Diagnosis of Conversion Disorder Using Diffusion Tensor Tractography and Transcranial Magnetic Stimulation in a Patient with Mild Traumatic Brain Injury
Abstract
:1. Introduction
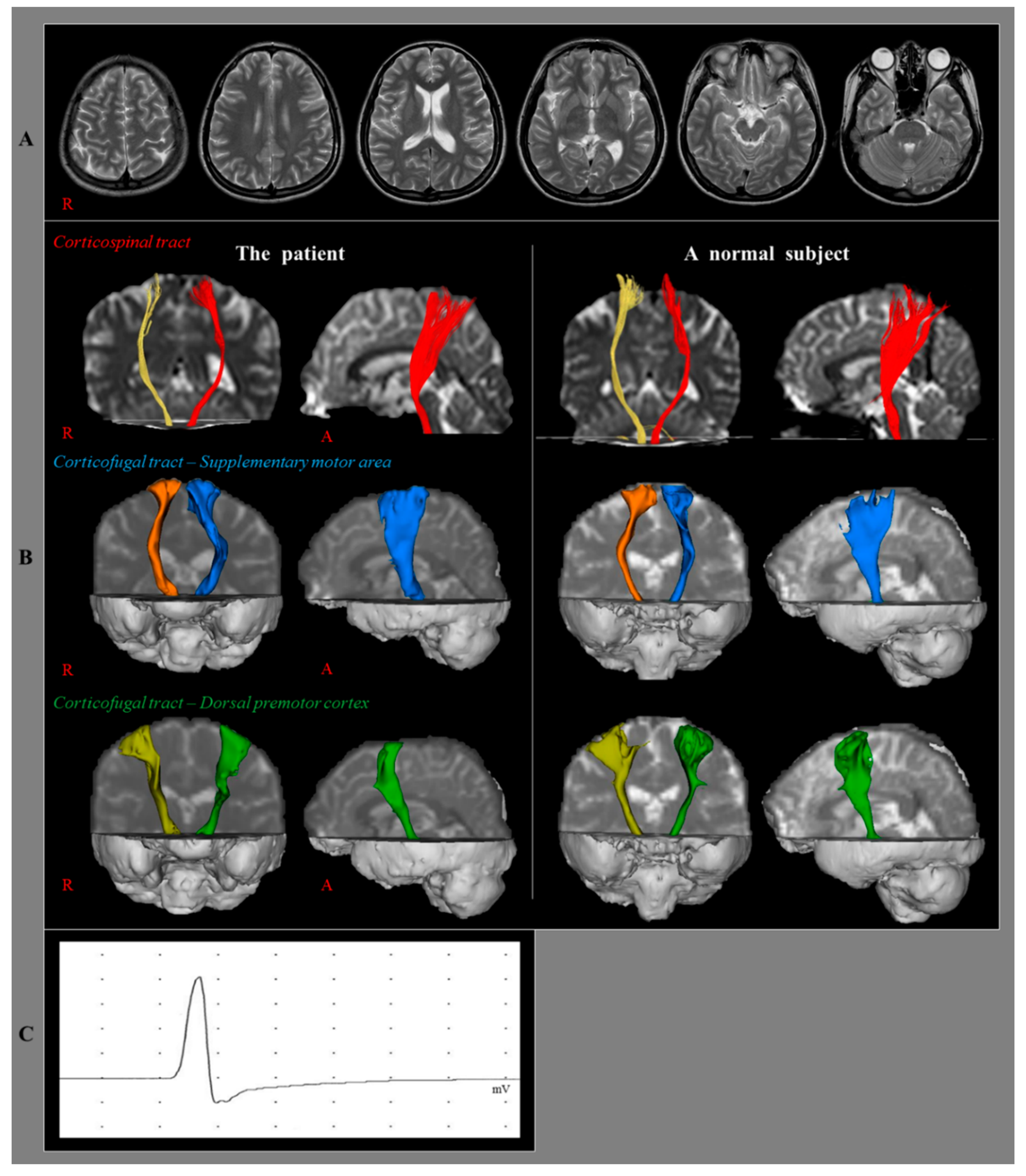
2. Case Report
3. Diffusion Tensor Imaging
4. Transcranial Magnetic Stimulation
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Krem, M.M. Motor conversion disorders reviewed from a neuropsychiatric perspective. J. Clin. Psychiatry 2004, 65, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lanska, D.J. Functional weakness and sensory loss. Semin. Neurol. 2006, 26, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Carson, A.; Sharpe, M. Functional symptoms and signs in neurology: Assessment and diagnosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, i2–i12. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Choi, B.Y.; Chang, C.H.; Kim, S.H.; Lee, J.; Chang, M.C.; Son, S.M.; Jang, S.H. The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation 2012, 31, 207–213. [Google Scholar] [PubMed]
- Jang, S.H.; Kim, K.; Kim, S.H.; Son, S.M.; Jang, W.H.; Kwon, H.G. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci. Lett 2014, 572, 1–6. [Google Scholar] [CrossRef]
- Leiguarda, R.C.; Marsden, C.D. Limb apraxias - Higher-order disorders of sensorimotor integration. Brain 2000, 123, 860–879. [Google Scholar] [CrossRef]
- Newton, J.M.; Ward, N.S.; Parker, G.J.M.; Deichmann, R.; Alexander, D.C.; Friston, K.J.; Frackowiak, R.S.J. Non-invasive mapping of corticofugal fibres from multiple motor areas - relevance to stroke recovery. Brain 2006, 129, 1844–1858. [Google Scholar] [CrossRef]
- Kunimatsu, A.; Aoki, S.; Masutani, Y.; Abe, O.; Mori, H.; Ohtomo, K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology 2003, 45, 532–535. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an ifcn committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Shahar, E.; Ravid, S.; Hafner, H.; Chistyakov, A.; Shcif, A. Diagnostic value of Hoover sign and motor-evoked potentials in acute somatoform unilateral weakness and sensory impairment mimicking vascular stroke. J. Clin. Neurosci. 2012, 19, 980–983. [Google Scholar] [CrossRef]
- Liepert, J.; Hassa, T.; Tuescher, O.; Schmidt, R. Motor excitability during movement imagination and movement observation in psychogenic lower limb paresis. J. Psychosom. Res. 2011, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Hassa, T.; Tuscher, O.; Schmidt, R. Abnormal motor excitability in patients with psychogenic paresis. J. Neurol. 2009, 256, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Shimojima, Y.; Nishikawa, N.; Hagiwara, N.; Amano, N.; Ikeda, S.I. Size variance of motor evoked potential at initiation of voluntary contraction in palsy of conversion disorder. Psychiatry Clin. Neurosci. 2008, 62, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liepert, J.; Hassa, T.; Tuscher, O.; Schmidt, R. Electrophysiological Correlates of Motor Conversion Disorder. Mov. Disord. 2008, 23, 2171–2176. [Google Scholar] [CrossRef]
- Schoenfeldt-Lecuona, C.; Lefaucheur, J.P.; Lepping, P.; Liepert, J.; Connemann, B.J.; Sartorius, A.; Nowak, D.A.; Gahr, M. Non-Invasive Brain Stimulation in Conversion (Functional) Weakness and Paralysis: A Systematic Review and Future Perspectives. Front Neurosci. 2016, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; Seo, J.P. Demonstration of Injury of the Corticospinal Tract in a Patient with Suspected Motor Conversion Disorder. Am. J. Phys. Med. Rehabil. 2017, 96, E53–E54. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional structural MR image analysis implementation as, F.S.L. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Mayka, M.A.; Corcos, D.M.; Leurgans, S.E.; Vaillancourt, D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. Neuroimage 2006, 31, 1453–1474. [Google Scholar] [CrossRef] [Green Version]
- Yoshor, D.; Mizrahi, E. Clinical Brain Mapping; McGrawHill: New York, NY, USA, 2012. [Google Scholar]
- Crawford, J.R.; Garthwaite, P.H.; Porter, S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cogn. Neuropsychol. 2010, 27, 245–260. [Google Scholar] [CrossRef]
- Nijland, R.H.M.; van Wegen, E.E.H.; Harmeling-van der Wel, B.C.; Kwakkel, G.; Investigators, E. Presence of Finger Extension and Shoulder Abduction Within 72 Hours After Stroke Predicts Functional Recovery Early Prediction of Functional Outcome After Stroke: The EPOS Cohort Study. Stroke 2010, 41, 745–750. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P. Limb-kinetic apraxia due to injury of corticofugal tracts from secondary motor area in patients with corona radiata infarct. Acta Neurol. Belg. 2016, 116, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Chang, C.H.; Lee, H.D. Limb-kinetic apraxia due to injury of the corticofugal tract from the secondary motor area in a stroke patient. Am. J. Phys. Med. Rehabil. 2016, 95, e115–e116. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Braass, H.; Liuzzi, G.; Hoerniss, V.; Lechner, P.; Gerloff, C.; Hummel, F.C. White matter integrity of premotor-motor connections is associated with motor output in chronic stroke patients. Neuroimage Clin. 2015, 7, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Cho, S.H.; Kim, Y.H.; You, S.H.; Kim, S.H.; Kim, O.; Yang, D.S.; Son, S. Motor recovery mechanism of diffuse axonal injury: A combined study of transcranial magnetic stimulation functional, M.R.I. Restor. Neurol. Neurosci. 2005, 23, 51–56. [Google Scholar]
- Parker, G.J.M.; Alexander, D.C. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 893–902. [Google Scholar] [CrossRef]
- Yamada, K.; Sakai, K.; Akazawa, K.; Yuen, S.; Nishimura, T. MR Tractography: A Review of Its Clinical Applications. Magn. Reson. Med. Sci. 2009, 8, 165–174. [Google Scholar] [CrossRef] [Green Version]
| Diffusion Tensor Tractography | ||||
|---|---|---|---|---|
| Patient | Control | p-Value | ||
| CST | FA | 0.47 | 0.49 | 0.58 |
| MD | 0.81 | 0.83 | 0.57 | |
| TV | 1695 | 1780.25 | 0.50 | |
| SMA-CFT | FA | 0.37 | 0.39 | 0.60 |
| MD | 1.01 | 0.99 | 0.75 | |
| TV | 6111 | 6329.25 | 0.87 | |
| dPMC-CFT | FA | 0.35 | 0.37 | 0.55 |
| MD | 1.08 | 1.01 | 0.37 | |
| TV | 6130 | 6537.5 | 0.79 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H.; Seo, Y.S. Diagnosis of Conversion Disorder Using Diffusion Tensor Tractography and Transcranial Magnetic Stimulation in a Patient with Mild Traumatic Brain Injury. Diagnostics 2019, 9, 155. https://doi.org/10.3390/diagnostics9040155
Jang SH, Seo YS. Diagnosis of Conversion Disorder Using Diffusion Tensor Tractography and Transcranial Magnetic Stimulation in a Patient with Mild Traumatic Brain Injury. Diagnostics. 2019; 9(4):155. https://doi.org/10.3390/diagnostics9040155
Chicago/Turabian StyleJang, Sung Ho, and You Sung Seo. 2019. "Diagnosis of Conversion Disorder Using Diffusion Tensor Tractography and Transcranial Magnetic Stimulation in a Patient with Mild Traumatic Brain Injury" Diagnostics 9, no. 4: 155. https://doi.org/10.3390/diagnostics9040155
APA StyleJang, S. H., & Seo, Y. S. (2019). Diagnosis of Conversion Disorder Using Diffusion Tensor Tractography and Transcranial Magnetic Stimulation in a Patient with Mild Traumatic Brain Injury. Diagnostics, 9(4), 155. https://doi.org/10.3390/diagnostics9040155





