Rapid Extraction Method of Mycobacterium ulcerans DNA from Clinical Samples of Suspected Buruli Ulcer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. M. ulcerans Genolyse DNA Extraction Protocol
2.3. RPA Assay
2.4. Clinical Sensitivity and Specificity of Mu DNA GenoLyse RPA Assay
2.5. Statistics
2.6. Ethics Statement
3. Results
3.1. Demographic of Patients
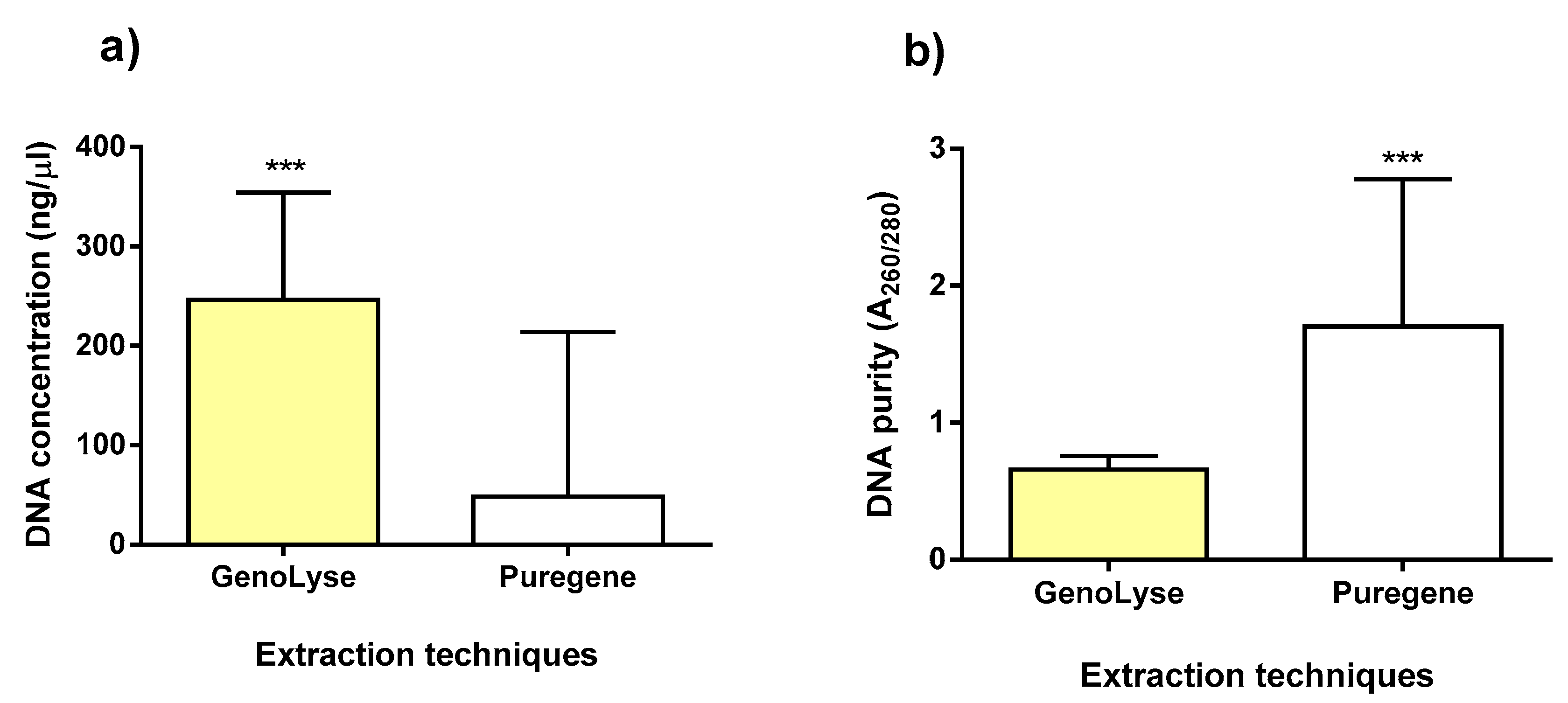
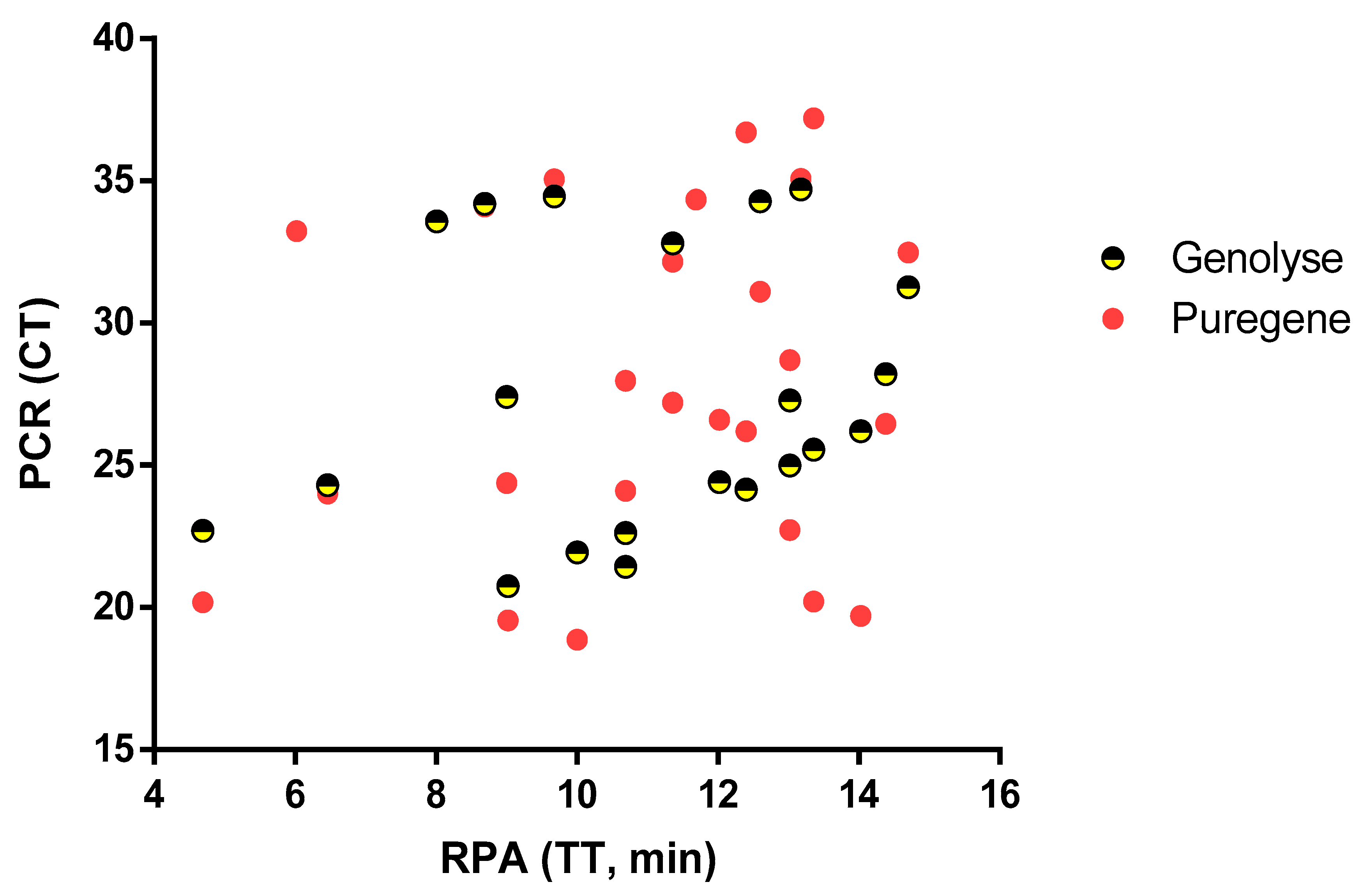
3.2. Quantity and Quality of DNA
3.3. Clinical Sensitivity and Specificity of Mu DNA GenoLyse RPA Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iddrisah, F.N.; Yeboah-Manu, D.; Nortey, P.A.; Nyarko, K.M.; Anim, J.; Antara, S.N.; Kenu, E.; Wurapa, F.; Afari, E.A. Outcome of Streptomycin-Rifampicin treatment of Buruli Ulcer in two Ghanaian districts. Pan Afr. Med. J. 2016, 25. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.O.; Sarfo, F.S.; Abass, M.K.; Abotsi, J.; Wilson, T.; Forson, M.; Amoako, Y.A.; Thompson, W.; Asiedu, K.; Wansbrough-Jones, M. Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob. Agents Chemother. 2014, 58, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, M.; Ahor, H.S.; Abd El Wahed, A.; Agbavor, B.; Sarpong, F.N.; Laing, K.; Wansbrough-Jones, M.; Phillips, R.O. Rapid detection of Mycobacterium ulcerans with isothermal recombinase polymerase amplification assay. PLoS Negl. Trop. Dis. 2019, 13, e0007155. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, M.; Beissner, M.; Loglo, A.D.; Tannor, E.; Awauh, N.Y.; Frempong, M.; Adjei, O.; Bretzel, G.; Phillips, R.O. Microscopy for Acid Fast Bacilli: A Useful but Neglected Tool in Routine Laboratory Diagnosis of Buruli Ulcer. J. Trop. Dis. 2015, 3, 3–6. [Google Scholar] [CrossRef]
- Beissner, M.; Phillips, R.O.; Battke, F.; Bauer, M.; Badziklou, K.; Sarfo, F.S.; Maman, I.; Rhomberg, A.; Piten, E.; Frimpong, M.; et al. Loop-Mediated Isothermal Amplification for Laboratory Confirmation of Buruli Ulcer Disease—Towards a Point-of-Care Test. PLoS Negl. Trop. Dis. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Njiru, Z.K.; Yeboah-Manu, D.; Stinear, T.P.; Fyfe, J.A.M. Rapid and Sensitive Detection of Mycobacterium ulcerans by Use of a Loop-Mediated Isothermal Amplification Test. J. Clin. Microbiol. 2012, 50, 1737–1741. [Google Scholar] [CrossRef]
- Ablordey, A.; Amissah, D.A.; Aboagye, I.F.; Hatano, B.; Yamazaki, T.; Sata, T.; Ishikawa, K.; Katano, H. Detection of Mycobacterium ulcerans by the Loop Mediated Isothermal Amplification Method. PLoS Negl. Trop. Dis. 2012, 6, 3–8. [Google Scholar] [CrossRef]
- World Health Organization. Report of a WHO–FIND Meeting on Diagnostics for Buruli Ulcer, Geneva, 26–27 March 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kotlowski, R.; Martin, A.; Ablordey, A.; Chemlal, K.; Fonteyne, P.A.; Portaels, F. One-tube cell lysis and DNA Extraction Procedure for PCR-Based Detection of Mycobacterium ulcerans in Aquatic Insects, Molluscs and Fish. J. Med. Microbiol. 2004, 53, 927–933. [Google Scholar] [CrossRef]
- Durnez, L.; Stragier, P.; Roebben, K.; Ablordey, A.; Leirs, H.; Portaels, F. A comparison of DNA Extraction Procedures for the Detection of Mycobacterium ulcerans, the Causative Agent of Buruli Ulcer, in Clinical and Environmental Specimens. J. Microbiol. Methods 2009, 76, 152–158. [Google Scholar] [CrossRef]
- Fyfe, J.A.M.; Lavender, C.J.; Handasyde, K.A.; Legione, A.R.; O’Brien, C.R.; Stinear, T.P.; Pidot, S.J.; Seemann, T.; Benbow, M.E.; Wallace, J.R.; et al. A Major Role for Mammals in the Ecology of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2010, 4, e791. [Google Scholar] [CrossRef]
- Fyfe, J.A.M.; Lavender, C.J.; Johnson, P.D.R.; Globan, M.; Sievers, A.; Azuolas, J.; Stinear, T.P. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl. Environ. Microbiol. 2007, 73, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.O.; Sarfo, F.S.; Osei-Sarpong, F.; Boateng, A.; Tetteh, I.; Lartey, A.; Adentwe, E.; Opare, W.; Asiedu, K.B.; Wansbrough-Jones, M. Sensitivity of PCR Targeting Mycobacterium ulcerans by use of Fine-Needle Aspirates for Diagnosis of Buruli Ulcer. J. Clin. Microbiol. 2009, 47, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.O.; Horsfield, C.; Kuijper, S.; Lartey, A.; Tetteh, I.; Etuaful, S.; Nyamekye, B.; Awuah, P.; Nyarko, K.M.; Osei-Sarpong, F.; et al. Sensitivity of PCR Targeting the IS2404 Insertion Sequence of Mycobacterium ulcerans in an Assay Using Punch Biopsy Specimens for Diagnosis of Buruli Ulcer. J. Clin. Microbiol. 2005, 43, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, V.; Adjei, O.; Nitschke, J.; Thompson, W.; Klutse, E.; Herbinger, K.H.; Thompson, R.; van Vloten, F.; Racz, P.; Fleischer, B.; et al. Dry Reagent-Based Polymerase Chain Reaction Compared with Other Laboratory Methods Available for the Diagnosis of Buruli Ulcer Disease. Clin. Infect. Dis. 2007, 45, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, V.; Adjei, O.; Racz, P.; Berberich, C.; Klutse, E.; van Vloten, F.; Kruppa, T.; Fleischer, B.; Bretzel, G. Dry-reagent-based PCR as a novel tool for laboratory confirmation of clinically diagnosed Mycobacterium ulcerans-associated disease in areas in the tropics where M. ulcerans is endemic. J. Clin. Microbiol. 2005, 43, 271–276. [Google Scholar] [CrossRef]
- Herbinger, K.; Adjei, O.; Awua-Boateng, N.; Nienhuis, W.A.; Kunaa, L.; Siegmund, V.; Nitschke, J.; Thompson, W.; Klutse, E.; Agbenorku, P.; et al. Comparative Study of the Sensitivity of Different Diagnostic Methods for the Laboratory Diagnosis of Buruli Ulcer Disease. Clin. Infect. Dis. 2009, 48, 1055–1064. [Google Scholar] [CrossRef]
- Beissner, M.; Symank, D.; Phillips, R.O.; Amoako, Y.A.; Awua-Boateng, N.Y.; Sarfo, F.S.; Jansson, M.; Huber, K.L.; Herbinger, K.H.; Battke, F.; et al. Detection of Viable Mycobacterium ulcerans in Clinical Samples by a Novel Combined 16S rRNA Reverse Transcriptase/IS2404 Real-Time qPCR Assay. PLoS Negl. Trop. Dis. 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Mondal, D.; Ghosh, P.; Khan, M.A.A.; Hossain, F.; Böhlken-Fascher, S.; Matlashewski, G.; Kroeger, A.; Olliaro, P.; Abd El Wahed, A. Mobile Suitcase Laboratory for Rapid Detection of Leishmania donovani using Recombinase Polymerase Amplification Assay. Parasit. Vectors 2016, 9, 281. [Google Scholar] [CrossRef]
- Weidmann, M.; Faye, O.; Faye, O.; Abd El Wahed, A.; Patel, P.; Batejat, C.; Manugerra, J.C.; Adjami, A.; Niedrig, M.; Hufert, F.T.; et al. Development of mobile laboratory for viral haemorrhagic fever detection in Africa. J. Infect. Dis. 2018, 218, 1622–1630. [Google Scholar] [CrossRef]
- WHO. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Diseases 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Crudu, V.; Stratan, E.; Romancenco, E.; Allerheiligen, V.; Hillemann, A.; Moraru, N. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J. Clin. Microbiol. 2012, 50, 1264–1269. [Google Scholar] [CrossRef]
- Matabane, M.M.Z.; Ismail, F.; Strydom, K.A.; Onwuegbuna, O.; Omar, S.V.; Ismail, N. Performance evaluation of three commercial molecular assays for the detection of Mycobacterium tuberculosis from clinical specimens in a high TB-HIV-burden setting. BMC Infect. Dis. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.K.; Quaye, C.; Mosi, L.; Addo, P.; Boakye, D.A. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infect. Dis. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Kersting, S.; Rausch, V.; Bier, F.; Von Nickisch-Rosenegk, M. Rapid detection of Plasmodium falciparum with Isothermal Recombinase Polymerase Amplification and Lateral Flow Analysis. Malar J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Krõlov, K.; Frolova, J.; Tudoran, O.; Suhorutsenko, J.; Lehto, T.; Sibul, H.; Mäger, I.; Laanpere, M.; Tulp, I.; Langel, Ü. Sensitive and Rapid Detection of Chlamydia trachomatis by Recombinase Polymerase Amplification Directly from Urine Samples. J. Mol. Diagn. 2014, 16, 127–135. [Google Scholar] [CrossRef]
- Rohrman, B.; Richards-Kortum, R. Inhibition of Recombinase Polymerase Amplification by Background DNA: A lateral Flow-Based Method for Enriching Target DNA. Anal. Chem. 2015, 87, 1963–1967. [Google Scholar] [CrossRef]
| Parameters | No. (%) of Total Lesions (n = 58) |
|---|---|
| Sex | |
| Male | 23 (40) |
| Female | 35 (60) |
| Sample Type | |
| Swab | 33 (57) |
| FNA | 25 (43) |
| Age in Years | |
| Median (IQR) | 17 (8-39) |
| Type of Lesion | |
| Ulcer | 33 (57) |
| Nodule | 4 (7) |
| Plaque | 18 (31) |
| Edema | 3 (5) |
| Category of Lesion | |
| I | 27 (47) |
| II | 16 (28) |
| III | 15 (26) |
| No. Positive/No. Clinical Confirmed as BU (% Positivity) | ||
|---|---|---|
| GenoLyse | Puregene * | |
| Polymerase chain reaction (PCR) | 12/15 (80) | 12/15 (80) |
| Recombinase polymerase amplification (RPA) | 12/15 (80) | 12/15 (80) |
| Puregene DNA qPCR * | Total | Sensitivity % | Specificity % | PPV % | NPV % | ||||
|---|---|---|---|---|---|---|---|---|---|
| +ve | −ve | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||
| Mu DNA GenoLyse qPCR | +ve | 22 | 0 | 22 | 73 (54–87) | 100 (75–100) | 100 (85–100) | 62 (38–81) | |
| −ve | 8 | 13 | 21 | ||||||
| Mu DNA GenoLyse RPA | Swab | +ve | 16 | 0 | 16 | 89 (65–99) | 100 (66–100) | 100 (79–100) | 82 (48–98) |
| −ve | 2 | 9 | 11 | ||||||
| FNA | +ve | 10 | 0 | 10 | 83 (52–98) | 100 (40–100) | 100 (69–100) | 67 (22–96) | |
| −ve | 2 | 4 | 6 | ||||||
| Total | +ve | 26 | 0 | 26 | 87 (69–96) | 100 (75–100) | 100 (87–100) | 75 (50–93) | |
| −ve | 4 | 13 | 17 | ||||||
| Reference | Kit/Extraction Method | Kit-Producing Company | Purification Method | Time Needed (min) a | Samples | Overnight Incubation Step | Heating Step (37–70 °C) | Proteinase K | Centrifugation | Pipetting Steps (≥10) | Costs per Reaction (€) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [9,10] | One-tube cell lysis | silica-cellulose membrane columns | 190 | tissue and environmental specimens | + | + | − | + | + | Unknown | |
| [10] | FastPrep® SPINKit | MP Biomedicals, Brussels, Belgium | silica filter column | 60 | tissue and environmental specimens | − | + | − | + | + | 4.42 |
| [10] | Modified Boom procedure | diatomaceous earth | 182 | tissue and environmental specimens | + | + | + | + | + | Unknown | |
| [10] | Maxwell® 16 kit | Promega, Leiden, Netherlands | MagneSil paramagnetic particles | 70 | tissue and environmental specimens | + | + | + | + | 5 # | 4.61 |
| [13,14] | Guanidinium thiocyanate (GuSCN)-diatoms method | diatom | 45 | FNA, swabs and tissue biopsies | + | + | + | + | + | Unknown | |
| [16] | Puregene Extraction Kit | Qiagen, Hilden, Germany | chemical (isopropanol +glycogen) | 300 | FNA, swabs and tissue biopsies | + | + | + | + | + | 2 |
| [7,24] | Boiling method | centrifugation | 15 | FNA and swabs | − | − | − | + | 2 | Unknown | |
| This study | Mu DNA GenoLyse | Hain Lifescience GmbH, Germany | centrifugation | 15 | FNA and swabs | − | − | − | + | 2 | 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frimpong, M.; Ahor, H.S.; Sakyi, S.A.; Agbavor, B.; Akowuah, E.; Phillips, R.O. Rapid Extraction Method of Mycobacterium ulcerans DNA from Clinical Samples of Suspected Buruli Ulcer Patients. Diagnostics 2019, 9, 204. https://doi.org/10.3390/diagnostics9040204
Frimpong M, Ahor HS, Sakyi SA, Agbavor B, Akowuah E, Phillips RO. Rapid Extraction Method of Mycobacterium ulcerans DNA from Clinical Samples of Suspected Buruli Ulcer Patients. Diagnostics. 2019; 9(4):204. https://doi.org/10.3390/diagnostics9040204
Chicago/Turabian StyleFrimpong, Michael, Hubert Senanu Ahor, Samuel Asamoah Sakyi, Bernadette Agbavor, Emmanuel Akowuah, and Richard Odame Phillips. 2019. "Rapid Extraction Method of Mycobacterium ulcerans DNA from Clinical Samples of Suspected Buruli Ulcer Patients" Diagnostics 9, no. 4: 204. https://doi.org/10.3390/diagnostics9040204
APA StyleFrimpong, M., Ahor, H. S., Sakyi, S. A., Agbavor, B., Akowuah, E., & Phillips, R. O. (2019). Rapid Extraction Method of Mycobacterium ulcerans DNA from Clinical Samples of Suspected Buruli Ulcer Patients. Diagnostics, 9(4), 204. https://doi.org/10.3390/diagnostics9040204







