti. Infect. Ther. 2014, 12, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. J. Respir. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Ciesielczuk, H.; Kouvas, N.; North, N.; Buchanan, R.; Tiberi, S. Evaluation of the BD MAX™ MDR-TB assay in a real-world setting for the diagnosis of pulmonary and extra-pulmonary TB. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Yang, X.; Yang, J.; Chen, L. Evaluation of the GenoType MTBDRplus and MTBDRsl for the detection of drug-resistant Mycobacterium tuberculosis on isolates from Beijing, China. Infect. Drug. Resist. 2018, 11, 1627–1634. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, X.Y.; Xu, L.; Xu, X.X.; Ruan, Z.F.; Huang, M.X.; Chen, L.; Jiang, X.W. Accurate and affordable detection of rifampicin and isoniazid resistance in Tuberculosis sputum specimens by multiplex PCR-multiple probes melting analysis. Infection 2024, 52, 2371–2398. [Google Scholar] [CrossRef]
- Shah, M.; Paradis, S.; Betz, J.; Beylis, N.; Bharadwaj, R.; Caceres, T.; Gotuzzo, E.; Joloba, M.; Mave, V.; Nakiyingi, L.; et al. Multicenter Study of the Accuracy of the BD MAX Multidrug-resistant Tuberculosis Assay for Detection of Mycobacterium tuberculosis Complex and Mutations Associated With Resistance to Rifampin and Isoniazid. Clin. Infect. Dis. 2020, 71, 1161–1167. [Google Scholar] [CrossRef]
- Bu, Q.; Qiang, R.; Fang, L.; Peng, X.; Zhang, H.; Cheng, H. Global trends in the incidence rates of MDR and XDR tuberculosis: Findings from the global burden of disease study 2019. Front Pharmacol. 2023, 14, 1156249. [Google Scholar] [CrossRef]
- Mvelase, N.R.; Balakrishna, Y.; Lutchminarain, K.; Mlisana, K. Evolving rifampicin and isoniazid mono-resistance in a high multidrug-resistant and extensively drug-resistant tuberculosis region: A retrospective data analysis. BMJ Open 2019, 9, e031663. [Google Scholar] [CrossRef]
- David, A.; de Vos, M.; Scott, L.; da Silva, P.; Trollip, A.; Ruhwald, M.; Schumacher, S.; Stevens, W. Feasibility, Ease-of-Use, and Operational Characteristics of World Health Organization-Recommended Moderate-Complexity Automated Nucleic Acid Amplification Tests for the Detection of Tuberculosis and Resistance to Rifampicin and Isoniazid. J. Mol. Diagn. 2023, 25, 46–56. [Google Scholar] [CrossRef]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef]
- Wali, A.; Safdar, N.; Ambreen, A.; Tahseen, S.; Mustafa, T. Care-seeking pathways and diagnostic delays in extrapulmonary TB patients. Public Health Action 2023, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]

| Test | Gene | Codon Range | Targeted Mutation Region | Identified Amino Acid Alterations |
|---|---|---|---|---|
| Hain MTBDRplus | rpoB | 505–509 | Rifampicin Resistance Determining Region (RRDR) | F505L, T508A, S509T |
| 510–513 | Q510H, L511P | |||
| 510–517 | Q513L, Q513P, del514-516 | |||
| 513–319 | D516V, D516Y, Del515, | |||
| 516–522 | Del518, N518, | |||
| 518–525 | S522L, S522Q | |||
| 526–529 | H526Y, H526D, H526R, H526P, H526Q, H526N, H526L, H526S, H526C, | |||
| 530–533 | S531L, S531Q, S531W, L533P, | |||
| katG | 944–945 | Isoniazid Resistance | ||
| inhA | −15, −8 | Isoniazid Resistance Promoter | C15T | |
| GeneXpert® MTB/RIF | rpoB | 426–452 | Rifampicin Resistance Determining Region (RRDR) | |
| BD MAX MDR-TB | rpoB | 507–533 | Rifampicin Resistance Determining Region (RRDR) | |
| katG | 944–945 | Isoniazid Resistance | ||
| inhA | inhA promoter region | Isoniazid Promoter | ||
| Xpert® MTB/XDR | katG | 315 | Isoniazid Resistance | codon 315, S315T |
| fabG1 | 199–210 | fabG1 promoter region | G609A | |
| oxyR–ahpC | 5 to −50, −47 to −92 | Intergenic region between oxyR and ahpC | ||
| inhA | 1 to −32 | Isoniazid and Ethionamide Resistance Promoter | C15T | |
| gyrA, gyrB | 87–95, 531–544 | Fluoroquinolone Resistance | D94G, A90V | |
| rrs | 1396–1417 | Resistance to Second-Line Drugs Amikacin, Kanamycin, and Capreomycin | A1401G | |
| eis | 6 to −42 | eis promoter | G-10A, C-12T |
| Sample Obtained for Testing | No. of Tested Samples | |
|---|---|---|
| Pulmonary samples | 4891 | |
| Bronchoalveolar Lavage (BAL) | 413 | |
| Sputum | 1018 | |
| Bronchial wash | 3460 | |
| Extrapulmonary samples | 1383 | |
| Pleural fluid | 826 | |
| Lymph node biopsy | 188 | |
| Tumor puncture | 117 | |
| Intraoperative specimen | 80 | |
| Urine | 64 | |
| Gastric lavage | 56 | |
| Tissue sample (other) | 30 | |
| Cerebrospinal fluid | 17 | |
| Vertebral bone specimen | 5 | |
| Total | 6274 | |
| Samples | AFB-Positive n (%) | LJ-Positive n (%) | BBL-Positive n (%) |
|---|---|---|---|
| Total (n = 6274) | 258 (4.11%) | 299 (4.77%) | 347 (5.53%) |
| Pulmonary (n = 4891) | 233 (3.55%) | 269 (4.28%) | 311 (4.95%) |
| Extrapulmonary (n = 1383) | 25 (0.39%) | 30 (0.48%) | 36 (0.57%) |
| Samples | Positive Results for the Genetic Probe | Positive Results for the Genetic Probe with Confirmed Growth on LJ Media | Positive Results for the Genetic Probe with No Confirmation on LJ Growth | Growth on LJ Solid Media with Negative Genetic Probe Assay |
|---|---|---|---|---|
| Total (n = 6274) | 425 | 277 | 140 | 23 |
| Pulmonary (n = 4891) | 376 | 249 | 118 | 21 |
| Extrapulmonary (n = 1383) | 49 | 27 | 22 | 2 |
| Tested Parameter | Total | Pulmonary Samples | Extrapulmonary Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BD MAX | BBL | AFB | BD MAX | BBL | AFB | BD MAX | BBL | AFB | |
| TP | 275 | 298 | 209 | 248 | 268 | 190 | 27 | 30 | 19 |
| FP | 140 | 40 | 31 | 118 | 34 | 25 | 22 | 6 | 6 |
| FN | 23 | 1 | 90 | 21 | 1 | 79 | 2 | 0 | 11 |
| TN | 5835 | 5933 | 5945 | 4501 | 4582 | 4594 | 1334 | 1351 | 1351 |
| Sensitivity (95% CI) | 92.3% (88.6–95.0%) | 99.7% (98.2–100%) | 69.9% (64.4–75%) | 92.2% (88.3–95.1%) | 99.6% (97.9–100%) | 70.6% (64.8–76.0%) | 93.1% (77.2–99.2%) | 100% (88.4–100%) | 63.3% (43.9–80.1%) |
| Specificity (95% CI) | 97.7% (97.2–98.0%) | 99.3% (99.1–99.5%) | 99.5% (99.3–99.6%) | 97.4% (96.9–97.9%) | 99.3% (99.0–99.5%) | 99.5% (99.2–99.6%) | 98.4% (97.6–99.0%) | 99.6% (99.0–99.8%) | 99.6% (99.0–99.8%) |
| PPV (95% CI) | 0.66 (0.61–0.71) | 0.88 (0.84–0.91) | 0.87 (0.82–0.91) | 0.68 (0.63–0.73) | 0.89 (0.85–0.92) | 0.88 (0.83–0.92) | 0.55 (0.40–0.69) | 0.83 (0.67–0.94) | 0.76 (0.55–0.91) |
| NPV (95% CI) | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 0.99 (0.98–0.99) | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 0.98 (0.98–0.99) | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 0.99 (0.99–1) |
| LR (+) (95% CI) | 39.38 (33.33–46.54) | 148.83 (109.27–202.70) | 134.75 (94.11–192.93) | 36.09 (30.10–43.27) | 135.26 (96.76–189.08) | 130.50 (87.61–194.38) | 57.39 (37.47–87.88) | 226.17 (101.79–502.53) | 143.24 (61.62–332.97) |
| LR (-) (95% CI) | 0.08 (0.05–0.12) | 0.003 (0.001–0.026) | 0.30 (0.25–0.36) | 0.08 (0.05–0.12) | 0.004 (0.001–0.0027) | 0.30 (0.25–0.36) | 0.07 (0.02–0.27) | - | 0.37 (0.23–0.59) |
| ACC (95% CI) | 97.4% (97.0–97.8%) | 99.3% (99.1–99.5%) | 98.1% (97.7–98.4%) | 97.2% (96.7–97.6%) | 99.3% (99–99.5%) | 97.9% (97.4–98.3%) | 98.3% (97.4–98.9%) | 99.6% (99.1–99.8%) | 98.8% (98.0–99.3%) |
| Anti-Tuberculosis Drug | Number of Resistant Isolates (%) |
|---|---|
| Streptomycin | 27 (7.78%) |
| Isoniazid | 30 (8.65%) |
| Rifampicin | 19 (5.48%) |
| Ethambutol | 12 (3.46%) |
| Pyrazinamide | 14 (4.03%) |
| Results of the BD MAX MDR-TB Assay | All Tested Samples | Percentage Value (%) | ||||
|---|---|---|---|---|---|---|
| Total | T (n = 6274) | P (n = 4891) | EX (n = 1383) | T [%] | P | EX |
| MTBC ND | 5848 | 4515 | 1333 | 93.21 | 92.31 | 96.38 |
| MTBC (TOTAL) | 426 | 376 | 50 | 6.79 | 7.68 | 3.62 |
| MTBC L-P in TOTAL | 119 | 103 | 16 | 1.90 | 2.10 | 1.16 |
| RIF R-ND | 270 | 240 | 30 | 4.30 | 4.9 | 2.17 |
| RIF R-D | 21 | 20 | 1 | 0.33 | 0.41 | 0.072 |
| RIF R-UNR | 135 | 116 | 19 | 2.15 | 2.37 | 1.37 |
| INH R-ND | 254 | 225 | 29 | 4.0 | 4.6 | 2.10 |
| INH R-D | 27 | 24 | 3 | 0.43 | 0.49 | 0.22 |
| katG Mut-D | 22 | - | - | 0.35 | - | - |
| inhApr Mut-D | 14 | - | - | 0.22 | - | - |
| INH R-UNR | 145 | 127 | 18 | 2.31 | 2.6 | 1.3 |
| Assay Performed | No. of Isolates RIF-R | No. of Isolates INH-R |
|---|---|---|
| BD MAX | 21 | 34 |
| GenoType MTBDRplus | 17 | 33 |
| GeneXpert MTB/XDR | 18 | 33 |
| BACTEC MGIT | 17 | 34 |
| AP | 18 | 32 |
| Test Applied | Results | RIF | κ | p | INH | κ | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||||
| GenoType MTBDRplus | Positive | 15 | 1 | 0.69 | <0.001 | 33 | 0 | 0.89 | <0.001 |
| Negative | 5 | 18 | 1 | 5 | |||||
| GeneXpert MTB/XDR | Positive | 16 | 1 | 0.74 | <0.001 | 33 | 0 | 0.89 | <0.001 |
| Negative | 4 | 18 | 1 | 5 | |||||
| BACTEC MGIT | Positive | 16 | 1 | 0.74 | <0.001 | 33 | 1 | 0.77 | <0.001 |
| Negative | 4 | 18 | 1 | 4 | |||||
| AP | Positive | 17 | 1 | 0.80 | <0.001 | 32 | 0 | 0.80 | <0.001 |
| Negative | 3 | 18 | 2 | 5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, K.; Wójcik, K.; Drożdż, K.; Klesiewicz, K. Utility of Rapid Molecular Assays for Detecting Multidrug-Resistant Mycobacterium tuberculosis in Extrapulmonary Samples. Diagnostics 2025, 15, 1113. https://doi.org/10.3390/diagnostics15091113
Kania K, Wójcik K, Drożdż K, Klesiewicz K. Utility of Rapid Molecular Assays for Detecting Multidrug-Resistant Mycobacterium tuberculosis in Extrapulmonary Samples. Diagnostics. 2025; 15(9):1113. https://doi.org/10.3390/diagnostics15091113
Chicago/Turabian StyleKania, Katarzyna, Katarzyna Wójcik, Kamil Drożdż, and Karolina Klesiewicz. 2025. "Utility of Rapid Molecular Assays for Detecting Multidrug-Resistant Mycobacterium tuberculosis in Extrapulmonary Samples" Diagnostics 15, no. 9: 1113. https://doi.org/10.3390/diagnostics15091113
APA StyleKania, K., Wójcik, K., Drożdż, K., & Klesiewicz, K. (2025). Utility of Rapid Molecular Assays for Detecting Multidrug-Resistant Mycobacterium tuberculosis in Extrapulmonary Samples. Diagnostics, 15(9), 1113. https://doi.org/10.3390/diagnostics15091113





