Mobile Diagnostics Based on Motion? A Close Look at Motility Patterns in the Schistosome Life Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscopy and Video Recordings
2.2. Mini-Microscopes and Video
2.3. Target Organisms
2.4. Image Analysis
2.4.1. Image Acquisition
2.4.2. Image Preprocessing for Removal of Background Noise
2.4.3. Image Segmentation Aiming at Creating Candidate Areas for Further Analysis
2.4.4. Processing the Segmented Targets to Analyze How the Object Behaves over Time
3. Results
3.1. Motility Patterns of Schistosoma mansoni Life Cycle Stages: From Slow Motion to Ultra Rapid
3.1.1. Eggs and Miracidia
3.1.2. Cercaria
3.1.3. Adult Worms
3.2. Videos Captured Using Mini-Microscopes
4. Discussion
- Motility patterns observed in schistosomes and in two apathogenic species: Turbatrix, a nematode, and Paramecium, a protozoan.
- Video recording capability of unsophisticated imaging devices and target processing.
- The image analysis process as time and frequency signal processing for identification of motility patterns, “diagnostic fingerprints.”
- Possible target organisms and apathogenic model organisms for a motility analysis-based approach to diagnostics.
4.1. Motility Patterns Observed
4.1.1. Schistosomes
4.1.2. Turbatrix and Paramecium
4.2. Image Capture and Video Recording
4.3. Image Analysis
4.4. Motility-Based Diagnostics—Obvious and Not-So-Obvious Applications
4.5. Model Organisms and Future Studies
5. Conclusions
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Supplementary File 7Supplementary File 8Supplementary File 9Supplementary File 10Supplementary File 11Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| POC | Point of Care |
| LED | Light-Emitting Diode |
| VHS | Video Home System |
| FPS | Frames per Second |
| KI | Karolinska Institutet, Stockholm, Sweden |
| FIMM | Institute for Molecular Medicine Finland |
References
- Bergquist, R.; Johansen, M.V.; Utzinger, J. Diagnostic dilemmas in helminthology: What tools to use and when? Trends Parasitol. 2009, 25, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ochodo, E.; Gopalakrishna, G.; Spek, B.; Reitsma, J.; van Lieshout, L.; Lamberton, P.; Bossuyt, P.M.; Leeflang, M.M. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- King, C.H.; Dickman, K.; Tisch, D.J. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005, 365, 1561–1569. [Google Scholar] [CrossRef]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Salim, N.; Schindler, T.; Karagiannis Voules, D.A.; Rothen, J.; Lweno, O.; Mohammed, A.S.; Singo, R.; Benninghoff, M.; et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am. J. Trop. Med. Hyg. 2014, 90, 535–545. [Google Scholar] [CrossRef] [PubMed]
- London Declaration 2012. Available online: http://unitingtocombatntds.org/resource/london-declaration (accessed on 10 March 2016).
- Hotez, P.J.; Herricks, J.R. Helminth elimination in the pursuit of sustainable development goals: A “Worm Index” for human development. PLoS Negl. Trop. Dis. 2015, 9, e0003618. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Bergquist, R.; Shu-Hua, X.; Singer, B.H.; Tanner, M. Sustainable schistosomiasis control—The way forward. Lancet 2003, 362, 1932–1934. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, H.; Jiang, Q. A diagnostic challenge for Schistosomiasis japonica in China: Consequences on praziquantel-based morbidity control. Parasite Vectors 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Thors, C.; Holmblad, P.; Maleki, M.; Carlson, J.; Linder, E. Schistosomiasis in Swedish travellers to sub-Saharan Africa: Can we rely on serology? Scand. J. Infect. Dis. 2006, 38, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.V.; Lier, T.; Sithithaworn, P. Towards improved diagnosis of neglected zoonotic trematodes using a One Health approach. Acta Trop. 2015, 141, 161–169. [Google Scholar] [CrossRef] [PubMed]
- King, C. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Comment in PubMed Commons. Cochrane Database Syst Rev. 2015. [Google Scholar] [CrossRef]
- Fung, M.S.; Xiao, N.; Wang, S.; Carlton, E.J. Field evaluation of a PCR test for Schistosoma japonicum egg detection in low-prevalence regions of China. Am. J. Trop. Med. Hyg. 2012, 87, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, L.; Roestenberg, M. Clinical consequences of new diagnostic tools for intestinal parasites. Clin. Microbiol. Infect. 2015, 21, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Zumla, A. Rapid infectious diseases diagnostics using Smartphones. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Abdul-Ghani, R. Towards e-parasitology: Making use of virtual microscopy. Trop. Med. Int. Health 2015, 20, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.F.; Su, T.-W.; Ozcan, A. Wide field-of-view lens-free fluorescent imaging on a chip. Lab Chip 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Greenbaum, A.; Luo, W.; Ozcan, A. Wide-field pathology imaging using on-chip microscopy. Virchows Arch. 2015, 467, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Switz, N.A.; D’Ambrosio, M.V.; Fletcher, D.A. Low-cost mobile phone microscopy with a reversed mobile phone camera lens. PLoS ONE 2014, 9, e95330. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, M.V.; Bakalar, M.; Bennuru, S.; Reber, C.; Skandarajah, A.; Nilsson, L.; Switz, N.; Kamgno, J.; Pion, S.; Boussinesq, M.; et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Linder, E.; Grote, A.; Varjo, S.; Linder, N.; Lebbad, M.; Lundin, M.; Diwan, V.; Hannuksela, J.; Lundin, J. On-chip imaging of Schistosoma haematobium eggs in urine for diagnosis by computer vision. PLoS Negl. Trop. Dis. 2013, 7, e2547. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Lai, H. Mao Zedong’s fight against schistosomiasis. Perspect. Biol. Med. 2008, 51, 176–187. [Google Scholar] [PubMed]
- Yu, J.M.; de Vlas, S.J.; Jiang, Q.W.; Gryseels, B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 2006, 56, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Dönges, J. [The miracidia hatch test for bilharziasis]. Dtsch. Med. Wochenschr. 1966, 91, 2212–2214. [Google Scholar] [CrossRef] [PubMed]
- Justesen, D.; van Sloterdijck, D. A quantitative and qualitative hatching test for schistosomiasis. Acta Leiden 1977, 45, 61–66. [Google Scholar] [PubMed]
- Handbook on the Prevention and Treatment of Schistosomiasis; U.S. Government Printing Office: Washington, DC, USA, 1977.
- Malone, J.B. Biology-based mapping of vector-borne parasites by Geographic Information Systems and Remote Sensing. Parassitologia 2005, 47, 27–50. [Google Scholar] [PubMed]
- Hotez, P.J.; Fenwick, A.; Savioli, L.; Molyneux, D.H. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 2009, 373, 1570–1575. [Google Scholar] [CrossRef]
- Hürlimann, E.; Schur, N.; Boutsika, K.; Stensgaard, A.-S.; Laserna de Himpsl, M.; Ziegelbauer, K.; Laizer, N.; Camenzind, L.; Di Pasquale, A.; Ekpo, U.F.; et al. Toward an open-access global database for mapping, control, and surveillance of neglected tropical diseases. PLoS Negl. Trop. Dis. 2011, 5, e1404. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.C.; Hale, E.; Donald, W.; Batarii, W.; Bugoro, H.; Nausien, J.; Smale, J.; Palmer, K.; Bobogare, A.; Taleo, G.; et al. A high-resolution geospatial surveillance-response system for malaria elimination in Solomon Islands and Vanuatu. Malar. J. 2013, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, L.; Huang, Y.; Yang, G.; Wu, F.; Hang, D.; Li, W.; Zhang, J.; Liang, Y.; Zhou, X. A real-time platform for monitoring schistosomiasis transmission supported by Google Earth and a web-based geographical information system. Geospat. Health 2015, 6, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dendere, R.; Myburg, N.; Douglas, T.S. A review of cellphone microscopy for disease detection. J. Microsc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Walz, Y.; Wegmann, M.; Dech, S.; Raso, G.; Utzinger, J. Risk profiling of schistosomiasis using remote sensing: Approaches, challenges and outlook. Parasit. Vectors 2015, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Thors, C. Serodiagnostics of Schistosomiasis Using Keyhole Limpet Hemocyanin (KLH) as Antigen; Karolinska Institutet: Stockholm, Sweden, 2006. [Google Scholar]
- Lim, W.; Isa, N.A.M. A novel adaptive color to grayscale conversion algorithm for digital images. Sci. Res. Essays 2012, 7, 2718–2730. [Google Scholar]
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Bali, S.; Singh, S.N. A review on the strategies and techniques of image segmentation. In Proceedings of the Fifth International Conference on Advanced Computing & Communication Technologies, Rohtak, India, 21–22 February 2015.
- Cheng, E.D.; Challa, S.; Chakravorty, R. Parallel microscopic cell image segmentation and multiple fusions. Int. J. Signal Imaging Syst. Eng. 2011, 4, 96. [Google Scholar] [CrossRef]
- Kaakinen, M.; Huttunen, S.; Paavolainen, L.; Marjomäki, V.; Heikkilä, J.; Eklund, L. Automatic detection and analysis of cell motility in phase-contrast time-lapse images using a combination of maximally stable extremal regions and Kalman filter approaches. J. Microsc. 2014, 253, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Khammari, H.; Poyil, A.T. Spectral characterization of ctride-to-stride variability in children gait motion. IJCSI 2013, 10, 216–223. [Google Scholar]
- Kekre, H.B.; Kulkanii, V.; Gaikar, P.; Gupta, N. Speaker identification using spectrograms of varying frame sizes. Int. J. Comput. Appl. 2012, 50, 27–33. [Google Scholar]
- Linder, E.; Lundin, M.; Thors, C.; Lebbad, M.; Winiecka-Krusnell, J.; Helin, H.; Leiva, B.; Isola, J.; Lundin, J. Web-based virtual microscopy for parasitology: A novel tool for education and quality assurance. PLoS Negl. Trop. Dis. 2008, 2, e315. [Google Scholar] [CrossRef]
- Farah Ahmed, S. Urinary tract complications of chronic Schistosoma haematobium infection and immunohistology of granuloma formation. Master’s Thesis, Karolinska Institutet and Somali National University, Mogadishu, Somalia, 1986. [Google Scholar]
- Rajagopalan, A.N.; Chellappa, R. Higher-order spectral analysis of human motion. In Proceedings of the International Conference on Image Processing, Vancouver, BC, Canada, 10–13 September 2000; Volume 3, pp. 230–233.
- Khunarsal, P.; Lursinsap, C.; Raicharoen, T. Singing voice recognition based on matching of spectrogram pattern. In Proceedings of the International Joint Conference on Neural Networks, Atlanta, GA, USA, 14–19 June 2009; pp. 1595–1599.
- Gryseels, B. Schistosomiasis. Infect. Dis. Clin. N. Am. 2012, 26, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.D. Introduction to Animal Parasitology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Ghaleba, A.M.; James Atwood, J., II; Jorge Morales-Montorc, J.; Damiand, R. A 3 kDa peptide is involved in the chemoattraction in vitro of the male Schistosoma mansoni to the female. Microbes Infect. 2006, 8, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- LoVerde, P.T.; Osman, A.; Hinck, A. Schistosoma mansoni: TGF-β Signaling Pathways. Exp. Parasitol. 2007, 117, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Linder, E. Fluorochrome-labelled lectins reveal secreted glycoconjugates of schistosome larvae. Parasitol. Today 1986, 2, 219–221. [Google Scholar] [CrossRef]
- Linder, E.; Thors, C.; Lundin, L. Isolation of an SBA lectin-reactive glycoprotein (GP50) and its identification in Schistosoma mansoni larval and adult worm secretions. J. Parasitol. 1991, 77, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Powers, T.R.; Valles, J.M.; Valles, J.M., Jr. Evidence for two extremes of ciliary motor response in a single swimming microorganism. Biophys. J. 2014, 106, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-W.; Erlinger, A.; Tseng, D.; Ozcan, A. Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy. Anal. Chem. 2010, 82, 8307–8312. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Choi, I.; Feng, J.; Huang, K.; Mcleod, E.; Ozcan, A. Sperm trajectories form chiral ribbons. Sci. Rep. 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taute, K.M.; Gude, S.; Tans, S.J.; Shimizu, T.S. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clavel, A.; Varea, M.; Doiz, O.; López, L.; Quílez, J.; Castillo, F.J.; Rubio, C.; Gómez-Lus, R. Visualization of hydatid elements: Comparison of several techniques. J. Clin. Microbiol. 1999, 37, 1561–1563. [Google Scholar] [PubMed]
- Thomas, A. Aquatics under Polarization. Available online: http://www.microscopy-uk.org.uk/mag/artdec11/tt-pol-aquatic.pdf (accessed on 6 June 2016).
- Varea, M.; Clavel, A.; Doiz, O.; Castillo, F.J.; Rubio, M.C.; Gómez-Lus, R. Fuchsin fluorescence and autofluorescence in Cryptosporidium, Isospora and Cyclospora oocysts. Int. J. Parasitol. 1998, 28, 1881–1883. [Google Scholar] [CrossRef]
- Park, S.; Hwang, H.; Nam, S.W.; Martinez, F.; Austin, R.H.; Ryu, W.S. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS ONE 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Butykai, A.; Orbán, A.; Kocsis, V.; Szaller, D.; Bordács, S.; Tátrai-Szekeres, E.; Kiss, L.F.; Bóta, A.; Vértessy, B.G.; Zelles, T.; et al. Malaria pigment crystals as magnetic micro-rotors: Key for high-sensitivity diagnosis. Sci. Rep. 2013, 3, 1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, E. Cercarial kissing marks-no superficial make-up. Parasitol. Today 1990, 6, 393–395. [Google Scholar] [CrossRef]
- Linder, E. Identification of schistosomal eggs: Description of an immunological spot assay for hatch fluid antigen. J. Immunol. Methods 1986, 88, 137–140. [Google Scholar] [CrossRef]
- Skandarajah, A.; Reber, C.C.D.; Switz, N.A.N.N.A.; Fletcher, D.A. Quantitative imaging with a mobile phone microscope. PLoS ONE 2014, 9, e96906. [Google Scholar] [CrossRef] [PubMed]
- Breslauer, D.N.; Maamari, R.N.; Switz, N.A.; Lam, W.A.; Fletcher, D.A. Mobile phone based clinical microscopy for global health applications. PLoS ONE 2009, 4, e6320. [Google Scholar] [CrossRef] [PubMed]
- Varjo, S.; Hannuksela, J.; Silven, O. Direct imaging with Printed Microlens Arrays. In Proceedings of the 21st International Conference on Pattern Recognition, Tsukuba, Japan, 11–15 November 2012; pp. 1355–1358.
- Vilmi, P.; Varjo, S.; Sliz, R.; Hannuksela, J.; Fabritius, T. Disposable optics for microscopy diagnostics. Sci. Rep. 2015, 5, 16957. [Google Scholar] [CrossRef] [PubMed]
- Varjo, S.; Hannuksela, J. A mobile imaging system for medical diagnostics. In Advanced Concepts for Intelligent Vision Systems, ACIVS; Springer International Publishing: New York, NY, USA, 2013; pp. 215–226. [Google Scholar]
- Cui, X.; Lee, L.M.; Heng, X.; Zhong, W.; Sternberg, P.W.; Psaltis, D.; Yang, C. Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 10670–10675. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.; Erickson, D.; Baugh, L.R.; Yaqoob, Z.; Sternberg, P.W.; Psaltis, D.; Yang, C. Optofluidic microscopy-a method for implementing a high resolution optical microscope on a chip. Lab Chip 2006, 6, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Arpa, A.; Wetzstein, G.; Lanman, D.; Raskar, R. Single lens off-chip cellphone microscopy. In Proceedings of the IEEE Computer Soceity Conference Computer Vision Pattern Recognition Workshops, Providence, RI, USA, 16–21 June 2012; pp. 23–28.
- Mudanyali, O.; Tseng, D.; Oh, C.; Isikman, S.O.; Sencan, I.; Bishara, W.; Oztoprak, C.; Seo, S.; Khademhosseini, B.; Ozcan, A. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip 2010, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Mudanyali, O.; Oztoprak, C.; Tseng, D.; Erlinger, A.; Ozcan, A. Detection of waterborne parasites using field-portable and cost-effective lensfree microscopy. Lab Chip 2010, 10, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, N.; Smal, I.; de Chaumont, F.; Maška, M.; Sbalzarini, I.F.; Gong, Y.; Cardinale, J.; Carthel, C.; Coraluppi, S.; Winter, M.; et al. Objective comparison of particle tracking methods. Nat. Methods 2014, 11, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, A.E.; Triajianto, J.; Routledge, E. Low-cost motility tracking system (LOCOMOTIS) for time-lapse microscopy applications and cell visualisation. PLoS ONE 2014, 9, e103547. [Google Scholar] [CrossRef] [PubMed]
- Gabor, D. Theory of communication. Part 1: The analysis of information. J. Inst. Electr. Eng. III Radio Commun. Eng. 1946, 93, 429–441. [Google Scholar] [CrossRef]
- Asarnow, D.E.D.E.; Singh, R. Segmenting the etiological agent of schistosomiasis for high-content screening. IEEE Trans. Med. Imaging 2013, 32, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Duin, R.P.W.; Mao, J. Statistical pattern recognition: A review. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 22, 4–37. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Freeman, W.T.; Rubinstein, M. A World of Movement. Sci. Am. 2014, 312, 46–51. [Google Scholar] [CrossRef]
- Kotze, A.C.; Steinmann, P.; Zhou, H.; Du, Z.W.; Zhou, X.N. The effect of egg embryonation on field-use of a hookworm benzimidazole-sensitivity egg hatch assay in Yunnan province, people’s republic of China. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Von Samson-Himmelstjerna, G.; Coles, G.; Jackson, F.; Bauer, C.; Borgsteede, F.; Cirak, V.Y.; Demeler, J.; Donnan, A.; Dorny, P.; Epe, C.; et al. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol. Res. 2009, 105, 825–834. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Bertsch, D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium Infection, Including Low-Prevalence and Previously-Treated Populations—Open Access Library. Available online: http://www.jourlib.org/paper/3001234#.VulOmhiKvpU (accessed on 16 March 2016).
- Upatham, E.S. Rapidity and duration of hatching of St. Lucian Schistosoma mansoni eggs in outdoor habitats. J. Helminthol. 1972, 46, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.; Webbe, G.; Sturrock, R.F. Human Schistosomiasis; CAB International: Oxon, UK, 1993. [Google Scholar]
- Braun-Munzinger, R.; Southgate, B. Egg viability in urinary schistosomiasis. I. New methods compared with available methods. Trop. Med. Hyg. 1993, 96, 22–27. [Google Scholar]
- Braun-Munzinger, R.A.; Southgate, B.A. Egg viability in urinary schistosomiasis. II. Simplifying modifications and standardization of new methods. J. Trop. Med. Hyg. 1993, 96, 179–185. [Google Scholar] [PubMed]
- Heddergott, N.; Krüger, T.; Babu, S.B.; Wei, A.; Stellamanns, E.; Uppaluri, S.; Pfohl, T.; Stark, H.; Engstler, M. Trypanosome motion represents an adaptation to the crowded environment of the vertebrate bloodstream. PLoS Pathog. 2012, 8, e1003023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Torralba, A.; Freeman, W.T.; Durand, F.; Adelson, E.H. Motion magnification. ACM Trans. Graph. 2005, 24, 519. [Google Scholar] [CrossRef]
- Kuehn, A.; Pradel, G. The coming-out of malaria gametocytes. J. Biomed. Biotechnol. 2010, 2010, 976827. [Google Scholar] [CrossRef] [PubMed]
- Vincensini, L.; Blisnick, T.; Bastin, P. 1001 Model organisms to study cilia and flagella. Biol. Cell 2011, 103, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Culturing Vinegar Eels Live Material Care Guide. Available online: http://www.flinnsci.com/media/406398/bf10587.pdf (accessed on 6 June 2016).
- Ephraim, R.K.D.; Duah, E.; Andrews, J.R.; Bogoch, I.I. Ultra-low-cost urine filtration for Schistosoma haematobium diagnosis: A proof-of-concept study. Am. J. Trop. Med. Hyg. 2014, 91, 544–546. [Google Scholar] [CrossRef] [PubMed]








| Environment | Location in Host | Stage | Purpose of Motility | Type of Motility | Reference |
|---|---|---|---|---|---|
| Definitive Host/Human, Mammalian | Tissue | Schistosomulum | Migration to mesenteric vessels | Inchworm | - |
| Blood | Adult worms | Ingestion of RBCs | Contractions | Videos; Figure 4a,c,d | |
| Metabolism | Peristaltics | ||||
| Mating | Attraction | - | |||
| Excretory function of protonephridia | Ciliary beating | - | |||
| Adult female only | Egg maturation and shell formation | Ootype contractions | Videos; Figure 4d | ||
| Egg ejection/Deposition in tissues | Transport/Dorsoflexion/Expulsion | Videos; Figure 4c | |||
| Adult male only | Sperm | (?) | - | ||
| Tissue | Eggs | Egg migration into excreta | Granuloma formation | Videos; Figure 5 | |
| Water | Eggs | Hatching/Bursting of egg shell | Rotation of intraoval miracidium | Video S1 | |
| Miracidia | Finding intermediate host | Swimming | Video S1; Video 2 | ||
| Intermediate host/Snail | Tissue | Sporocyst | - | - | - |
| Water | Cercaria | Host finding | Swimming | - | |
| Attachment/Penetration into host | Wiggling/Tail loss/ | Video S3; Figure 3b | |||
| Inchworm | - | ||||
| Frame | Time (s) | Velocity (mm/s) | Acceleration (mm/s2) |
|---|---|---|---|
| 75 | 2.503 | 0.076 | 2.269 |
| 76 | 2.536 | 0.034 | −1.253 |
| 78 | 2.569 | 0.104 | 2.104 |
| 79 | 2.603 | 0.076 | −0.851 |
| 80 | 2.636 | 0.096 | 0.620 |
| 81 | 2.669 | 0.069 | −0.830 |
| 82 | 2.703 | 0.217 | 4.439 |
| 83 | 2.736 | 0.058 | −4.774 |
| 84 | 2.769 | 0.295 | 7.130 |
| 85 | 2.803 | 0.349 | 1.594 |
| 86 | 2.836 | 0.455 | 3.189 |
| Trajectory ID | Mean Velocity (μm/s) | Track Length (mm) |
|---|---|---|
| 1 | 123.6 | 10.49 |
| 2 | 329.0 | 3.77 |
| 3 | 152.6 | 12.60 |
| 4 | 261.2 | 12.60 |
| 5 | 213.0 | 12.60 |
| 6 | 139.3 | 12.60 |
| 11 | 356.0 | 5.12 |
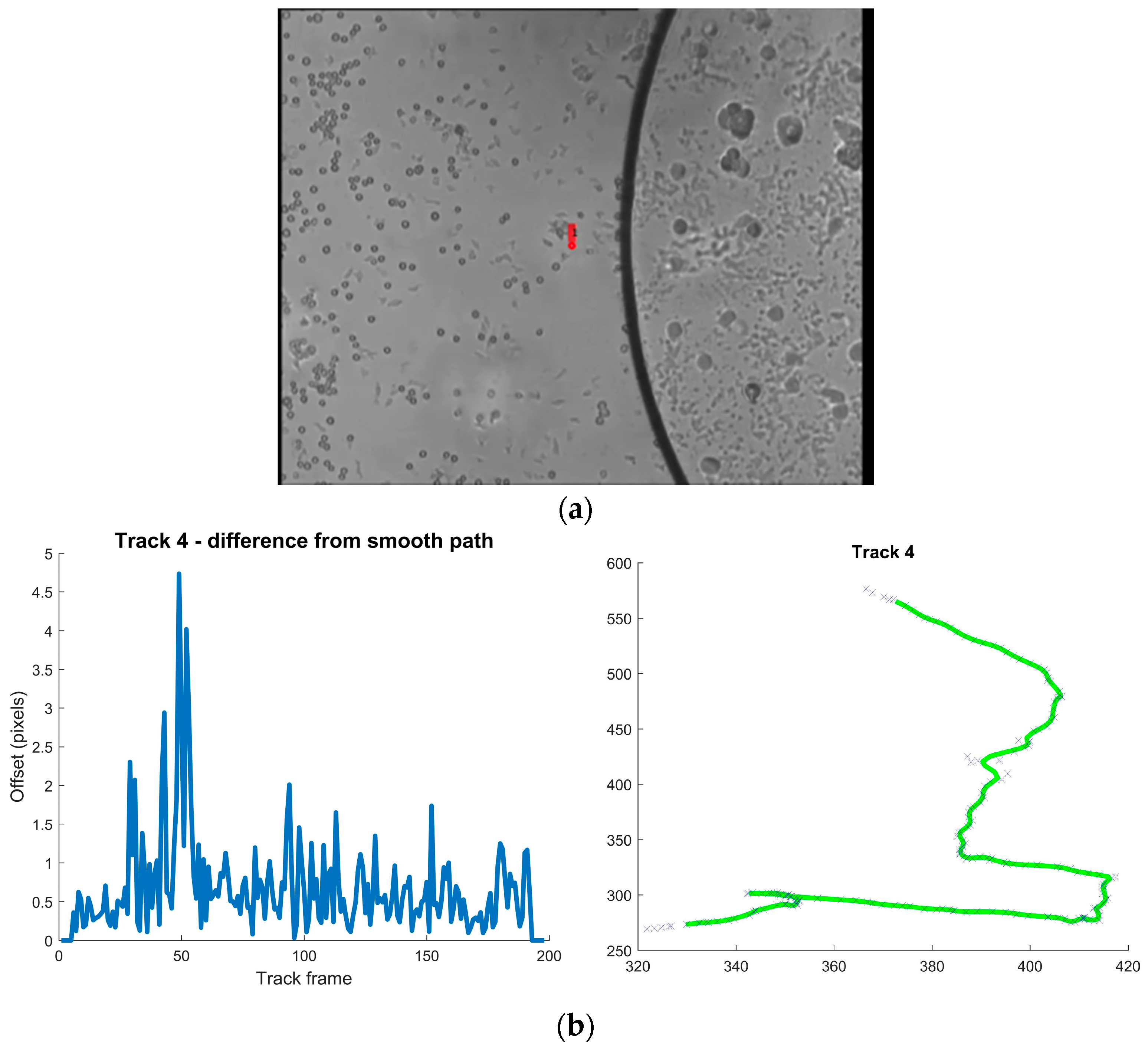
| Track | Mean Difference from Smoothed Path Pixels | Max Pixels | Direction Changes (mean) Degrees/ms | Max Degrees/ms |
|---|---|---|---|---|
| 1 | 0.464 | 1.623 | 0.407 | 2.814 |
| 3 | 0.428 | 1.543 | 0.199 | 1.044 |
| 4 | 0.664 | 4.737 | 0.177 | 1.066 |
| 5 | 0.601 | 5.547 | 0.210 | 1.027 |
| 6 | 0.355 | 2.060 | 0.176 | 1.041 |
| 11 | 0.887 | 7.668 | 0.091 | 0.428 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linder, E.; Varjo, S.; Thors, C. Mobile Diagnostics Based on Motion? A Close Look at Motility Patterns in the Schistosome Life Cycle. Diagnostics 2016, 6, 24. https://doi.org/10.3390/diagnostics6020024
Linder E, Varjo S, Thors C. Mobile Diagnostics Based on Motion? A Close Look at Motility Patterns in the Schistosome Life Cycle. Diagnostics. 2016; 6(2):24. https://doi.org/10.3390/diagnostics6020024
Chicago/Turabian StyleLinder, Ewert, Sami Varjo, and Cecilia Thors. 2016. "Mobile Diagnostics Based on Motion? A Close Look at Motility Patterns in the Schistosome Life Cycle" Diagnostics 6, no. 2: 24. https://doi.org/10.3390/diagnostics6020024
APA StyleLinder, E., Varjo, S., & Thors, C. (2016). Mobile Diagnostics Based on Motion? A Close Look at Motility Patterns in the Schistosome Life Cycle. Diagnostics, 6(2), 24. https://doi.org/10.3390/diagnostics6020024





