Codex (Cognitive Disorders Examination) Decision Tree Modified for the Detection of Dementia and MCI
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belmin, J.; Pariel-Madjlessi, S.; Surun, P.; Bentot, C.; Feteanu, D.; Lefebvre des Noettes, V.; Onen, F.; Drunat, O.; Trivalle, C.; Chassagne, P.; et al. The cognitive disorders examination (Codex) is a reliable 3-minute test for detection of dementia in the elderly (validation study in 323 subjects). Presse Med. 2007, 36, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Belmin, J.; Oasi, C.; Folio, P.; Pariel-Madjlessi, S. Codex, un test ultra-rapide pour le repérage des démences chez les sujets âgés. Revue Geriatr. 2007, 32, 627–631. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Larner, A.J. Codex (cognitive disorders examination) for the detection of dementia and mild cognitive impairment. Codex pour la détection de la démence et du mild cognitive impairment. Presse Med. 2013, 42, e425–e428. [Google Scholar] [CrossRef] [PubMed]
- Ziso, B.; Larner, A.J. Codex (cognitive disorders examination) for the detection of dementia and mild cognitive impairment: Diagnostic utility. J. Neurol. Neurosurg. Psychiatry 2013, 84, e2. [Google Scholar] [CrossRef]
- Larner, A.J. Dementia in Clinical Practice: A Neurological Perspective. Pragmatic Studies in the Cognitive Function Clinic, 3rd ed.; Springer: London, UK, 2018; pp. 89–90. [Google Scholar]
- Avgerinou, C.; Koufogianni, K.; Solini-Kosti, E.; Belmin, J. Validation of the Greek translation of the Cognitive Disorders Examination (Codex) for the detection of dementia in primary care. Ann. Hellenic Med. 2017, 34, 334–342. [Google Scholar]
- Mézière, A.; Paillaud, E.; Belmin, J.; Pariel, S.; Herbaud, S.; Canouï-Poitrine, F.; Le Thuautd, A.; Martyce, J.; Plaud, B. Delirium in older people after proximal femoral fracture repair: role of a preoperative screening cognitive test. Ann. Fr. Anesth. Reanim. 2013, 32, e91–e96. [Google Scholar] [CrossRef]
- Ambert-Dahan, E.; Routier, S.; Marot, L.; Bouccara, D.; Sterkers, O.; Ferrary, E.; Mosnier, I. Cognitive evaluation of cochlear implanted adults using CODEX and MoCA screening tests. Otol. Neurotol. 2017, 38, e282–e284. [Google Scholar] [CrossRef] [PubMed]
- Vannier-Nitenberg, C.; Dauphinot, V.; Bongue, B.; Sass, C.; Rouch, I.; Beuachet, O.; Krolak-Salmon, P.; Fantino, B. Early detection of memory impairment in people over 65 years old consulting at Health Examination Centers for the French health insurance: the EVATEM protocol. BMC Geriatr. 2013, 13, 55. [Google Scholar] [CrossRef]
- Vannier-Nitenberg, C.; Dauphinot, V.; Bongue, B.; Sass, C.; Bathsavanis, A.; Rouch, I.; Deville, N.; Beauchet, O.; Krolak-Salmon, P.; Fantino, B. Performance of cognitive tests, individually and combined, for the detection of cognitive disorders amongst community-dwelling elderly people with memory complaints: The EVATEM study. Eur. J. Neurol. 2016, 23, 554–561. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Larner, A.J. Comparing diagnostic accuracy of cognitive screening instruments: A weighted comparison approach. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. MACE versus MoCA: equivalence or superiority? Pragmatic diagnostic test accuracy study. Int. Psychogeriatr. 2017, 29, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Free-Cog: Pragmatic test accuracy study. Dement. Geriatr. Cogn. Disord. 2019, 48. accepted. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Moher, D.; Rennie, D.; de Vet, H.C.W.; Lijmer, J.G. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin. Chem. 2003, 49, 7–18. [Google Scholar] [CrossRef]
- Noel-Storr, A.H.; McCleery, J.M.; Richard, E.; Ritchie, C.W.; Flicker, L.; Cullum, S.J.; Davis, D.; Quinn, T.J.; Hyde, C.; Rutjes, A.W.; et al. Reporting standards for studies of diagnostic test accuracy in dementia: The STARDdem Initiative. Neurology 2014, 83, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Evaluating cognitive screening instruments with the “likelihood to be diagnosed or misdiagnosed” measure. Int. J. Clin. Pract. 2019, 73, e13265. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Kalbe, E.; Kessler, J. DemTect. In Cognitive Screening Instruments. A Practical Approach, 2nd ed.; Larner, A.J., Ed.; Springer: London, UK, 2017; pp. 197–208. [Google Scholar]
- Pellegrini, E.; Ballerini, L.; Hernandez, M.D.C.V.; Chappell, F.M.; Gonzalez-Castro, V.; Anblagan, D.; Danso, S.; Muñoz-Maniega, S.; Job, D.; Pernet, C.; et al. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: A systematic review. Alzheimers Dement. (Amst) 2018, 10, 519–535. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Tang, H.; Wei, C.Y.; Zhang, C.; Hung, G.U.; Zhou, W. NMD-12: A new machine-learning derived screening instrument to detect mild cognitive impairment and dementia. PLoS ONE 2019, 14, e0213430. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Performance-based cognitive screening instruments: an extended analysis of the time versus accuracy trade-off. Diagnostics (Basel) 2015, 5, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; McGrory, S.; Leslie, F.; Dawson, K.; Ahmed, S.; Butler, C.R.; Rowe, J.B.; Mioshi, E.; Hodges, J.R. The Mini-Addenbrooke’s Cognitive Examination: A new assessment tool for dementia. Dement. Geriatr. Cogn. Disord. 2015, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Gao, Y.; McGlade, C.; Henry, L.; Gallagher, P.; Timmons, S.; Molloy, D.W. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 2012, 41, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
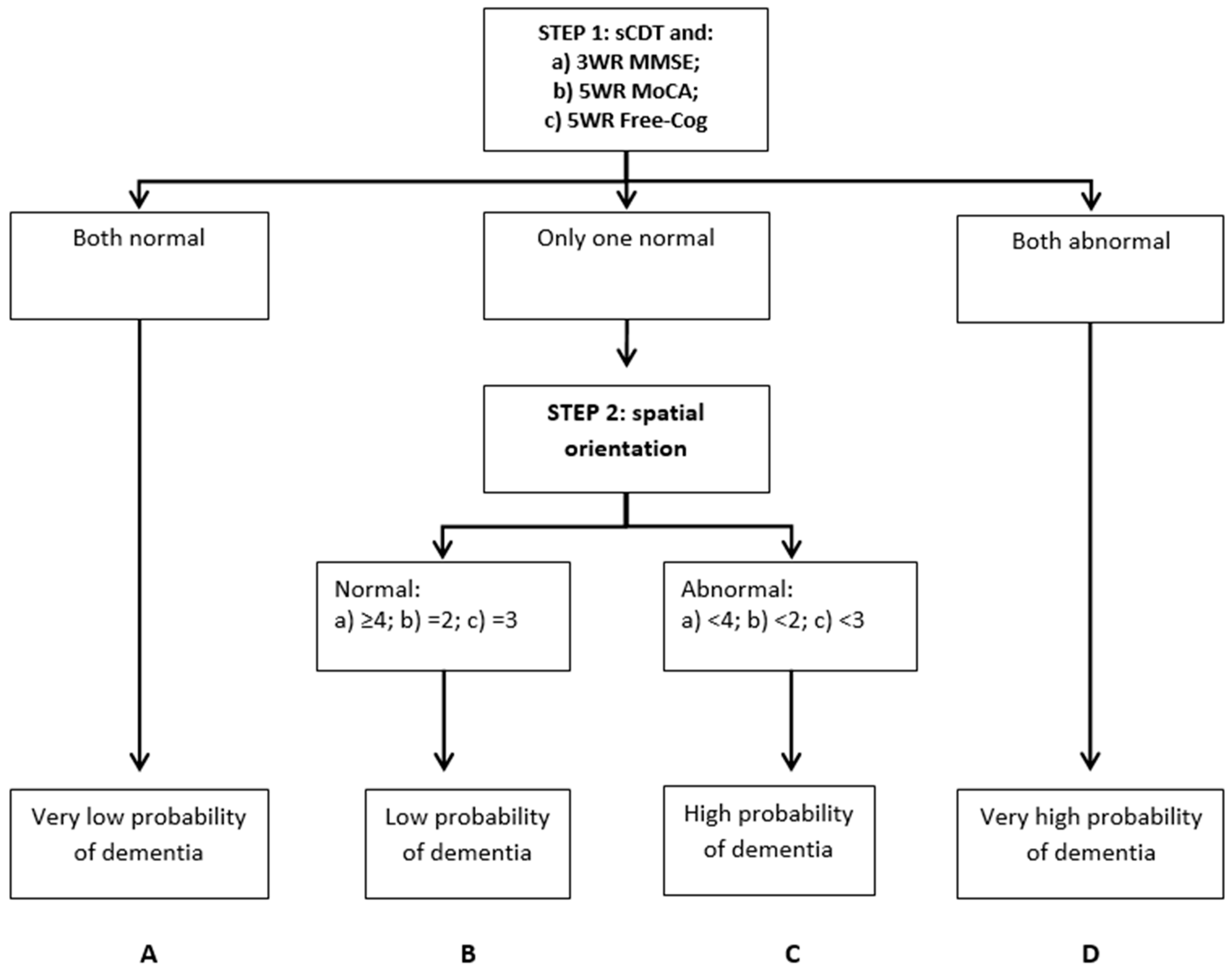
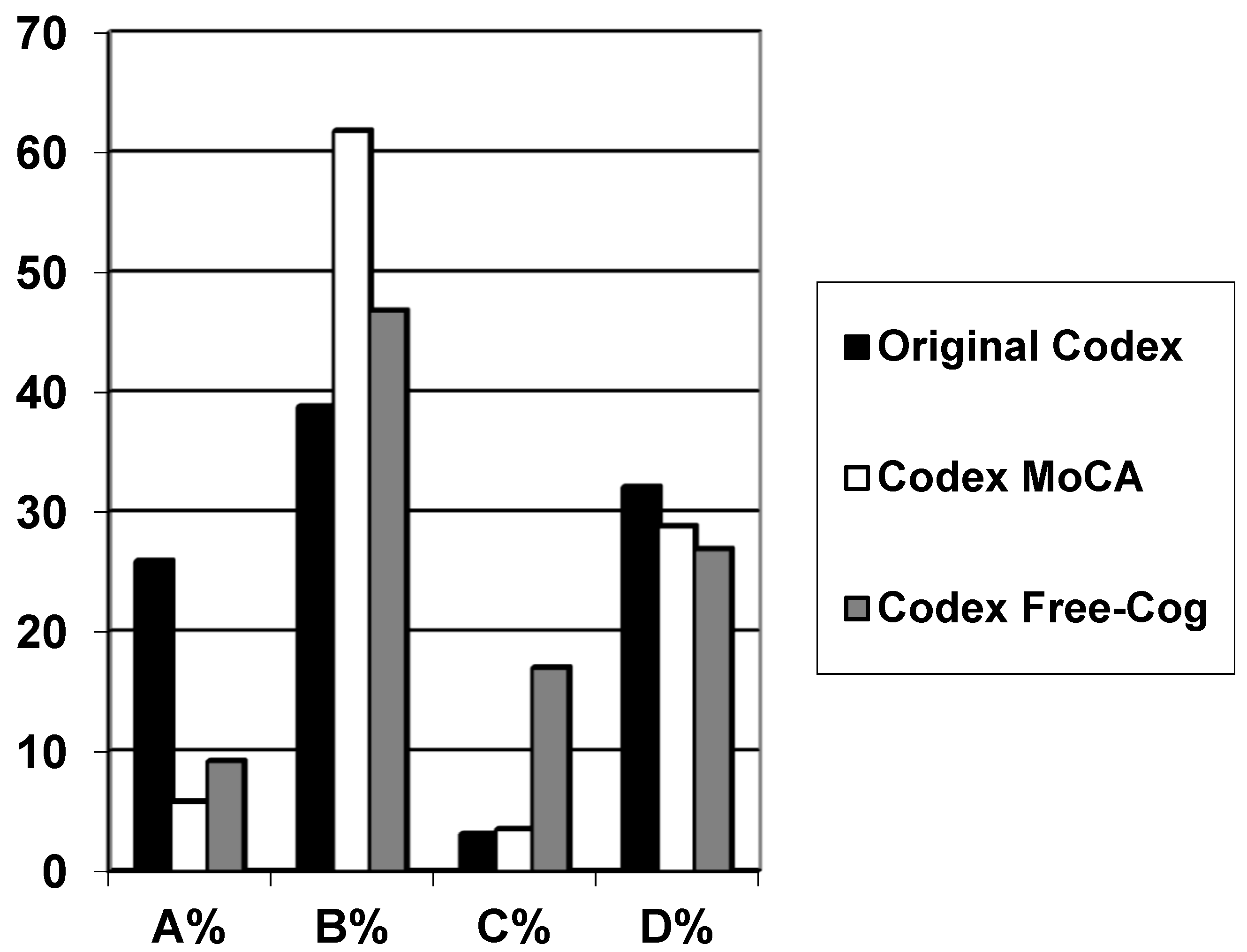
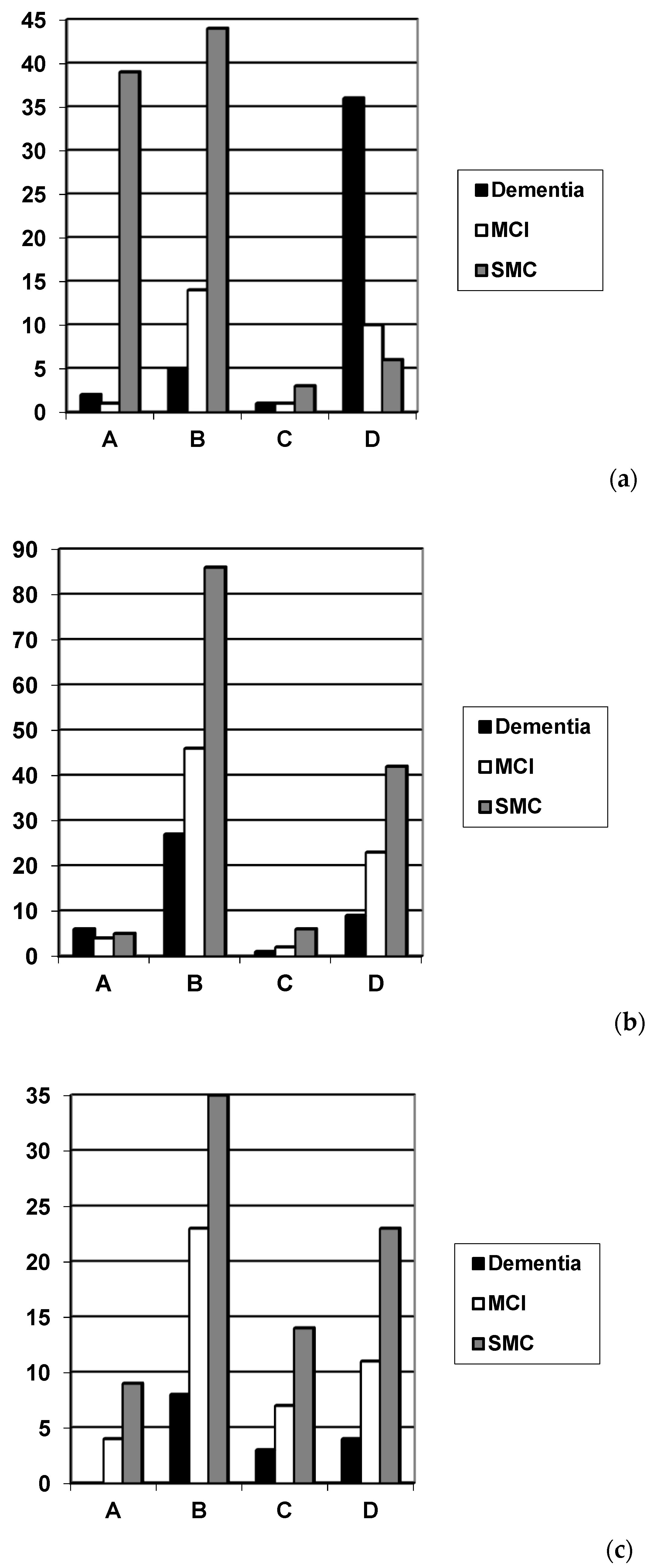
| Codex | N | Gender F:M (% Female) | Age Range (Median) | Diagnosis Dementia/MCI/SMC (%) | Codex Category | |||
|---|---|---|---|---|---|---|---|---|
| A (%) | B (%) | C (%) | D (%) | |||||
| Original | 162 | 79:83 (49) | 20–89 (61) | 44/26/92 (27/16/57) | 42 (25.9) | 63 (38.8) | 5 (3.1) | 52 (32.1) |
| Modified from MoCA | 257 | 116:141 (45) | 22–89 (59) | 43/75/139 (17/29/54) | 15 (5.8) | 159 (61.8) | 9 (3.5) | 74 (28.8) |
| Modified from Free-Cog | 141 | 61:80 (43) | 28–88 (62) | 15/45/81 (11/32/57) | 13 (9.2) | 66 (46.8) | 24 (17.0) | 38 (26.9) |
| Diagnosis of Dementia vs. No Dementia (MCI + SMC) | Diagnosis of Dementia vs. MCI | Diagnosis of MCI vs. No Cognitive Impairment (SMC) | |
|---|---|---|---|
| N | 162 (44 vs. 118) | 70 (44 vs. 26) | 118 (26 vs. 92) |
| Prevalence (P = pre-test probability) | Dementia 0.27 | Dementia 0.63 | MCI 0.22 |
| Pre-test odds (= P/1 − P) | Dementia 0.37 | Dementia 1.69 | MCI 0.28 |
| Sensitivity (Se) | 0.84 (0.73–0.95) | 0.84 (0.73–0.95) | 0.42 (0.23–0.61) |
| Specificity (Sp) | 0.83 (0.76–0.90) | 0.58 (0.39–0.77) | 0.90 (0.84–0.96) |
| Y | 0.67 | 0.42 | 0.32 |
| PPV (= post-test probability) | 0.65 (0.53–0.77) | 0.77 (0.65–0.89) | 0.55 (0.33–0.77) |
| NPV | 0.93 (0.89–0.98) | 0.68 (0.49–0.88) | 0.85 (0.78–0.92) |
| PSI | 0.58 | 0.45 | 0.40 |
| Accuracy (Acc) | 0.83 (0.78–0.89) | 0.74 (0.64–0.85) | 0.80 (0.72–0.87) |
| Net Reclassification Improvement (NRI = Acc − P) | 0.56 | 0.11 | 0.58 |
| LDM (= NNM/NND, NNM/NNP) | 4.02, 3.48 | 1.63, 1.75 | 1.57, 1.97 |
| LR+ | 4.96 (3.29–7.49) = moderate | 1.99 (1.25–3.17) = slight | 4.32 (2.01–9.30) = moderate |
| LR− | 0.19 (0.13–0.29) = large | 0.28 (0.17–0.44) = moderate | 0.64 (0.30–1.38) = slight |
| DOR | 25.9 (17.2–39.1) | 7.21 (4.52–11.5) | 6.76 (3.14–14.5) |
| Post-test odds (= pre-test odds × LR+) | Dementia 1.85 | Dementia 3.36 | MCI 1.21 |
| CUI+ (= Se × PPV) | 0.55 = adequate | 0.65 = good | 0.23 = very poor |
| CUI− (= Sp × NPV) | 0.78 = good | 0.39 = poor | 0.76 = good |
| Diagnosis of Dementia vs. No Dementia (MCI + SMC) | Diagnosis of Dementia vs. MCI | Diagnosis of MCI vs. No Cognitive Impairment (SMC) | |
|---|---|---|---|
| N | 257 (43 vs. 214) | 118 (43 vs. 75) | 214 (75 vs. 139) |
| Prevalence (P = pre-test probability) | Dementia 0.17 | Dementia 0.36 | MCI 0.35 |
| Pre-test odds (= P/1 − P) | Dementia 0.20 | Dementia 0.57 | MCI 0.54 |
| Sensitivity (Se) | 0.23 (0.11–0.36) | 0.23 (0.11–0.36) | 0.33 (0.23–0.44) |
| Specificity (Sp) | 0.66 (0.60–0.72) | 0.67 (0.56–0.77) | 0.65 (0.58–0.73) |
| Y | −0.11 | −0.10 | −0.02 |
| PPV (= post-test probability) | 0.12 (0.05–0.19) | 0.29 (0.14–0.44) | 0.34 (0.23–0.45) |
| NPV | 0.81 (0.75–0.87) | 0.60 (0.50–0.71) | 0.65 (0.57–0.72) |
| PSI | −0.07 | −0.11 | −0.01 |
| Accuracy (Acc) | 0.59 (0.53–0.65) | 0.51 (0.42–0.60) | 0.54 (0.48–0.61) |
| Net Reclassification Improvement (NRI = Acc − P) | 0.42 | 0.15 | 0.19 |
| LDM (= NNM/NND, NNM/NNP) | −0.27, −0.17 | −0.20, −0.22 | −0.04, −0.02 |
| LR+ | 0.68 (0.38–1.21) = slight | 0.70 (0.37–1.31) = slight | 0.97 (0.65–1.43) = slight |
| LR− | 1.16 (0.66–2.07) = slight | 1.15 (0.61–2.16) = slight | 1.02 (0.69–1.51) = slight |
| DOR | 0.59 (0.33–1.04) | 0.61 (0.32–1.14) | 0.95 (0.64–1.40) |
| Post-test odds (= pre-test odds × LR+) | Dementia 0.14 | Dementia 0.40 | MCI 0.52 |
| CUI+ (= Se × PPV) | 0.03 = very poor | 0.07 = very poor | 0.11 = very poor |
| CUI− (= Sp × NPV) | 0.53 = adequate | 0.40 = poor | 0.42 = poor |
| Diagnosis of Dementia vs. No Dementia (MCI + SMC) | Diagnosis of Dementia vs. MCI | Diagnosis of MCI vs. No Cognitive Impairment (SMC) | |
|---|---|---|---|
| N | 141 (15 vs. 126) | 60 (15 vs. 45) | 126 (45 vs. 81) |
| Prevalence (P = pre-test probability) | Dementia 0.11 | Dementia 0.25 | MCI 0.36 |
| Pre-test odds (= P/1 − P) | Dementia 0.12 | Dementia 0.33 | MCI 0.56 |
| Sensitivity (Se) | 0.47 (0.21–0.72) | 0.47 (0.21–0.72) | 0.40 (0.26–0.54) |
| Specificity (Sp) | 0.56 (0.48–0.65) | 0.60 (0.47–0.74) | 0.54 (0.43–0.65) |
| Y | 0.03 | 0.07 | −0.06 |
| PPV (= post-test probability) | 0.11 (0.03–0.19) | 0.28 (0.10–0.46) | 0.33 (0.20–0.45) |
| NPV | 0.90 (0.83–0.97) | 0.77 (0.63–0.91) | 0.62 (0.51–0.73) |
| PSI | 0.01 | 0.05 | −0.05 |
| Accuracy (Acc) | 0.55 (0.47–0.64) | 0.57 (0.44–0.69) | 0.49 (0.40–0.58) |
| Net Reclassification Improvement (NRI = Acc − P) | 0.44 | 0.32 | 0.13 |
| LDM (= NNM/NND, NNM/NNP) | 0.07, 0.02 | 0.63, 0.12 | −0.12, −0.10 |
| LR+ | 1.07 (0.60–1.91) = slight | 1.17 (0.61–2.23) = slight | 0.88 (0.57–1.35) = slight |
| LR− | 0.95 (0.53–1.68) = slight | 0.89 (0.46–1.70) = slight | 1.10 (0.72–1.70) = slight |
| DOR | 1.13 (0.63–2.01) | 1.31 (0.69–2.51) | 0.79 (0.52–1.22) |
| Post-test odds (= pre-test odds × LR+) | Dementia 0.13 | Dementia 0.38 | MCI 0.49 |
| CUI+ (= Se × PPV) | 0.05 = very poor | 0.13 = very poor | 0.13 = very poor |
| CUI− (= Sp × NPV) | 0.51 = adequate | 0.46 = poor | 0.34 = very poor |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziso, B.; Larner, A.J. Codex (Cognitive Disorders Examination) Decision Tree Modified for the Detection of Dementia and MCI. Diagnostics 2019, 9, 58. https://doi.org/10.3390/diagnostics9020058
Ziso B, Larner AJ. Codex (Cognitive Disorders Examination) Decision Tree Modified for the Detection of Dementia and MCI. Diagnostics. 2019; 9(2):58. https://doi.org/10.3390/diagnostics9020058
Chicago/Turabian StyleZiso, Besa, and Andrew J. Larner. 2019. "Codex (Cognitive Disorders Examination) Decision Tree Modified for the Detection of Dementia and MCI" Diagnostics 9, no. 2: 58. https://doi.org/10.3390/diagnostics9020058
APA StyleZiso, B., & Larner, A. J. (2019). Codex (Cognitive Disorders Examination) Decision Tree Modified for the Detection of Dementia and MCI. Diagnostics, 9(2), 58. https://doi.org/10.3390/diagnostics9020058




