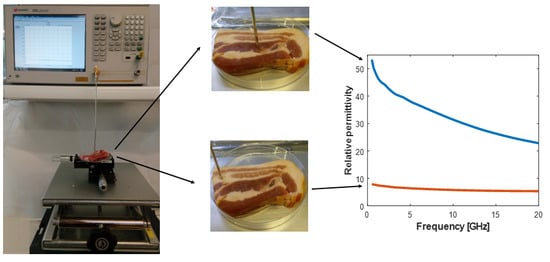
Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices
Abstract
1. Introduction
2. Tissue Dielectric Properties: Background and Relevant Works
2.1. Basics of Dielectric Properties
2.2. Dielectric Property Studies in the Literature
3. Measurement Approaches
3.1. Overview of Measurement Techniques
3.1.1. Transmission Line
3.1.2. Cavity Perturbation
3.1.3. Tetrapolar Impedance
3.1.4. Open-ended Coaxial Probe
3.2. Evolution of the Coaxial Probe Design and Fabrication
4. Calibration and Confounders
4.1. Standard Calibration
4.2. Calibration Procedure and Confounders
4.2.1. Equipment Set-Up and Confounders
4.2.2. Signal Settings and Confounders
4.2.3. Measurement of the Three Standards and Confounders
Temperature of the Liquid
Other Confounders in the Liquid Measurement
4.3. Confounders Introduced in the System after Calibration
5. Validation and Measurement Uncertainty
5.1. Validation Liquids: Models, and Their Advantages and Disadvantages
5.1.1. Alcohols
5.1.2. Saline
5.1.3. Other Liquids
5.2. Uncertainty Calculation
6. Tissue Sample Preparation and Measurement Procedure
6.1. Probe Selection Considerations
6.1.1. Sample Size, Sensing Volume, and Heterogeneity
6.1.2. Tissue Surface Characteristics
6.2. Tissue Preparation and Handling
6.2.1. In Vivo vs. Ex Vivo Measurements
6.2.2. Surgical Intervention, Sample Access, and Excision
6.2.3. Tissue Transportation
6.2.4. Tissue Handling
6.3. Procedure for Tissue Measurements
6.3.1. Measurement Region Choice Confounders
6.3.2. Probe-Tissue Contact
6.3.3. Temperature Effects
7. Tissue Sample Histological Analysis
7.1. Factors Impacting Histological Analysis
7.2. The Link between Heterogeneity, Histology, and Sensing Volume
7.3. Histological Analysis Techniques in Dielectric Studies
8. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Formica, D.; Silvestri, S. Biological Effects of Exposure to Magnetic Resonance Imaging: An Overview. Biomed. Eng. Online 2004, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A. Exposure Limits and Dielectric Contrast for Breast Cancer Tissues: Experimental Results up to 50 GHz. In Proceedings of the 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 667–671. [Google Scholar]
- Nikolova, N.K. Microwave Imaging for Breast Cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Pastorino, M. Microwave Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Noghanian, S. Introduction to Microwave Imaging; Springer: New York, NY, USA, 2014. [Google Scholar]
- Zou, Y.; Guo, Z. A Review of Electrical Impedance Techniques for Breast Cancer Detection. Med. Eng. Phys. 2003, 25, 79–90. [Google Scholar] [CrossRef]
- Brown, B. Electrical Impedance Tomography (EIT): A Review. J. Med. Eng. Technol. 2003, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, A.D.; Ortola, C.F.; Martinez, M.M.; Vidal, A.; Santos, A.; Marquez, M.P.; Roka, P.L.; Bohm, S.H.; Suarez-Sipmann, F. Position-Dependent Distribution of Lung Ventilation—A Feasability Study. In Proceedings of the 2015 IEEE Sensors Applications Symposium (SAS), Zadar, Croatia, 13–15 April 2015. [Google Scholar]
- Avery, J.; Dowrick, T.; Faulkner, M.; Goren, N.; Holder, D. A Versatile and Reproducible Multi-Frequency Electrical Impedance Tomography System. Sensors 2017, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Halter, R.J.; Zhou, T.; Meaney, P.M.; Hartov, A.; Barth, R.J.; Rosenkranz, K.M.; Wells, W.A.; Kogel, C.A.; Borsic, A.; Rizzo, E.J.; et al. The Correlation of in Vivo and Ex Vivo Tissue Dielectric Properties to Validate Electromagnetic Breast Imaging: Initial Clinical Experience. Physiol. Meas. 2009, 30, S121–S136. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A Large-Scale Study of the Ultrawideband Microwave Dielectric Properties of Normal Breast Tissue Obtained from Reduction Surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, T.; Kubota, S.; Kuroki, S.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex Permittivities of Breast Tumor Tissues Obtained from Cancer Surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Porter, E.; Kirshin, E.; Santorelli, A.; Coates, M.; Popović, M. Time-Domain Multistatic Radar System for Microwave Breast Screening. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 229–232. [Google Scholar] [CrossRef]
- Scapaticci, R.; Bellizzi, G.; Catapano, I.; Crocco, L.; Bucci, O.M. An Effective Procedure for MNP-Enhanced Breast Cancer Microwave Imaging. IEEE Trans. Biomed. Eng. 2014, 61, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, M.; Morgan, F.; Flores-Tapia, D.; Byrne, D.; Glavin, M.; Jones, E. Prototype Ultra Wideband Radar System for Bladder Monitoring Applications. Prog. Electromagn. Res. C 2012, 33, 17–28. [Google Scholar] [CrossRef]
- Arunachalam, K.; MacCarini, P.; De Luca, V.; Tognolatti, P.; Bardati, F.; Snow, B.; Stauffer, P. Detection of Vesicoureteral Reflux Using Microwave Radiometrysystem Characterization with Tissue Phantoms. IEEE Trans. Biomed. Eng. 2011, 58, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Bialkowski, M.E. Microwave Head Imaging for Stroke Detection. Prog. Electromagn. Res. M 2011, 21, 163–175. [Google Scholar] [CrossRef]
- Persson, M.; Fhager, A.; Trefna, H.D.; Yu, Y.; McKelvey, T.; Pegenius, G.; Karlsson, J.E.; Elam, M. Microwave-Based Stroke Diagnosis Making Global Prehospital Thrombolytic Treatment Possible. IEEE Trans. Biomed. Eng. 2014, 61, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Dowrick, T.; Blochet, C.; Holder, D. In Vivo Bioimpedance Measurement of Healthy and Ischaemic Rat Brain: Implications for Stroke Imaging Using Electrical Impedance Tomography. Physiol. Meas. 2015, 36, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Scapaticci, R.; Bucci, O.M.; Catapano, I.; Crocco, L. Differential Microwave Imaging for Brain Stroke Followup. Int. J. Antennas Propag. 2014. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local Hyperthermia Combined with Radiotherapy And-/or Chemotherapy: Recent Advances and Promises for the Future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Ghadjar, P.; Reichardt, P.; Hohenberger, P.; Verweij, J.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Vujaskovic, Z.; et al. 13LBA Improved Overall Survival by Adding Regional Hyperthermia to Neo-Adjuvant Chemotherapy in Patients with Localized High-Risk Soft Tissue Sarcoma (HR-STS): Long-Term Outcomes of the EORTC 62961/ESHO Randomized Phase III Study. Eur. J. Cancer 2015, 51, S716. [Google Scholar] [CrossRef]
- Wessalowski, R.; Schneider, D.T.; Mils, O.; Friemann, V.; Kyrillopoulou, O.; Schaper, J.; Matuschek, C.; Rothe, K.; Leuschner, I.; Willers, R.; et al. Regional Deep Hyperthermia for Salvage Treatment of Children and Adolescents with Refractory or Recurrent Non-Testicular Malignant Germ-Cell Tumours: An Open-Label, Non-Randomised, Single-Institution, Phase 2 Study. Lancet Oncol. 2013, 14, 843–852. [Google Scholar] [CrossRef]
- Ekstrand, V.; Wiksell, H.; Schultz, I.; Sandstedt, B.; Rotstein, S.; Eriksson, A. Influence of Electrical and Thermal Properties on RF Ablation of Breast Cancer: Is the Tumour Preferentially Heated? Biomed. Eng. Online 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, I.; Bozzi, E.; Cioni, R.; Parentini, B.; Bartolozzi, C. Radiofrequency Ablation of Lung Tumours. Insights Imaging 2011, 2, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A.; Marra, P.; Beaty, K.; Ellis, L.M.; Vauthey, J.N.; Abdalla, E.K.; Scaife, C.; Raut, C.; Wolff, R.; Choi, H.; et al. Early and Late Complications after Radiofrequency Ablation of Malignant Liver Tumors in 608 Patients. Ann. Surg. 2004, 239, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Rossetto, F.; Prakash, M.; Neuman, D.G.; Lee, T. Phantom and Animal Tissues for Modelling the Electrical Properties of Human Liver. Int. J. Hyperth. 2003, 19, 89–101. [Google Scholar] [CrossRef]
- Yang, D.; Converse, M.; Mahvi, D.; Webster, J. Measurement and Analysis of Tissue Temperature during Microwave Liver Ablation. IEEE Trans. Biomed. Eng. 2007, 54, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Lopresto, V.; Pinto, R.; Lovisolo, G.; Cavagnaro, M. Changes in the Dielectric Properties of Ex Vivo Bovine Liver during Microwave Thermal Ablation at 2.45 GHz. Phys. Med. Biol. 2012, 57, 2309–2327. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Converse, M.; Booske, J.H.; Hagness, S.C. Ultrawideband Temperature-Dependent Dielectric Properties of Animal Liver Tissue in the Microwave Frequency Range. Phys. Med. Biol. 2006, 51, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L. Temperature-Dependent Dielectric Properties of Liver Tissue Measured during Thermal Ablation: Toward an Improved Numerical Model. In Proceedings of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 230–233. [Google Scholar]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in Combined Treatment of Cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, D.E. Image-Guided Thermal Ablation of Lung Malignancies. Radiology 2011, 260, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Brace, C.L. Expanded Modeling of Temperature-Dependent Dielectric Properties for Microwave Thermal Ablation. Phys. Med. Biol. 2011, 56, 5249–5264. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical Models to Evaluate the Temperature Increase Induced by Ex Vivo Microwave Thermal Ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric Properties of Human Normal, Malignant and Cirrhotic Liver Tissue: In Vivo and Ex Vivo Measurements from 0.5 to 20 GHz Using a Precision Open-Ended Coaxial Probe. Phys. Med. Biol. 2007, 52, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Stuchly, M.A.; Athey, T.W.; Samaras, G.M.; Taylor, G.E. Measurement of Radio Frequency Permittivity of Biological Tissues with an Open-Ended Coaxial Line: Part II—Experimental Results. IEEE Trans. Microw. Theory Tech. 1982, 30, 87–92. [Google Scholar] [CrossRef]
- Burdette, E.; Cain, F.; Seals, J. In Vivo Probe Measurement Technique for Determining Dielectric Properties at VHF through Microwave Frequencies. IEEE Trans. Microw. Theory Tech. 1980, 28, 414–427. [Google Scholar] [CrossRef]
- Kraszewski, A.; Stuchly, M.A.; Stuchly, S.S.; Smith, A.M. In Vivo and in Vitro Dielectric Properties of Animal Tissues at Radio Frequencies. Bioelectromagnetics 1982, 3, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Mealing, G.A. Dielectric Properties of Frog Tissues in Vivo and in Vitro. Phys. Med. Biol. 1985, 30, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: II. Measurements in the Frequency Range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Holden, S.; Gabriel, C. Mobile Telecommunications and Health Research Programme: Dielectric Properties of Tissues at Microwave Frequencies; Microwave Consultants Limited: London, UK, 2005. [Google Scholar]
- Abdilla, L.; Sammut, C.; Mangion, L. Dielectric Properties of Muscle and Liver from 500 MHz–40 GHz. Electromagn. Biol. Med. 2013, 32, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P.; Foster, K.R. RF Field Interactions with Biological Systems: Electrical Properties and Biophysical Mechanisms. Proc. IEEE 1980, 68, 104–113. [Google Scholar] [CrossRef]
- Foster, K.; Schwan, H. Dielectric Properties of Tissues and Biological Materials: A Critical Review. Crit. Rev. Biomed. Eng. 1989, 17, 25–104. [Google Scholar] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: III. Parametric Models for the Dielectric Spectrum of Tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Clarke, R.; Hodgetts, T.; Symm, G. RF and Microwave Dielectric Measurements upon Layered Materials Using Coaxial Sensors; NPL Report MAT 13; National Physical Laboratory: Teddington, UK, 2008. [Google Scholar]
- Gulich, R.; Köhler, M.; Lunkenheimer, P.; Loidl, A. Dielectric Spectroscopy on Aqueous Electrolytic Solutions. Radiat. Environ. Biophys. 2009, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- England, T.S.; Sharples, N.A.A. Dielectric Properties of the Human Body in the Microwave Region of the Spectrum. Nature 1949, 163, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.F. The Dielectric Behaviour of Some Types of Human Tissues at Microwave Frequencies. Br. J. Appl. Phys. 1951, 2, 295–300. [Google Scholar] [CrossRef]
- Schwan, H.P. Electrical Properties of Tissue and Cell Suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P.; Li, K. Capacity and Conductivity of Body Tissues at Ultrahigh Frequencies. Proc. IRE 1953, 41, 1735–1740. [Google Scholar] [CrossRef]
- Stuchly, M.A.; Stuchly, S.S. Dielectric Properties of Biological Substances—Tabulated. J. Microw. Power 1980, 15, 19–25. [Google Scholar] [CrossRef]
- Burdette, E.C.; Friederich, P.G.; Seaman, R.L.; Larsen, L.E. In Situ Permittivity of Canine Brain: Regional Variations and Postmortem Changes. IEEE Trans. Microw. Theory Tech. 1986, 34, 38–50. [Google Scholar] [CrossRef]
- Smith, S.R.; Foster, K.R. Dielectric Properties of Low-Water-Content Tissues. Phys. Med. Biol. 1985, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Zhadobov, M.; Augustine, R.; Sauleau, R.; Alekseev, S.; Di Paola, A.; Le Quément, C.; Mahamoud, Y.S.; Le Dréan, Y. Complex Permittivity of Representative Biological Solutions in the 2–67 GHz Range. Bioelectromagnetics 2012, 33, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.; Renne, G.; Preda, L.; et al. Experimental Validation of the Dielectric Permittivity of Breast Cancer Tissues up to 50 GHz. In Proceedings of the IEEE MTT-S International Microwave Workshop Advanced Materials and Processes for RF and THz Applications (IMWS-AMP), Pavia, Italy, 20–22 September 2017; pp. 20–22. [Google Scholar]
- Stuchly, M.A.; Stuchly, S.S. Coaxial Line Reflection Methods for Measuring Dielectric Properties of Biological Substances at Radio and Microwave Frequencies-A Review. IEEE Trans. Instrum. Meas. 1980, 29, 176–183. [Google Scholar] [CrossRef]
- Athey, T.W.; Stuchly, M.A.; Stuchly, S.S. Measurement of Radio Frequency Permittivity of Biological Tissues with an Open-Ended Coaxial Line: Part I. IEEE Trans. Microw. Theory Tech. 1982, 30, 82–86. [Google Scholar] [CrossRef]
- Gabriel, C.; Grant, E.H.; Young, I.R. Use of Time Domain Spectroscopy for Measuring Dielectric Properties with a Coaxial Probe. J. Phys. E 1986, 19, 843–846. [Google Scholar] [CrossRef]
- Foster, K.R.; Schepps, J.L.; Stoy, R.D.; Schwan, H.P. Dielectric Properties of Brain Tissue between 0.01 and 10 GHz. Phys. Med. Biol. 1979, 24, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, A.; Stuchly, S.S.; Eidus, L.; Swarup, A. In Vitro Dielectric Properties of Human Tissues at Radiofrequencies. Phys. Med. Biol. 1987, 32, 615. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Dielectric Properties of Biological Materials: Biophysical and Medical Applications. IEEE Trans. Electr. Insul. 1984, EI-19, 453–474. [Google Scholar] [CrossRef]
- Schepps, J.L.; Foster, K.R. The UHF and Microwave Dielectric Properties of Normal and Tumour Tissues: Variation in Dielectric Properties with Tissue Water Content. Phys. Med. Biol. 1980, 25, 1149. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, E. The Dielectric Properties of Biological Tissues: I. Literature Survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; Report N.AL/OE-TR-1996-0037; Occupational and Environmental Health Directorate, Radiofrequency Radiation Division: Brooks Air Force Base, TX, USA, 1996. [Google Scholar]
- Federal Communications Commission. Tissue Dielectric Properties; FCC: Washington, DC, USA, 2008. Available online: https://www.fcc.gov/general/body-tissue-dielectric-parameters (accessed on 30 October 2017).
- Andreuccetti, D.; Fossi, R.; Petrucci, C. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10 Hz–100 GHz; IFAC-CNR: Florence, Italy, 1997; Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 4 June 2018).
- Alanen, E.; Lahtinen, T.; Nuutinen, J. Variational Formulation of Open-Ended Coaxial Line in Contact with Layered Biological Medium. IEEE Trans. Biomed. Eng. 1998, 45, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Hagl, D.; Popovic, D.; Hagness, S.C.; Booske, J.H.; Okoniewski, M. Sensing Volume of Open-Ended Coaxial Probes for Dielectric Characterization of Breast Tissue at Microwave Frequencies. IEEE Trans. Microw. Theory Tech. 2003, 51, 1194–1206. [Google Scholar] [CrossRef]
- Popovic, D.; Okoniewski, M.; Hagl, D.; Booske, J.H.; Hagness, S.C. Volume Sensing Properties of Open Ended Coaxial Probes for Dielectric Spectroscopy of Breast Tissue. In Proceedings of the IEEE Antennas and Propagation Society, Boston, MA, USA, 8–13 July 2001; pp. 254–257. [Google Scholar]
- Popovic, D.; McCartney, L.; Beasley, C.; Lazebnik, M.; Okoniewski, M.; Hagness, S.C.; Booske, J.H. Precision Open-Ended Coaxial Probes for in Vivo and Ex Vivo Dielectric Spectroscopy of Biological Tissues at Microwave Frequencies. IEEE Trans. Microw. Theory Tech. 2005, 53, 1713–1721. [Google Scholar] [CrossRef]
- Taylor, B.N.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results; NIST Technical Note 1297; US Department of Commerce, Technology Administration, National Institute of Standards and Technology: Gaithersburg, MD, USA, 1994. [Google Scholar]
- Gabriel, C.; Peyman, A. Dielectric Measurement: Error Analysis and Assessment of Uncertainty. Phys. Med. Biol. 2006, 51, 6033–6046. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A Large-Scale Study of the Ultrawideband Microwave Dielectric Properties of Normal, Benign and Malignant Breast Tissues Obtained from Cancer Surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.S.; Mishra, R.K.; Swarup, A.; Thomas, J.M. Dielectric Properties of Normal & Malignant Human Breast Tissues at Radiowave & Microwave Frequencies. Indian J. Biochem. Biophys. 1984, 21, 76–79. [Google Scholar] [PubMed]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The Measured Electrical Properties of Normal and Malignant Human Tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric Properties Characterization from 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Meaney, P.M.; Gregory, A.; Epstein, N.; Paulsen, K.D. Microwave Open-Ended Coaxial Dielectric Probe: Interpretation of the Sensing Volume Re-Visited. BMC Med. Phys. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Gregory, A.P.; Seppälä, J.; Lahtinen, T. Open-Ended Coaxial Dielectric Probe Effective Penetration Depth Determination. IEEE Trans. Microw. Theory Tech. 2016, 64, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; La Gioia, A.; Santorelli, A.; O’Halloran, M. Modeling of the Dielectric Properties of Biological Tissues within the Histology Region. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3290–3301. [Google Scholar] [CrossRef]
- Porter, E.; O’Halloran, M. Investigation of Histology Region in Dielectric Measurements of Heterogeneous Tissues. IEEE Trans. Dielectr. Electr. Insul. 2017, 65, 5541–5552. [Google Scholar] [CrossRef]
- Peyman, A.; Kos, B.; Djokić, M.; Trotovšek, B.; Limbaeck-Stokin, C.; Serša, G.; Miklavčič, D. Variation in Dielectric Properties Due to Pathological Changes in Human Liver. Bioelectromagnetics 2015, 36, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, T.; Arihiro, K.; Kikkawa, T. Comparative Study on Dielectric Constants and Conductivities of Invasive Ductal Carcinoma Tissues. IEEE Eng. Med. Biol. Soc. 2015, 4387–4390. [Google Scholar] [CrossRef]
- Sabouni, A.; Hahn, C.; Noghanian, S.; Sauter, E.; Weiland, T. Study of the Effects of Changing Physiological Conditions on Dielectric Properties of Breast Tissues. ISRN Biomed. Imaging 2013, 2013, 894153. [Google Scholar] [CrossRef]
- Reinecke, T.; Hagemeier, L.; Schulte, V.; Klintschar, M.; Zimmermann, S. Quantification of Edema in Human Brain Tissue by Determination of Electromagnetic Parameters. In Proceedings of the IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Nicolson, A.; Ross, G.F. Measurement of the Intrinsic Properties of Materials by Time-Domain Techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Weir, W.B. Automatic Measurement of Complex Dielectric Constant and Permeability. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Vanzura, E.J.; Kissick, W.A. Improved Technique for Determining Complex Permittivity with the Transmission/Reflection Method. IEEE Trans. Microw. Theory Tech. 1990, 38, 1096–1103. [Google Scholar] [CrossRef]
- Kim, S.; Baker-Jarvis, J. An Approximate Approach To Determining the Permittivity and Permeability near λ/2 Resonances in Transmission/Reflection Measurements. Prog. Electromagn. Res. B 2014, 58, 95–109. [Google Scholar] [CrossRef]
- Boughriet, A.H.; Legrand, C.; Chapoton, A. Noniterative Stable Transmission/Reflection Method for Low-Loss Material Complex Permittivity Determination. IEEE Trans. Microw. Theory Tech. 1997, 45, 52–57. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Janezic, M.; Domich, P.; Geyer, R. Analysis of an Open-Ended Coaxial Probe with Lift-off for Non Destructive Testing. IEEE Trans. Instrum. Meas. 1994, 43, 1–8. [Google Scholar] [CrossRef]
- Gregory, A.; Clarke, R. A Review of RF and Microwave Techniques for Dielectric Measurements on Polar Liquids. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 727–743. [Google Scholar] [CrossRef]
- Agilent. Basics of Measuring the Dielectric Properties of Materials; Agilent Technologies: Santa Clara, CA, USA, 2005. [Google Scholar]
- Land, D.V.; Campbell, A.M. A Quick Accurate Method for Measuring the Microwave Dielectric Properties of Small Tissue Samples. Phys. Med. Biol. 1992, 37, 183. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Land, D.V. Dielectric Properties of Female Human Breast Tissue Measured in Vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hwang, J.Y.; Andriese, M. Maximum Sample Volume for Permittivity Measurements by Cavity Perturbation Technique. IEEE Trans. Instrum. Meas. 2014, 63, 450–455. [Google Scholar] [CrossRef]
- Campbell, A. Measurements and Analysis of the Microwave Dielectric Properties of Tissues. J. Appl. Phys. 1990, 22, 95. [Google Scholar]
- Ramos, A.; Bertemes-Filho, P. Numerical Sensitivity Modeling for the Detection of Skin Tumors by Using Tetrapolar Probe. Electromagn. Biol. Med. 2011, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, K.; Porterfield, J.E.; Kottam, A.T.G.; Feldman, M.D.; Escobedo, D.; Valvano, J.W.; Pearce, J.A. Electrical Conductivity and Permittivity of Murine Myocardium. IEEE Trans. Biomed. Eng. 2009, 56, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Wi, H.; McEwan, A.; Kwon, H.; Oh, T.I.; Woo, E.J.; Seo, J.K. Evaluation of a Multi-Electrode Bioimpedance Spectroscopy Tensor Probe to Detect the Anisotropic Conductivity Spectra of Biological Tissues. Meas. Sci. Technol. 2014, 25, 075702. [Google Scholar] [CrossRef]
- Misra, D.K. A Quasi-Static Analysis of Open-Ended Coaxial Lines. IEEE Trans. Microw. Theory Tech. 1987, 35, 925–928. [Google Scholar] [CrossRef]
- Grant, J.P.; Clarke, R.N.; Symm, G.T.; Spyron, N.M. A Critical Study of the Open-Ended Coaxial-Line Sensor Technique for RF and Microwave Complex Permittivity Measurements. J. Phys. E Sci. Instrum. 1989, 22, 757–770. [Google Scholar] [CrossRef]
- Jenkins, S.; Preece, A.W.; Hodgetts, T.E.; Symm, G.T.; Warham, A.G.P.; Clarke, R.N. Comparison of Three Numerical Treatments for the Open-Ended Coaxial Line Sensor. Electron. Lett. 1990, 26, 234–236. [Google Scholar] [CrossRef]
- Misra, D. On the Measurement of the Complex Permittivity of Materials by an Open-Ended Coaxial Probe. IEEE Microw. Guid. Wave Lett. 1995, 5, 161–163. [Google Scholar] [CrossRef]
- Perez Cesaretti, M.D. General Effective Medium Model for the Complex Permittivity Extraction with an Open-Ended Coaxial Probe in Presence of a Multilayer Material under Test. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2012. [Google Scholar]
- Keysight Technologies. Keysight E5063A ENA Series Network Analyzer; Keysight Technologies: Santa Clara, CA, USA, 2015. [Google Scholar]
- Gabriel, C.; Chan, T.Y.; Grant, E.H. Admittance Models for Open Ended Coaxial Probes and Their Place in Dielectric Spectroscopy. Phys. Med. Biol. 1994, 39, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Berube, D.; Ghannouchi, F.M.; Savard, P. A Comparative Study of Four Open-Ended Coaxial Probe Models for Permittivity Measurements of Lossy Dielectric/Biological Materials at Microwave Frequencies. IEEE Trans. Microw. Theory Tech. 1996, 44, 1928–1934. [Google Scholar] [CrossRef]
- Zajíček, R.; Oppl, L.; Vrba, J. Broadband Measurement of Complex Permitivity Using Reflection Method and Coaxial Probes. Radioengineering 2008, 17, 14–19. [Google Scholar]
- Schwan, H.P.; Foster, K.R. Microwave Dielectric Properties of Tissue. Some Comments on the Rotational Mobility of Tissue Water. Biophys. J. 1977, 17, 193–197. [Google Scholar] [CrossRef]
- Peyman, A. Dielectric Properties of Tissues; Variation with Structure and Composition. In Proceedings of the International Conference on Electromagnetics in Advanced Applications (ICEAA), Torino, Italy, 14–18 September 2009; pp. 863–864. [Google Scholar]
- Popovic, D.; Okoniewski, M. Effects of Mechanical Flaws in Open-Ended Coaxial Probes for Dielectric Spectroscopy. IEEE Microw. Wirel. Components Lett. 2002, 12, 401–403. [Google Scholar] [CrossRef]
- Keysight. N1501A Dielectric Probe Kit 10 MHz to 50 GHz: Technical Overview. 2015. Available online: http://www.Keysight.Com/En/Pd-2492144-Pn-N1501A/Dielectric-Probe-Kit (accessed on 30 October 2017).
- Karacolak, T.; Cooper, R.; Unlu, E.S.; Topsakal, E. Dielectric Properties of Porcine Skin Tissue and in Vivo Testing of Implantable Antennas Using Pigs as Model Animals. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1686–1689. [Google Scholar] [CrossRef]
- Nyshadham, A.; Sibbald, C.L.; Stuchly, S.S. Permittivity Measurements Using Open-Ended Sensors and Reference Liquid Calibration—An Uncertainty Analysis. IEEE Trans. Microw. Theory Tech. 1992, 40, 305–314. [Google Scholar] [CrossRef]
- Marsland, T.P.; Evans, S. Dielectric Measurements with an Open-Ended Coaxial Probe. IEE Proc. H Microw. Antennas Propag. 1987, 134, 341–349. [Google Scholar] [CrossRef]
- Piuzzi, E.; Merla, C.; Cannazza, G.; Zambotti, A.; Apollonio, F.; Cataldo, A.; D’Atanasio, P.; De Benedetto, E.; Liberti, M. A Comparative Analysis between Customized and Commercial Systems for Complex Permittivity Measurements on Liquid Samples at Microwave Frequencies. IEEE Trans. Instrum. Meas. 2013, 62, 1034–1046. [Google Scholar] [CrossRef]
- Packard, H. Automating the HP 8410B Microwave Network Analyzer. Appl. Note 1980, 221, 1–25. [Google Scholar]
- Bobowski, J.S.; Johnson, T. Permittivity Measurements of Biological Samples by an Open-Ended Coaxial Line. Prog. Electromagn. Res. 2012, 40, 159–183. [Google Scholar] [CrossRef]
- Peyman, A.; Holden, S.J.; Watts, S.; Perrott, R.; Gabriel, C. Dielectric Properties of Porcine Cerebrospinal Tissues at Microwave Frequencies: In Vivo, in Vitro and Systematic Variation with Age. Phys. Med. Biol. 2007, 52, 2229–2245. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H. Transmission Line Calculator. Electronics 1939, 12, 29–31. [Google Scholar]
- Kaatze, U. Complex Permittivity of Water as a Function of Frequency and Temperature. J. Chem. Eng. Data 1989, 34, 371–374. [Google Scholar] [CrossRef]
- Anderson, J.M.; Sibbald, C.L.; Stuchly, S.S. Dielectric Measurements Using a Rational Function Model. IEEE Trans. Microw. Theory Tech. 1994, 42, 199–204. [Google Scholar] [CrossRef]
- De Langhe, P.; Blomme, K.; Martens, L.; De Zutter, D. Measurement of Low-Permittivity Materials Based on a Spectral-Domain Analysis for the Open-Ended Coaxial Probe. IEEE Trans. Instrum. Meas. 1993, 42, 879–886. [Google Scholar] [CrossRef]
- Peyman, A.; Gabriel, C.; Grant, E.H.; Vermeeren, G.; Martens, L. Variation of the Dielectric Properties of Tissues with Age: The Effect on the Values of SAR in Children When Exposed to Walkie-Talkie Devices. Phys. Med. Biol. 2009, 54, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.; Porter, E.; Meaney, P.M.; O’Halloran, M. Effect of Logarithmic and Linear Frequency Scales on Parametric Modelling of Tissue Dielectric Data. Biomed. Phys. Eng. Express 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kraszewski, A.; Stuchly, M.A.; Stuchly, S.S. ANA Calibration Method for Measurements of Dielectric Properties. IEEE Trans. Instrum. Meas. 1983, 32, 385–387. [Google Scholar] [CrossRef]
- Buchner, R.; Hefter, G.T.; May, M.P. Dielectric Relaxation of Aqueous NaCl Solutions. J. Phys. Chem. 1999, 103, 1–9. [Google Scholar] [CrossRef]
- Wei, Y.Z.; Sridhar, S. Radiation-Corrected Open-Ended Coax Line Technique for Dielectric Measurements of Liquids up to 20 GHZ. IEEE Trans. Microw. Theory Tech. 1991, 39, 526–531. [Google Scholar] [CrossRef]
- Gregory, A.P.; Clarke, R.N. Tables of the Complex Permittivity of Dielectric Reference Liquids at Frequencies up to 5 GHz; NPL Report MAT 23; National Physical Laboratory: Teddington, UK, 2012. [Google Scholar]
- Peyman, A.; Gabriel, C.; Grant, E.H. Complex Permittivity of Sodium Chloride Solutions at Microwave Frequencies. Bioelectromagnetics 2007, 28, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.P.; Sheppard, R.J.; Szwarnowski, S. The Dielectric Properties of Formamide, Ethanediol and Methanol. J. Phys. D Appl. Phys. 1978, 11, 695–701. [Google Scholar] [CrossRef]
- Barthel, J.; Buchner, R. High Frequency Permittivity and Its Use in the Investigation of Solution Properties. Pure Appl. Chem. 1991, 63, 1473–1482. [Google Scholar] [CrossRef]
- Stogryn, A. Equations for Calculating the Dielectric Constant of Saline Water. IEEE Trans. Microw. Theory Tech. 1971, 19, 733–736. [Google Scholar] [CrossRef]
- Nortemann, K.; Hilland, J.; Kaatze, U. Dielectric Properties of Aqueous NaCl Solutions at Microwave Frequencies. J. Phys. Chem. A 1997, 101, 6864–6869. [Google Scholar] [CrossRef]
- Lamkaouchi, K.; Balana, A.; Delbos, G.; Ellison, W.J. Permittivity Measurements of Lossy Liquids in the Range 26-110 GHz. Meas. Sci. Technol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Kaatze, U.; Pottel, R.; Schaefer, M. Dielectric Spectrum of Dimethyl Sulfoxide/Water Mixtures as a Function of Composition. J. Phys. Chem. 1989, 93, 5623–5627. [Google Scholar] [CrossRef]
- Vij, J.K.; Grochulski, T.; Kocot, A.; Hufnagel, F. Complex Permittivity Measurements of Acetone in the Frequency Region 50–310 GHz. Mol. Phys. 1991, 72, 353–361. [Google Scholar] [CrossRef]
- Gregory, A.P.; Clarke, R.N. Dielectric Metrology with Coaxial Sensors. Meas. Sci. Technol. 2007, 18, 1372–1386. [Google Scholar] [CrossRef]
- Peyman, A.; Rezazadeh, A.; Gabriel, C. Changes in the Dielectric Properties of Rat Tissue as a Function of Age at Microwave Frequencies. Phys. Med. Biol. 2001, 46, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, K.; Ji, Z. Bilayered Dielectric Measurement With an Open-Ended Coaxial Probe. IEEE Trans. Microw. Theory Tech. 1994, 42, 966–971. [Google Scholar] [CrossRef]
- Huclova, S.; Baumann, D.; Talary, M.; Fröhlich, J. Sensitivity and Specificity Analysis of Fringing-Field Dielectric Spectroscopy Applied to a Multi-Layer System Modelling the Human Skin. Phys. Med. Biol. 2011, 56, 7777–7793. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Golnabi, A.; Fanning, M.W.; Geimer, S.D.; Paulsen, K.D. Dielectric Volume Measurements for Biomedical Applications. In Proceedings of the 13th International Symposium on Antenna Technology and Applied Electromagnetics and the Canadian Radio Sciences Meeting, Toronto, ON, Canada, 15–18 February 2009. [Google Scholar]
- Johnson, C.C.; Guy, A.W. Nonionizing Electromagnetic Wave Effects in Biological Materials and Systems. Proc. IEEE 1972, 60, 692–718. [Google Scholar] [CrossRef]
- Shahzad, A.; Sonja, K.; Jones, M.; Dwyer, R.M.; O’Halloran, M. Investigation of the Effect of Dehydration on Tissue Dielectric Properties in Ex Vivo Measurements. Biomed. Phys. Eng. Express 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Farrugia, L.; Wismayer, P.S.; Mangion, L.Z.; Sammut, C.V. Accurate in Vivo Dielectric Properties of Liver from 500 MHz to 40 GHz and Their Correlation to Ex Vivo Measurements. Electromagn. Biol. Med. 2016, 8378, 1–9. [Google Scholar] [CrossRef]
- Nopp, P.; Rapp, E.; Pfützner, H.; Nakesch, H.; Ruhsam, C. Dielectric Properties of Lung Tissue as a Function of Air Content. Phys. Med. Biol. 1993, 38, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Peyman, A.; Grant, E.H. Electrical Conductivity of Tissue at Frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863–4878. [Google Scholar] [CrossRef] [PubMed]
- Haemmerich, D.; Ozkan, R.; Tungjitkusolmun, S.; Tsai, J.Z.; Mahvi, D.; Staelin, S.T.; Webster, J.G. Changes in Electrical Resistivity of Swine Liver after Occlusion and Postmortem. Med. Biol. Eng. Comput. 2002, 40, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.B.; Bement, S.L. The Specific Impedance of the Dorsal Columns of Cat: An Anisotropic Medium. Exp. Neurol. 1965, 11, 451–463. [Google Scholar] [CrossRef]
- Hart, F.X.; Dunfee, W.R. In Vivo Measurement of the Low-Frequency Dielectric Spectra of Frog Skeletal Muscle. Phys. Med. Biol. 1993, 38, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Lopresto, V.; Pinto, R.; Farina, L.; Cavagnaro, M. Treatment Planning in Microwave Thermal Ablation: Clinical Gaps and Recent Research Advances. Int. J. Hyperth. 2017, 33, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Woodford, P.; O’Dowd, G. Wheater’s Functional Histology: A Text and Colour Atlas, 6th ed.; Elsevier Health Sciences: London, UK, 2013. [Google Scholar]
- Cross, S.S. Grading and Scoring in Histopathology. Histopathology 1998, 33, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Veta, M.; Pluim, J.P.W.; Van Diest, P.J.; Viergever, M.A. Breast Cancer Histopathology Image Analysis: A Review. IEEE Trans. Biomed. Eng. 2014, 61, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- National Health Service (NHS). Pathology; National Health Service (NHS): London, UK, 2016. [Google Scholar]
- Verkooijen, H.M.; Peterse, J.L.; Schipper, M.E.I.; Buskens, E.; Hendriks, J.H.C.L.; Pijnappel, R.M.; Peeters, P.H.M.; Borel Rinkes, I.H.M.; Mali, W.P.T.M.; Holland, R. Interobserver Variability between General and Expert Pathologists during the Histopathological Assessment of Large-Core Needle and Open Biopsies of Non-Palpable Breast Lesions. Eur. J. Cancer 2003, 39, 2187–2191. [Google Scholar] [CrossRef]
- Gomes, D.S.; Porto, S.S.; Balabram, D.; Gobbi, H. Inter-Observer Variability between General Pathologists and a Specialist in Breast Pathology in the Diagnosis of Lobular Neoplasia, Columnar Cell Lesions, Atypical Ductal Hyperplasia and Ductal Carcinoma in Situ of the Breast. Diagn. Pathol. 2014, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.C.; Schiffman, M.; Hunt, W.C.; Joste, N.; Ghosh, A.; Wentzensen, N.; Wheeler, C.M. Cervical Histopathology Variability among Laboratories: A Population-Based Statewide Investigation. Am. J. Clin. Pathol. 2013, 139, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, D.A.G. Berechnung Verschiedener Physikalischer Konstanten von Heterogenen Substanzen. 1. Dielektizitatskonstanten Und Leitfahigkeiten Der Mischkorper Aus Isotropen Substanzen. Ann. Phys. 1935, 24, 636–679. [Google Scholar] [CrossRef]



| Recent Works | Probe | Frequency [GHz] | Tissue Type | Sample Size | Relative Permittivity Range | Conductivity Range [S/m] |
|---|---|---|---|---|---|---|
| Halter et al. (2009) [10] | Slim form with 2.2 mm diameter (in vivo) | 0.1–8.5 | Ex vivo and in vivo | 5 mm thick | In vivo breast tissue: 95–45 | In vivo breast tissue: 0.1–10 |
| High temperature with 19 mm flange (ex vivo) | Breast tumour (human) | Ex vivo breast tissue: 50–35 | Ex vivo breast tissue: 0.1–8 | |||
| Karacolak et al. (2012) [116] | High temperature with 19 mm flange | 0.3–3 | Ex vivo skin (porcine) | 45 × 45 × 4 mm3 | 50–36 | 0.4–2.2 |
| Lopresto et al. (2012) [29] | Slim form with 2.2 mm diameter | 2.45 | Ex vivo liver tissue (bovine) | 20 × 20 × 50 mm3 | 44.98–26.11 (temperature incremented from 15 °C to 98.9 °C, then decremented to 39.6 °C) | 1.79–1.19 (temperature incremented from 15 °C to 98.9 °C, then decremented to 39.6 °C) |
| Sabouni et al. (2013) [86] | Performance with 9.5 mm diameter | 0.5–20 | Ex vivo breast tissue (human) | N/A | Breast tissue: 63–35 | Breast tissue: 0.2–32 |
| Fibroglandular breast tissue: 40–20 | Fibroglandular breast tissue: 0.2–16.3 | |||||
| Abdilla et al. (2013) [44] | Slim form with 2.2 mm diameter | 0.5–50 | Ex vivo muscle and liver (bovine, porcine) | 60 × 60 × 40 mm3 | Muscle tissue: 58–18 Liver tissue: 51–15 | N/A(Loss factor for muscle/liver tissue: 32–10) |
| Sugitani et al. (2014) [12] | Slim form with 2.2 mm diameter | 0.5–20 | Ex vivo breast tumour (human) | 50–300 mm diameter | Breast tumour tissue: 65–22 | Breast tumour tissue: 0.1–25 |
| Breast fibroglandular tissue: 40–18 | Breast fibroglandular tissue: 0.1–12 | |||||
| Breast fat tissue: 12–6 | Breast fat tissue: 0.1–3 | |||||
| Peyman et al. (2015) [84] | Slim form with 2.2 mm diameter | 0.1–5 | Ex vivo liver tissue (human) | 20 mm thick | Liver normal tissue: 68–43 Liver tumour tissue: 68–32 | Liver normal/tumour tissue: 0.7–5 |
| Martellosio et al. (2017) [79] | Slim form with 2.2 mm diameter | 0.5–50 | Ex vivo breast tumour (human) | 6 mm thick volume between 700 mm3 and 1500 mm3 | Breast normal tissue: 64–3 Breast tumour tissue: 69–9 | N/A (Breast normal tissue imaginary part: 41–0.1; Breast tumour tissue imaginary part: 45–4 |
| Calibration Steps | Error or Confounder | Action for Correction or Compensation |
|---|---|---|
| Equipment set-up |
| |
 Open |
|
|
 Short |
| |
 Load |
| Liquid | Models | Storage and Handling |
|---|---|---|
| Methanol (alcohol with intermediate permittivity values similar to breast tissue) | Debye model [132]:
| Inflammable and acute inhalation toxicity. Fire-proof storage cabinets required. Handling in fumehood required. |
Cole-Cole model [134]:
| Rapid evaporation may occur and should be avoided. | |
| Ethanediol (alcohol with high permittivity values similar to breast glandular tissue) | Cole-Davidson model [132]:
| Inflammable and acute inhalation toxicity. Fire-proof storage cabinets required. Handling in fumehood required. Ethanediol is hygroscopic and when it evaporates the liquid temperature increases, causing an increase in relative permittivity [132]. |
| Ethanol (alcohol with intermediate permittivity values similar to breast tissue) | Debye-Γ model [132]:
| Inflammable and acute inhalation toxicity. Fire-proof storage cabinets required. Handling in fumehood required. |
| Butanol (alcohol with low permittivity values similar to fat tissue) | Double Debye model [132]:
| Inflammable and acute inhalation toxicity. Fire-proof storage cabinets required. Handling in fumehood required. |
| Saline (NaCl) (polar liquid having dielectric properties similar to biological tissues) | Cole-Cole model [133]:
| Storage in sealed containers. No special handling required. |
Cole-Davidson model [49]:
| ||
| Formamide (polar organic solvent having wide permittivity spectrum at microwave frequencies) | Cole-Davidson model [135]:
| Toxic through inhalation, oral, or skin exposure. Fire-proof storage cabinets required. Handling in fumehood required. |
| DI water (polar liquid having well-known modelled properties) | Debye model [124]:
| Storage in sealed containers. No special handling required. |
| Dimethyl sulphoxide (DMSO) (highly polar organic reagent having high permittivity) | Debye model [132]:
| DMSO is exceptionally hygroscopic and needs to be measured as soon as the container is opened [132]. |
| Acetone (polar organic solvent having intermediate permittivity values) | Static permittivity (since acetone has very high relaxation frequency) [132]:
| Acetone boiling point is at 56 °C [132]. |
Budo model/confined rotator models [140]:
| Special handling is required, since it is a powerful liquid able to soften some plastics [94] and it is inflammable. |
| Case Scenarios | μT | μTFE | μA | μ |
|---|---|---|---|---|
| Known TFE, Known age, Unknown T (between 18 °C and 25 °C) | 0.91% | N/A | N/A | 0.91% |
| Known T, Known age, Unknown TFE (within 3.5 h) | N/A | 25% | N/A | 25% |
| Known T, Known TFE, Unknown age (within 70 days old) | N/A | N/A | 15% | 15% |
| Known T, Unknown TFE (within 3.5 h), Unknown age (within 70 days old) | N/A | 25% | 15% | 29.15% |
| Known TFE, Unknown age (within 70 days old), Unknown T (between 18 °C and 25 °C) | 0.91% | N/A | 15% | 15.02% |
| Unknown TFE (within 3.5 h), Unknown age (within 70 days old), Unknown T (between 18 °C and 25 °C) | 0.91% | 25% | 15% | 29.17% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. https://doi.org/10.3390/diagnostics8020040
La Gioia A, Porter E, Merunka I, Shahzad A, Salahuddin S, Jones M, O’Halloran M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics. 2018; 8(2):40. https://doi.org/10.3390/diagnostics8020040
Chicago/Turabian StyleLa Gioia, Alessandra, Emily Porter, Ilja Merunka, Atif Shahzad, Saqib Salahuddin, Marggie Jones, and Martin O’Halloran. 2018. "Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices" Diagnostics 8, no. 2: 40. https://doi.org/10.3390/diagnostics8020040
APA StyleLa Gioia, A., Porter, E., Merunka, I., Shahzad, A., Salahuddin, S., Jones, M., & O’Halloran, M. (2018). Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics, 8(2), 40. https://doi.org/10.3390/diagnostics8020040