A Pilot Study into the Association between Oral Health Status and Human Papillomavirus—16 Infection
Abstract
:1. Introduction
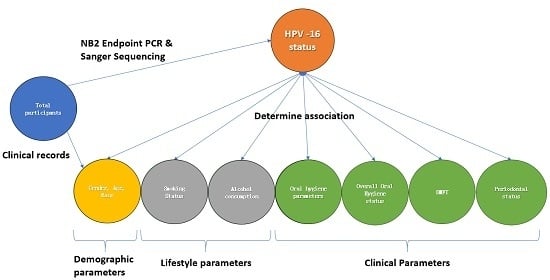
2. Methods
2.1. Participants
2.2. Participant Demographics, Lifestyle and Clinical Data
2.3. Salivary Oral Rinse Sample Collection and Processing
2.4. DNA Extraction from Oral Rinse Samples
2.5. End-Point PCR Detection and Sequence Confirmation of HPV-16 DNA
2.6. Statistical Methods
3. Results
3.1. Patient Demographics
3.2. Lifestyle Related Risk Factors/Parameters
3.3. Oral Health Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HPV | human papillomavirus |
| OPC | oropharyngeal cancer |
| HNC | head and neck cancers |
| OC | oral cavity cancer |
| BOP | bleeding on probing |
| PSR | periodontal screening record |
| DMFT | decayed, missing and filled teeth index |
References
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Menzin, J.; Lines, L.M.; Manning, L.N. The economics of squamous cell carcinoma of the head and neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.K.; Xiao, W.; Broutian, T.R.; He, X.; Gillison, M.L. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex. Transm. Dis. 2012, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hariri, S.; Unger, E.R.; Sternberg, M.; Dunne, E.F.; Swan, D.; Patel, S.; Markowitz, L.E. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J. Infect. Dis. 2011, 204, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lazcano-Ponce, E.; Villa, L.L.; Flores, R.; Salmeron, J.; Lee, J.H.; Papenfuss, M.R.; Abrahamsen, M.; Jolles, E.; Nielson, C.M.; et al. The human papillomavirus infection in men study: Human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, E.M.; Ang, K.K. The epidemic of HPV-associated oropharyngeal cancer is here: Is it time to change our treatment paradigms? J. Natl. Compr. Cancer Netw. 2011, 9, 665–673. [Google Scholar]
- Boscolo-Rizzo, P.; Del Mistro, A.; Bussu, F.; Lupato, V.; Baboci, L.; Almadori, G.; Da Mosto, M.C.; Paludetti, G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 77–87. [Google Scholar] [PubMed]
- Zaravinos, A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget 2014, 5, 3956–3969. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Lodi, G.; von Bültzingslöwen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Flake, C.; Arafa, J.; Hall, A.; Ence, E.; Howard, K.; Kingsley, K. Screening and detection of human papillomavirus (HPV) high-risk strains HPV16 and HPV18 in saliva samples from subjects under 18 years old in Nevada: A pilot study. BMC Oral Health 2012, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.-B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive Control of Human Papillomavirus Infections and Related Diseases. Vaccine 2013, 31, H1–H31. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.C.; Markham, C.M.; Ross, M.W.; Mullen, P.D. Examining the association between oral health and oral HPV infection. Cancer Prev. Res. 2013, 6, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.C.; Lambie, D.; Verma, M.; Punyadeera, C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015, 4, 596–607. [Google Scholar] [CrossRef]
- Chai, R.C.; Lim, Y.; Frazer, I.H.; Wan, Y.; Perry, C.; Jones, L.; Lambie, D.; Punyadeera, C. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16(INK4a) expression in head and neck squamous cell carcinoma patients. BMC Cancer 2016, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Calvopina, D.; Punyadeera, C. miRNAs in human papilloma virus associated oral and oropharyngeal squamous cell carcinomas. Expert Rev. Mol. Diagn. 2014, 14, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Sugiyama, M. Risk Factors for Oral Human Papillomavirus Infection in Healthy Individuals: A Systematic Review and Meta-Analysis. J. Clin. Med. Res. 2016, 8, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.S.R.; O’Regan, E.M.; Kennedy, S.; Martin, C.; O’Leary, J.J.; Timon, C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J. Clin. Cases 2014, 2, 172–193. [Google Scholar] [PubMed]
- Bui, T.C.; Tran, L.T.; Markham, C.M.; Huynh, T.T.; Tran, L.T.; Pham, V.T.; Tran, Q.M.; Hoang, N.H.; Hwang, L.Y.; Sturgis, E.M. Self-reported Oral Health, Oral Hygiene, and Oral HPV Infection in At-Risk Women in Ho Chi Minh City, Vietnam. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Janam, P.; Vijayamma, J.M.B. Prevalence of human papilloma virus in marginal periodontium and its association with periodontitis: A cross sectional study. J. Indian Soc. Periodontol. 2014, 18, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Mazul, A.L.; Taylor, J.M.; Divaris, K.; Weissler, M.C.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Olshan, A.F.; Zevallos, J.P. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2017, 123, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Australian Research Centre for Population Oral Health. Periodontal diseases in the Australian adult population. Aust. Dent. J. 2009, 54, 390–393. [Google Scholar]
- Ciarrocca, K.; Jackson, L.L.; de Rossi, S.S. Human papillomavirus: The fundamentals of HPV for oral health care providers. J. Calif. Dent. Assoc. 2013, 41, 349–355. [Google Scholar] [PubMed]
- Cleveland, J.L.; Junger, M.L.; Saraiya, M.; Markowitz, L.E.; Dunne, E.F.; Epstein, J.B. The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States: Implications for dentistry. J. Am. Dent. Assoc. 2011, 142, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Slade, G.D.; Patton, L.L. National prevalence of oral HPV infection and related risk factors in the U.S. adult population. Oral Dis. 2012, 18, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M.; Sullivan, M.A.; Stoler, D.L.; Melendy, T.; Hyland, A.; Smaldino, P.J.; Rigual, N.R.; Loree, T.R. Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 391–396. [Google Scholar] [PubMed]
- Cullinan, M.P.; Ford, P.J.; Seymour, G.J. Periodontal disease and systemic health: Current status. Aust. Dent. J. 2009, 54 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, C.A.; Adams, P.F.; Peregoy, J.A. Health behaviors of adults: United States, 2008–2010. Vital Health Stat. 2013, 257, 1–184. [Google Scholar]
- Dietary Guidelines for Americans; U.S. Government Printing Office: Washington, DC, USA, 2010.
- Preshaw, P.M. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health 2015, 15 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; MacFarlane, T.W.; Lamey, P.J.; Ferguson, M.M. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J. Oral Pathol. 1986, 15, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.J.; Tran, P.; Cockburn, N.; Keen, B.; Kavanagh, D.J.; Gartner, C. Survey of dental clinic patients: Smoking and preferences for cessation support. Aust. Dent. J. 2016, 61, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Cornford, M.; Perry, S.; Davis, M.; Dunne, M.P.; Whiteman, D.C. Prevalence and risk factors for oral HPV infection in young Australians. PLoS ONE 2014, 9, e91761. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M. Interaction between chronic inflammation and oral HPV infection in the etiology of head and neck cancers. Int. J. Otolaryngol. 2012, 2012, 575242. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Horewicz, V.V.; Feres, M.; Rapp, G.E.; Yasuda, V.; Cury, P.R. Human papillomavirus-16 prevalence in gingival tissue and its association with periodontal destruction: A case-control study. J. Periodontol. 2010, 81, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Hormia, M.; Willberg, J.; Ruokonen, H.; Syrjänen, S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J. Periodontol. 2005, 76, 358–363. [Google Scholar] [CrossRef]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Slots, J. Detection of human viruses in periodontal pockets using polymerase chain reaction. Oral Microbiol. Immunol. 1996, 11, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Daley, E.; DeBate, R.; Dodd, V.; Dyer, K.; Fuhrmann, H.; Helmy, H.; Smith, S.A. Exploring awareness, attitudes, and perceived role among oral health providers regarding HPV-related oral cancers. J. Public Health Dent. 2011, 71, 136–142. [Google Scholar] [CrossRef] [PubMed]

| Variable | HPV-16 DNA Positive (% within Variable) | HPV-16 DNA Negative (% within Variable) | Total Number (% within Total Population) | p-Value |
|---|---|---|---|---|
| Gender | 0.328 | |||
| Male | 5 (3.6) | 132 (96.4) | 137 (61.4) | |
| Female | 5 (5.8) | 81 (94.2) | 86 (38.6) | |
| Age | 0.130 | |||
| 18–30 | 1 (1.9) | 52 (98.1) | 53 (23.8) | |
| 31–50 | 3 (2.8) | 103 (97.2) | 106 (47.5) | |
| 51–70 | 6 (10.0) | 54 (90.0) | 60 (26.9) | |
| 71–90 | 0 (0) | 4 (100.00) | 4 (1.8) | |
| Race | 1.000 | |||
| Caucasian | 6 (3.6) | 160 (96.4) | 166 (78.3) | |
| Asian | 0 (0) | 26 (100.0) | 26 (12.3) | |
| South Asian | 0 (0) | 10 (100.0) | 10 (4.7) | |
| Aborigine/Pacific Islander | 0 (0) | 4 (100.0) | 4 (1.9) | |
| African | 0 (0) | 1 (100.0) | 1 (0.5) | |
| Latino | 0 (0) | 4 (100.0) | 4 (1.9) | |
| Other | 0 (0) | 1 (100.0) | 1 (0.5) | |
| Smoking | 0.595 | |||
| Never Smoker | 2 (2.1) | 95 (97.9) | 97 (45.8) | |
| Former Smoker | 4 (5.7) | 66 (94.3) | 70 (33.0) | |
| Current Daily Smoker <15 | 0 (0) | 26 (100.00) | 26 (12.3) | |
| Current Daily Smoker 15–24 | 0 (0) | 12 (100.0) | 12 (5.7) | |
| Current Daily Smoker 25–34 | 0 (0) | 1 (100.0) | 1 (0.5) | |
| Current Daily Smoker >35 | 0 (0) | 1 (100.0) | 1 (0.5) | |
| Current Non Daily Smoker | 0 (0) | 5 (100.0) | 5 (2.4) | |
| Alcohol consumption | 0.147 | |||
| Non Drinker | 1 (1.8) | 54 (98.2) | 55 (29.3) | |
| Infrequent Drinker | 3 (8.6) | 32 (91.4) | 35 (18.6) | |
| Regular Drinker (<1/day for F, <2/day for M) | 1 (1.3) | 77 (98.7) | 78 (41.5) | |
| Regular Drinker (>1/day for F, >2/day for M) | 1 (5.0) | 19 (95.0) | 20 (10.6) | |
| Plaque | 0.262 | |||
| Nil | 0 (0) | 3 (100.0) | 3 (1.4) | |
| Mild | 4 (3.6) | 108 (96.4) | 112 (50.9) | |
| Moderate | 6 (8.1) | 68 (91.9) | 74 (33.6) | |
| Severe | 0 (0) | 31 (100.0) | 31 (14.1) | |
| Calculus (Supra-gingival) | 0.182 | |||
| Nil | 0 (0) | 3 (100.0) | 3 (1.4) | |
| Mild | 2 (1.9) | 106 (98.1) | 108 (49.1) | |
| Moderate | 6 (7.3) | 76 (92.7) | 82 (37.3) | |
| Severe | 2 (7.4) | 25 (92.6) | 27 (12.3) | |
| Calculus (Sub-gingival) | 0.608 | |||
| Nil | 1 (4.5) | 21 (95.5) | 22 (10.0) | |
| Mild | 3 (3.1) | 95 (96.9) | 98 (44.5) | |
| Moderate | 4 (5.6) | 68 (94.4) | 72 (32.7) | |
| Severe | 2 (7.1) | 26 (92.9) | 28 (12.7) | |
| Oral Hygiene | 0.233 | |||
| Good to Excellent | 3 (2.9) | 99 (97.1) | 102 (46.4) | |
| Poor to Fair | 7 (5.9) | 111 (94.1) | 118 (53.6) | |
| DMFT | 0.259 | |||
| 0–9 | 6 (7.6) | 73 (92.4) | 79 (35.9) | |
| 10–19 | 2 (2.1) | 92 (97.9) | 94 (42.7) | |
| 20–28 | 2 (4.3) | 45 (95.7) | 47 (21.4) | |
| Periodontal Status | 0.367 | |||
| Non-Periodontal-Diseased | 7 (5.3) | 124 (94.7) | 131 (59.5) | |
| Periodontal Diseased | 3 (3.4) | 86 (96.6) | 89 (40.5) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.X.; Bennett, N.; Tran, P.; Tang, K.D.; Lim, Y.; Frazer, I.; Samaranayake, L.; Punyadeera, C. A Pilot Study into the Association between Oral Health Status and Human Papillomavirus—16 Infection. Diagnostics 2017, 7, 11. https://doi.org/10.3390/diagnostics7010011
Sun CX, Bennett N, Tran P, Tang KD, Lim Y, Frazer I, Samaranayake L, Punyadeera C. A Pilot Study into the Association between Oral Health Status and Human Papillomavirus—16 Infection. Diagnostics. 2017; 7(1):11. https://doi.org/10.3390/diagnostics7010011
Chicago/Turabian StyleSun, Charles Xiaohang, Nigel Bennett, Peter Tran, Kai Dun Tang, Yenkai Lim, Ian Frazer, Lakshman Samaranayake, and Chamindie Punyadeera. 2017. "A Pilot Study into the Association between Oral Health Status and Human Papillomavirus—16 Infection" Diagnostics 7, no. 1: 11. https://doi.org/10.3390/diagnostics7010011
APA StyleSun, C. X., Bennett, N., Tran, P., Tang, K. D., Lim, Y., Frazer, I., Samaranayake, L., & Punyadeera, C. (2017). A Pilot Study into the Association between Oral Health Status and Human Papillomavirus—16 Infection. Diagnostics, 7(1), 11. https://doi.org/10.3390/diagnostics7010011