Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Analysis
2.3. Analyzed Parameters
2.4. Statistical Analyses
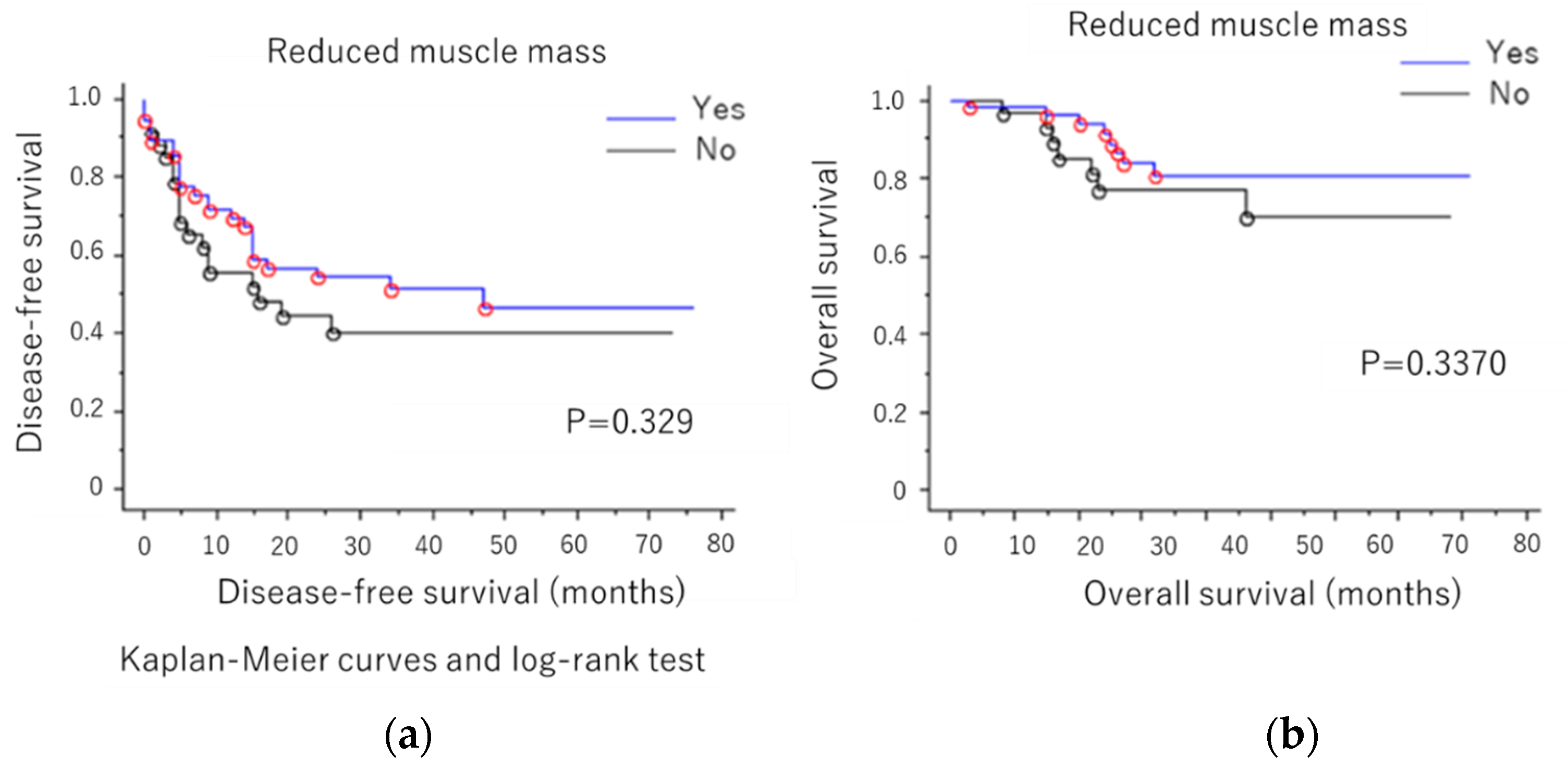
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| DFS | Disease-free survival |
| GI | Gastrointestinal |
| HCC | Hepatocellular carcinoma |
| HU | Hounsfield unit |
| IMAC | Intramuscular adipose tissue content |
| OS | Overall survival |
| QOL | Quality of life |
| SDs | Standard deviations |
| SMI | Skeletal muscle index |
| CA125 | Carbohydrate antigen 125 |
| STN | Sialyl Tn antigen |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
References
- Wingo, P.A.; Tong, T.; Bolden, S. Cancer Statistics. CA Cancer J. Clin. 1995, 45, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, N.; Katagiri, H.; Miyazaki, K. Mechanisms of ovarian cancer metastasis: Biochemical pathways. Int. J. Mol. Sci. 2012, 13, 11705–11717. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 27, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Shirai, H.; Kamo, N.; Yagi, S.; Taura, K.; Hideaki Okajima, H.; Uemoto, S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014, 20, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Takahashi, T.; Yokoyama, M.; Kinoshita, D.; Honda, Y.; Funayama, A.; Nishiyama, S.; Takahashi, H.; et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur. J. Intern. Med. 2015, 26, 118–122. [Google Scholar] [CrossRef]
- Voron, T.; Tselikas, L.; Pietrasz, D.; Pigneur, F.; Laurent, A.; Compagnon, P.; Salloum, C.; Luciani, A.; Azoulay, D. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann. Surg. 2015, 261, 1173–1183. [Google Scholar] [CrossRef]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.I.; Koga, F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Ida, S.; Watanabe, M.; Yoshida, N.; Baba, Y.; Umezaki, N.; Harada, K.; Karashima, R.; Imamura, Y.; Iwagami, S.; Baba, H. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann. Surg. Oncol. 2015, 22, 4432–4437. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Ito, T.; Fujimoto, Y.; Ogawa, K.; Mori, A.; Hammad, A.; Hatano, E.; Uemoto, S. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2015, 22, 475–485. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Masui, T.; Mizumoto, M.; Hammad, A.; Mori, A.; Takaori, K.; Uemoto, S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015, 157, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Kobayashi, A.; Iida, T.; Yagi, S.; Taura, K.; Hatano, E.; Uemoto, S. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery 2016, 159, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.S.; Kim, E.Y.; Jin, W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE 2018, 13, e0202700. [Google Scholar] [CrossRef] [PubMed]
- Rutten, I.J.G.; Ubachs, J.; Kruitwagen, R.F.; Beets-Tan, R.G.; Olde Damink, S.W.; Van Gorp, T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.; Knogler, T.; Pils, D.; Obermayr, E.; Reinthaller, A.; Zahn, L.; Radlgruber, I.; Mayerhoefer, M.E.; Grimm, C.; Polterauer, S. Skeletal Muscle Depletion and Markers for Cancer Cachexia Are Strong Prognostic Factors in Epithelial Ovarian Cancer. PLoS ONE 2015, 10, e0140403. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Nilsson, J.H.; Strandberg Holka, P.; Eberhard, J.; Keussen, I.; Sturesson, C. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford) 2017, 19, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hojan, K.; Milecki, P.; Molinska-Glura, M.; Roszak, A.; Leszczynski, P. Effect of physical activity on bone strength and body composition in breast cancer premenopausal women during endocrine therapy. Eur. J. Phys. Rehabil. Med. 2013, 49, 331–339. [Google Scholar]
- Aapro, M.; Arends, J.; Bozzetti, F.; Fearon, K.; Grunberg, S.M.; Herrstedt, J.; Hopkinson, J.; Jacquelin-Ravel, N.; Jatoi, A.; Kaasa, S.; et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann. Oncol. 2014, 25, 1492–1499. [Google Scholar] [CrossRef]
- Durán-Poveda, M.; Jimenez-Fonseca, P.; Sirvent-Ochando, M.; García-Luna, P.P.; Pereira-Cunill, J.L.; Lema-Marqués, B.; Parejo-Arrondo, M.T.; Belda-Iniesta, C. Integral nutritional approach to the care of cancer patients: Results from a Delphi panel. Clin. Transl. Oncol. 2018, 20, 1202–1211. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Shimokata, H.; Ando, F.; Yuki, A.; Otsuka, R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: A 12-year longitudinal study. Geriatr. Gerontol. Int. 2014, 14, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Mathis, K.M.; Rogers, C.J.; Schmitz, K.H.; Waning, D.L. Cancer and chemotherapy-induced musculoskeletal degradation. JBMR Plus 2019, 3, e10187. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Kitase, Y.; Mitra, S.; Erne, C.E.; Novinger, L.J.; Zimmers, T.A.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Martin, P.; Lewandowski, P.A. Cancer cachexia: Impact, mechanisms and emerging treatments. J. Cachexia Sarcopenia Muscle 2013, 4, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Watanabe, M.; et al. Negative impact of skeletal muscle loss after systematic chemotherapy in patients with unresectable colorectal cancer. PLoS ONE 2015, 10, e0129742. [Google Scholar] [CrossRef]
- Gagnon, B.; Murphy, J.; Eades, M.; Lemoignan, J.; Jelowicki, M.; Carney, S.; Amdouni, S.; Di Dio, P.; Chasen, M.; MacDonald, N. Prospective evaluation of an interdisciplinary nutrition–rehabilitation program for patients with advanced cancer. Curr. Oncol. 2013, 20, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.K.; Henly, S.J.; Shapiro, A.C.; Schroeder, L.M. Interventions to prevent loss of bone mineral density in women receiving chemotherapy for breast cancer. Clin. J. Oncol. Nurs. 2005, 9, 177–184. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Yingling, V.R. American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef]
- Hojan, K.; Milecki, P.; Leszczynski, P. The impact of aerobic exercises on bone mineral density in breast cancer women during endocrine therapy. Pol. Orthop. Traumatol. 2013, 78, 47–51. [Google Scholar]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.M.; Bennett, J.A.; Leo, M.C.; Torgrimson-Ojerio, B.; Luoh, S.W.; Schwartz, A. Impact of resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: A randomized controlled trial. Osteoporos. Int. 2013, 24, 1637–1646. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Bennett, J.A.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Skeletal response to resistance and impact training in prostate cancer survivors. Med. Sci. Sports Exerc. 2014, 46, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Saarto, T.; Sievanen, H.; Kellokumpu-Lehtinen, P.; Nikander, R.; Vehmanen, L.; Huovinen, R.; Kautiainen, H.; Järvenpää, S.; Penttinen, H.M.; Utriainen, M.; et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos. Int. 2012, 23, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Pedrazzoli, P.; Cereda, E.; Gavazzi, C.; Pinto, C.; Paccagnella, A.; Beretta, G.D.; Nardi, M.; Laviano, A.; Zagonel, V. Nutritional support in cancer patients: A position paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J. Cancer 2016, 7, 131–135. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V. Malnutrition in cancer patient: When to have a specialized consultation? Bull. Cancer 2009, 96, 615–623. [Google Scholar]
- Caccialanza, R.; De Lorenzo, F.; Gianotti, L.; Zagonel, V.; Gavazzi, C.; Farina, G.; Cotogni, P.; Cinieri, S.; Cereda, E.; Marchetti, P.; et al. Nutritional support for cancer patients: Still a neglected right? Support. Care Cancer 2017, 25, 3001–3004. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.F.P.M.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019. [Google Scholar] [CrossRef]

| Characteristics | Mean ± SD (%) | Characteristics | Number of Patients (%) |
|---|---|---|---|
| Age (Years) | 61.8 (range: 25–84) | FIGO stage | |
| Weight (kg) | 53 ± 8.6 | I | 37 (39.4) |
| Height (m) | 1.5 ± 0.06 | II | 9 (9.6) |
| BMI (kg/m2) | 22.9 ± 3.7 | III | 30 (31,9) |
| Initial tumor marker | IV | 18 (19.1) | |
| CA125 | 1250.5 ± 2659.7 | Histology | |
| CEA | 15.9 ± 89.9 | Serous | 45 (47.8) |
| STN | 216.1 ± 569.6 | Endometrioid | 21 (22.3) |
| CA19-9 | 448.4 ± 2898.1 | Mucinous | 12 (12.8) |
| Clear cell | 16 (17.1) | ||
| Other | 0 (0) | ||
| Tumor grade | |||
| Grade 1 | 11 (11.7) | ||
| Grade 2 | 32 (34) | ||
| Grade 3 (Clear cell included) | 51 (54.2) | ||
| Residual tumor | |||
| Positive | 45 (47.9) | ||
| Negative | 49 (52.1) | ||
| Recurrence | |||
| Yes | 44 (46.8) | ||
| No | 50 (53.2) |
| Reduced Muscle Mass | |||
|---|---|---|---|
| No (n = 32) | Yes (n = 62) | p-Value | |
| Age | 61.2 ± 10.5 | 61.6 ± 12.9 | p = 0.838 |
| BMI | 24.9 ± 5.8 | 21.5 ± 2.8 | P = 0.005 |
| CA125 | 1005.0 ± 1822.2 | 1379.4 ± 3014.7 | p = 0.493 |
| STN | 259.6 ± 864.0 | 193.4 ± 338.4 | p = 0.018 |
| CA19-9 | 203.4 ± 364.7 | 600.9 ± 3687.9 | p = 0.488 |
| CEA | 32.2 ± 141.2 | 4.96 ± 8.65 | p = 0.315 |
| FIGO stage III, IV (%) | 14/32(43.7%) | 34/62(54.8%) | p = 0.3851 |
| SMI | 42.6 ± 4.4 | 31.6 ± 4.2 | p = 0.000 |
| Visceral fat | 106.5 ± 65.2 | 52.0 ± 38.5 | p = 0.317 |
| Postoperative complication (%) | 5/32(15.6%) | 14/62(22.5%) | p = 0.5892 |
| Length of stay (days) | 17.4 ± 8.7 | 18.0 ± 10.3 | p = 0.766 |
| Reduced Muscle Quality | |||
|---|---|---|---|
| No (n = 73) | Yes (n = 21) | p-Value | |
| Age | 62.0 ± 12.0 | 62.6 ± 11.4 | p = 0.582 |
| BMI | 22.3 ± 3.4 | 23.5 ± 6.5 | p = 0.418 |
| CA125 | 1410.2 ± 2973.1 | 636.8 ± 1283.5 | p = 0.101 |
| STN | 207.5 ± 618.5 | 175.2 ± 298.3 | p = 0.808 |
| CA19-9 | 130.1 ± 281.8 | 1435.4 ± 5827.5 | p = 0.352 |
| CEA | 18.8 ± 104.0 | 5.7 ± 10.9 | p = 0.389 |
| FIGO stage III, IV (%) | 38/73 (52.0%) | 10/21 (47.6%) | p = 0.8065 |
| SMI | 34.3 ± 5.7 | 39.0 ± 8.4 | p = 0.028 |
| Visceral fat | 54.65 ± 40.7 | 126.7 ± 64.1 | p = 0.000 |
| Postoperative complication (%) | 17/73(23.2%) | 2/21(9.5%) | p = 0.2251 |
| Length of stay (days) | 17.4 ± 8.7 | 18.0 ± 10.3 | p = 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, N.; Nakayama, K.; Nakamura, K.; Razia, S.; Kyo, S. Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients. Diagnostics 2019, 9, 206. https://doi.org/10.3390/diagnostics9040206
Nakayama N, Nakayama K, Nakamura K, Razia S, Kyo S. Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients. Diagnostics. 2019; 9(4):206. https://doi.org/10.3390/diagnostics9040206
Chicago/Turabian StyleNakayama, Naomi, Kentaro Nakayama, Kohei Nakamura, Sultana Razia, and Satoru Kyo. 2019. "Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients" Diagnostics 9, no. 4: 206. https://doi.org/10.3390/diagnostics9040206
APA StyleNakayama, N., Nakayama, K., Nakamura, K., Razia, S., & Kyo, S. (2019). Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients. Diagnostics, 9(4), 206. https://doi.org/10.3390/diagnostics9040206





