Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors
Abstract
:1. Introduction
2. Available Infusion Therapies or Device-Aided Therapies (DAT) and Patient Selection
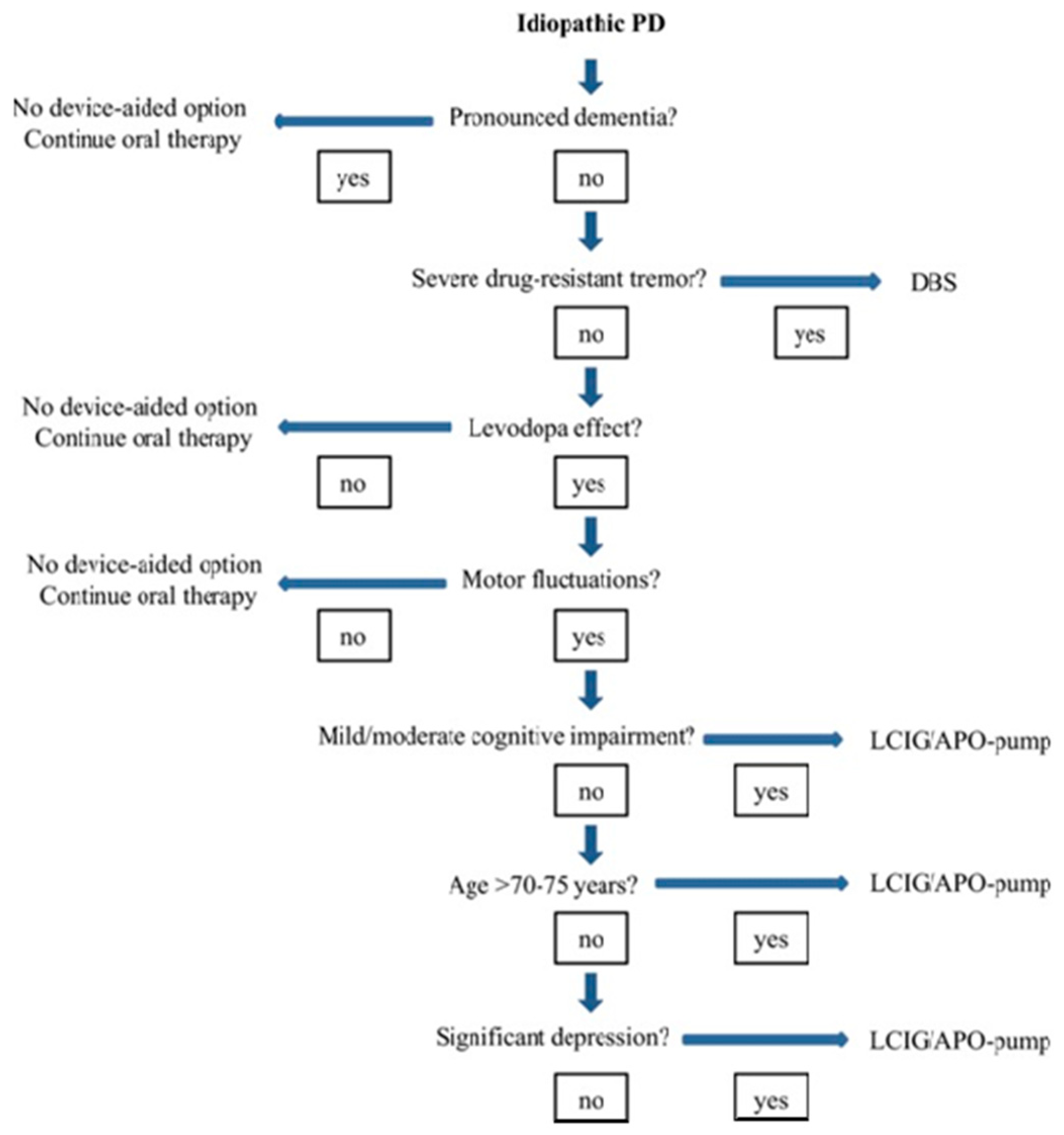
2.1. Selection of the Ideal Patient
2.2. Apomorphine: History and Molecular Structure
2.3. DAT Therapies: Evidence-Based Clinical Motor and Non-Motor Outcomes
2.4. Objective Measurements of Patient Outcomes in Parkinson’s Disease: Rating Scales
2.4.1. MDS-UPDRS Scale
2.4.2. Hoehn and Yahr Rating Scale
2.4.3. Short Parkinson’s Evaluation Scale/Scales for Outcomes in Parkinson’s Disease (SPES/SCOPA)
2.4.4. Non-Motor Symptoms Scale (NMSS)
2.4.5. PDSS (Parkinson’s Disease Sleep Scale)
2.4.6. King’s Parkinson’s Pain Scale (KPSS)
2.4.7. Montreal Cognitive Assessment (MoCA)
2.4.8. Hospital Anxiety and Depression Scale (HADS)
2.4.9. Parkinson’s Disease Questionnaires (PDQ-8 and PDQ-39)
2.4.10. Parkinson’s Disease Questionnaire (PDQ-8)
3. Continuous Objective Monitoring (COM) Using Wearable Sensors and Its Role in Identifying Potential Candidates for Device-Aided Therapies (DAT)
3.1. About PKG
3.2. Glossary of PKG Terms
- Median BKS. The median BKS was the 50th percentile of the BKS for all 6 days the PKG was worn (usually 6 days).
- The interquartile range of the BKS was a measure of the fluctuation of the BKS.
- The percent time in bradykinesia (PTB). Epochs whose BKS lay between 26.1 and 49.4 and whose 25th percentiles of the BKS were >18.5 and 90th percentiles, <80. Additionally, any epoch whose BKS was >49.9 but contained tremor was included.
- Median DKS: This is the 50th percentile for all the days that the PKG was worn. Brisk walking introducing resonant peaks may artificially increase the DKS. An algorithm was used to detect and remove epochs affected in this way.
- Interquartile range of DKS: calculates the median BKS and is a measure of the fluctuation of the DKS.
- Percent time in dyskinesia (PTD): Those DKS used to estimate the median DKS were passed through a median filter (most of the epochs in the filter period must be in the dyskinetic range (DKS > 7) for the centre to be classed as dyskinetic).
- Percent time with tremor (PTT): This was the percentage of 2 min epochs estimated over all the days that the PKG was worn that contained tremor. Tremor is likely to be present if the PTT score is >1%.
- The percent time immobile (PTI): This was the percentage of 2 min epochs with BKS > 80 from all the days that the PKG was worn. These scores were associated with daytime sleep.
- The doses of levodopa/day. These were calculated from the number of reminders programmed into the logger.
3.3. PKG Database and Associated Studies
4. Conclusions
4.1. Clinical Scenario 1
4.1.1. Current PD Medications
- Stalevo (l’dopa, 200 mg carbidopa, 50 mg; entacopone, 200 mg) QDS;
- Sinemet, controlled release, 250 mg (l’dopa, 200 mg; carbidopa, 50 mg) ON;
- Rotigotine, 8 mg (he responded very well initially and then started developing rashes, on rotigotine patches for 3 years);
- Previously tried a dopaminergic regime (selegiline, ropinorole, sinemet, etc.).
4.1.2. Current Ongoing Problems
- Troublesome dyskinesias;
- Unpredictable offs/freezing episodes;
- Attention/memory/cognitive problems;
- Apathy/hallucinations and non-intrusive perceptual issues.
4.2. Clinical Scenario 2
4.2.1. Current PD Medications
- Sinemet PLUS (l’dopa, 100 mg; carbidopa, 25 mg) at 7 am, 10 am, 1 pm, 4 pm, and 7 pm;
- Sinemet, controlled release, 250 mg (l’dopa, 200 mg; carbidopa, 50 mg) at 10 pm;
- Opicopone, 50 mg, 8 pm;
- Previously tried a dopaminergic regime (pramipexole, ropinorole, and entacopone).
4.2.2. Current Ongoing Problems
- Troublesome dyskinesias;
- Unpredictable offs/freezing episodes/falls;
- Cardiovascular, urinary, and gastrointestinal dysfunction;
- Severe sleep-related issues (excessive daytime sleepiness);
- Previous adverse reactions to dopamine agonists.
4.3. Discussion and Outcomes
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Parkinson’s Foundation. Statistics. Available online: https://www.parkinson.org/Understanding-Parkinsons/Statistics (accessed on 25 October 2019).
- Ray Chaudhuri, K.; Poewe, W.; Brooks, D. Motor and Nonmotor Complications of Levodopa: Phenomenology, Risk Factors, and Imaging Features. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Leta, V.; Jenner, P.; Chaudhuri, K.R.; Antonini, A. Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update. Expert Opin. Drug Saf. 2019, 18, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, S.; Ouchchane, L.; Metz, O.; Gerbaud, L.; Durif, F. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov. Disord. Off. J. Mov. Disord. Soc. 2005, 20, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Wu, K.; Molloy, S.; Bain, P.G.; Chaudhuri, K.R.; Piccini, P. Parkinson’s disease symptoms: The patient’s perspective. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Fung, V.S.C.; Lopiano, L.; Elibol, B.; Smolentseva, I.G.; Seppi, K.; Takáts, A.; Onuk, K.; Parra, J.C.; Bergmann, L.; et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef]
- Cloud, L.J.; Greene, J.G. Gastrointestinal Features of Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 379–384. [Google Scholar] [CrossRef]
- Dubow, J.S. Autonomic Dysfunction in Parkinson’s Disease. Dis. A Mon. 2007, 53, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M. Nonmotor symptoms in Parkinson’s disease. Int. J. Neurosci. 2011, 121 (Suppl. 2), 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; MacPhee, G.; MacMahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Fahn, S. Adverse effects of levodopa. In The Scientific Basis for the Treatment of Parkinson’s Disease; Olanow, C.W., Lieberman, A.N., Eds.; Parthenon Publishing Group: Carnforth, UK, 1992; pp. 89–112. [Google Scholar]
- Fahn, S.; Bressman, S.B. Should Levodopa Therapy for Parkinsonism be Started Early or Late? Evidence against Early Treatment. Can. J. Neurol. Sci. J. Can. Des. Sci. Neurol. 1984, 11 (Suppl. 1), 200–205. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Elton, R.L.; Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Calne, D.B., Goldstein, M., Eds.; MacMillan Healthcare Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–163. [Google Scholar]
- Timpka, J.; Nitu, B.; Datieva, V.; Odin, P. Antonini: ADevice-Aided Treatment Strategies in Advanced Parkinson’s DiseaseInternational Review of Neurobiology. Int. Rev. Neurobiol. 2017, 132, 453–474. [Google Scholar]
- Odin, P.; Chaudhuri, K.R.; Slevin, J.T.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.K.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Park. Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Nyholm, D. The rationale for continuous dopaminergic stimulation in advanced Parkinson’s disease. Park. Relat. Disord. 2007, 13, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Rätsch, C.; Hofmann, A. The Encyclopedia of Psychoactive Plants; Simon & Schuster: New York, NY, USA, 2005. [Google Scholar]
- Arppe, A.E. UebereinemerkwürdigeVeränderung des MorphinsdurchSchwefelsäure. Justus Liebigs Ann. Chem. 1845, 55, 96–101. [Google Scholar] [CrossRef]
- Matthiessen, A. Researches into the chemical constitution of the opium bases. Part I—On the action of hydrochloric. Proc. R. Soc. Lond. 1868, 17, 455–460. [Google Scholar]
- Ribarič, S. The pharmacological properties and therapeutic use of apomorphine. Molecules 2012, 17, 5289–5309. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, R.F.; Gutmann, L.; Hull, K.L.; Bottini, P.B.; Sherry, J.H. Continued efficacy and safety of subcutaneous apomorphine in patients with advanced Parkinson’s disease. Park. Relat. Disord. 2007, 13, 93–100. [Google Scholar] [CrossRef]
- Isaacson, S.; Lew, M.; Ondo, W.; Hubble, J.; Clinch, T.; Pagan, F. Apomorphine subcutaneous injection for the management of morning akinesia in Parkinson’s disease. Mov. Disord. Clin. Pract. 2017, 4, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenkwalder, C.; Chaudhuri, K.R.; Ruiz, P.J.G.; LeWitt, P.; Katzenschlager, R.; Sixel-Döring, F.; Henriksen, T.; Sesar, Á.; Poewe, W.; Baker, M.; et al. Expert Consensus Group for Use of Apomorphine in Parkinson’s Disease. Expert consensus group report on the use of apomorphine in the treatment of Parkinson’s disease—Clinical practice recommendations. Park. Relat. Disord. 2015, 21, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Katzenschlager, R.; Poewe, W.; Rascol, O.; Trenkwalder, C.; Deuschl, G.; Chaudhuri, K.R.; Henriksen, T.; Van Laar, T.; Spivey, K.; Vel, S.; et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): A multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018, 17, 749–759. [Google Scholar] [CrossRef]
- Nyholm, D. Duodopa® treatment for advanced Parkinson’s disease: A review of efficacy and safety. Park. Relat. Disord. 2012, 18, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Aldred, J.; Kovacs, N.; Pontieri, F.; Standaert, D.; Bourgeois, P.; Davis, T.; Cubo, E.; Anca-Herschkovitsch, M.; Iansek, R.; Siddiqui, M.; et al. Abstract. Improvements in Dyskinesia with Levodopa-Carbidopa Intestinal Gel in Advanced Parkinson’s Disease Patients in a ‘Real-World’ Study: Interim Results of the Multinational DUO GLOBE Study With up to 24 Months Follow-Up. Neurology 2020, 94 (Suppl. 15), 1824. [Google Scholar]
- Wirdefeldt, K.; Odin, P.; Nyholm, D. Levodopa–Carbidopa Intestinal Gel in Patients with Parkinson’s Disease: A Systematic Review. CNS Drugs 2016, 30, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Poewe, W.; Chaudhuri, K.R.; Jech, R.; Pickut, B.; Pirtošek, Z.; Szasz, J.; Valldeoriola, F.; Winkler, C.; Bergmann, L.; et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Park. Relat. Disord. 2017, 45, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Russo, G.S.; Hashimoto, T.; Zhang, J.; Vitek, J.L. Subthalamic Nucleus Stimulation Modulates Thalamic Neuronal Activity. J. Neurosci. 2008, 28, 11916–11924. [Google Scholar] [CrossRef] [PubMed]
- Perestelo-Pérez, L.; Rivero-Santana, A.; Pérez-Ramos, J.; Serrano-Pérez, P.; Panetta, J.; Hilarión, P. Deep brain stimulation in Parkinson’s disease: Meta-analysis of randomized controlled trials. J. Neurol. 2014, 261, 2051–2060. [Google Scholar] [CrossRef]
- Xie, C.-L.; Shao, B.; Chen, J.; Zhou, Y.; Lin, S.-Y.; Wang, W.-W. Effects of neurostimulation for advanced Parkinson’s disease patients on motor symptoms: A multiple-treatments meta-analysis of randomized controlled trials. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martin, P.; Reddy, P.; Katzenschlager, R.; Antonini, A.; Todorova, A.; Odin, P.; Henriksen, T.; Martin, A.; Calandrella, D.; Rizos, A.; et al. EuroInf: A multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 510–516. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Martinez-Martin, P.; Rizos, A.; Trost, M.; dos Santos Ghilardi, M.G.; Reddy, P.; Sauerbier, A.; Petry-Schmelzer, J.N.; Kramberger, M.; Borgemeester, R.W.; et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Stebbins, G.T.; Chmura, T.A.; Fahn, S.; Poewe, W.; Tanner, C.M. Teaching program for the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale:(MDS-UPDRS). Mov. Disord. 2010, 25, 1190–1194. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbaan, D.; van Rooden, S.; Benit, C.; van Zwet, E.; Marinus, J.; van Hilten, J. SPES/SCOPA and MDS-UPDRS: Formulas for converting scores of two motor scales in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 632–634. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Martin, P.; Benito-León, J.; Burguera, J.A.; Castro, A.; Linazasoro, G.; Martínez-Castrillo, J.C.; Valldeoriola, F.; Vázquez, A.; Vivancos, F.; del Val, J.; et al. The SCOPA–Motor Scale for assessment of Parkinson’s disease is a consistent and valid measure. J. Clin. Epidemiol 2005, 58, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Pal, S.; DiMarco, A.; Whately-Smith, C.; Bridgman, K.; Mathew, R.; Pezzela, F.R.; Forbes, A.; Högl, B.; Trenkwalder, C. The Parkinson’s disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.R.; Rizos, A.; Trenkwalder, C.; Rascol, O.; Pal, S.; Martino, D.; Carroll, C.; Paviour, D.; Falup-Pecurariu, C.; Kessel, B.; et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov. Disord. 2015, 30, 1623–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The PDQ-8: Development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health 1997, 12, 805–814. [Google Scholar] [CrossRef]
- Peto, V.; Jenkinson, C.; Fitzpatrick, R.; Greenhall, R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual. Life Res. 1995, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Storch, A.; Schneider, C.B.; Wolz, M.; Stürwald, Y.; Nebe, A.; Odin, P.; Mahler, A.; Fuchs, G.; Jost, W.H.; Chaudhuri, K.R.; et al. Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology 2013, 80, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S. (Spyros) Patient Diaries As a Clinical Endpoint in Parkinson’s Disease Clinical Trials. CNS Neurosci. Ther. 2011, 18, 380–387. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated Assessment of Bradykinesia and Dyskinesia in Parkinson’s Disease. J. Park. Dis. 2012, 2, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Kotschet, K.; Johnson, W.; McGregor, S.; Kettlewell, J.; Kyoong, A.; O’Driscoll, D.M.; Turton, A.R.; Griffiths, R.I.; Horne, M.K. Daytime sleep in Parkinson’s Disease measured by episodes of immobility. Park. Relat. Disord. 2014, 20, 578–583. [Google Scholar] [CrossRef]
- Braybrook, M.; O’Connor, S.; Churchward, P.; Perera, T.; Farzanehfar, P.; Horne, M. An Ambulatory Tremor Score for Parkinson’s Disease. J. Park. Dis. 2016, 6, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Farzanehfar, P.; Horne, M. Evaluation of the Parkinson’s KinetiGraph in monitoring and managing Parkinson’s disease. Expert Rev. Med. Devices 2017, 14, 583–591. [Google Scholar] [CrossRef]
- Horne, M.; Volkmann, J.; Sannelli, S.; Luyet, P.-P.; Moro, E. An evaluation of the parkinson’skinetigraph (pkg) as a tool to support deep brain stimulation eligibility assessment in patients with parkinson’s disease. Mov. Disord. 2017, 32 (Suppl. 2). [Google Scholar]
- Schuepbach, W.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Hälbig, T.; Hesekamp, H.; Navarro, S.; Meier, N.; et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, E.; Allert, N.; Eleopra, R.; Houeto, J.-L.; Phan, T.-M.; Stoevelaar, H.; International Study Group onReferral Criteria for DBS. A decision tool to support appropriate referral for deep brain stimulation in Parkinson’s disease. J. Neurol. 2009, 256, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.; Fernandez, H.H.; Pedraza, O.; Misra, M.; Lyons, K.E.; Pahwa, R.; Tarsy, D.; Scollins, L.; Corapi, K.; Friehs, G.M.; et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology 2004, 63, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.W.; Schootman, M.; Kung, N.; Wang, X.-Y.; Perlmutter, J.S.; Racette, B.A. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014, 82, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-Y.; O’Sullivan, S.S.; Kotschet, K.; Gallagher, D.A.; Lacey, C.; Lawrence, A.D.; Lees, A.J.; O’Sullivan, D.J.; Peppard, R.F.; Rodrigues, J.P.; et al. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J. Clin. Neurosci. 2009, 16, 1148–1152. [Google Scholar] [CrossRef]
- Jenner, P. Wearing Off, Dyskinesia, and the Use of Continuous Drug Delivery in Parkinson’s Disease. Neurol. Clin. 2013, 31, S17–S35. [Google Scholar] [CrossRef] [Green Version]
- Stacy, M.; Hauser, R.; Oertel, W.; Schapira, A.; Sethi, K.; Stocchi, F.; Tolosa, E. End-of-dose wearing off in parkinson disease: A 9-question survey assessment. Clin. Neuropharmacol. 2006, 29, 312–321. [Google Scholar] [CrossRef]
- Stocchi, F.; Antonini, A.; Barone, P.; Tinazzi, M.; Zappia, M.; Onofrj, M.; Ruggieri, S.; Morgante, L.; Bonuccelli, U.; Lopiano, L.; et al. Early DEtection of wEaring off in Parkinson disease: The DEEP study. Park. Relat. Disord. 2014, 20, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Florkowski, C.M. Sensitivity, Specificity, Receiver-Operating Characteristic (ROC) Curves and Likelihood Ratios: Communicating the Performance of Diagnostic Tests. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S83–S87. [Google Scholar]
- Khodakarami, H.; Farzanehfar, P.; Horne, M. The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies. Sensors 2019, 19, 2241. [Google Scholar] [CrossRef] [Green Version]
- Odin, P.; Chaudhuri, K.R.; Volkmann, J.; Antonini, A.; Storch, A.; Dietrichs, E.; Pirtošek, Z.; Henriksen, T.; Horne, M.; Devos, D.; et al. Viewpoint and practical recommendations from a movement disorder specialist panel on objective measurement in the clinical management of Parkinson’s disease. NPJ Park. Dis. 2018, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Isaacson, S.H.; Torres-Russotto, D.; Nahab, F.B.; Lynch, P.M.; Kotschet, K.E. Role of the Personal KinetiGraph in the routine clinical assessment of Parkinson’s disease: Recommendations from an expert panel. Expert Rev. Neurother. 2018, 18, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.C.; Lewis, A. Weight in Parkinson’s Disease: Phenotypic Significance. Int. Rev. Neurobiol. 2017, 134, 891–919. [Google Scholar] [PubMed]












| PEN (Figure 4) | PUMP (Figure 4) |
|---|---|
| Anticipated rescue when required during motor and non-motor “off” periods | Patient considers that rescue doses required too frequently |
| When absorption of oral levodopa is impaired or the patient has gastric emptying problems (gastroparesis) | Dyskinesias limit further therapy optimization |
| To treat delayed “on” | Simplify complex PD dosing regimens to improve convenience and compliance |
| To treat early-morning problems (akinesia and dystonia) | Alternative to surgical therapy or LCIG, if contraindicated, or due to patient preference |
| Absorption or gastric emptying of oral levodopa is impaired |
| Symptoms That Support Use | Symptoms That Discourage Use |
|---|---|
| Dyskinesias | Marked ongoing hallucinations/psychosis |
| Maintenance insomnia | Impulse-control disorders |
| Pronounced therapy-refractory depression | Drug-related daytime somnolence |
| Non-motor fluctuations | Orthostatic hypotension |
| Dysarthria | Marked ongoing hallucinations/psychosis |
| Restless legs |
| Symptoms That Support Use | Symptoms That Discourage Use |
|---|---|
| Dyskinesias | No specific symptoms (like severe dementia) to discourage use; presence of some symptoms may require further investigation |
| Drug-related hallucinations and/or delusions in patient history | |
| Impulse-control disorders | |
| Maintenance insomnia | |
| Mild cognitive impairment | |
| Pronounced therapy-refractory depression | |
| Dysarthria | |
| Restless legs |
| Symptoms That Support Use | Symptoms That Discourage Use |
|---|---|
| Dyskinesias | Marked ongoing hallucinations |
| Drug-related hallucinations and/or delusions in patient history | Dementia |
| Impulse-control disorders | Pronounced therapy-refractory depression |
| Maintenance insomnia | Dysphagia |
| Non-motor fluctuations | Dysarthria |
| L-dopa-unresponsive postural and gait problems, falls | |
| Marked ongoing hallucinations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metta, V.; Batzu, L.; Leta, V.; Trivedi, D.; Powdleska, A.; Mridula, K.R.; Kukle, P.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; et al. Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors. J. Pers. Med. 2021, 11, 680. https://doi.org/10.3390/jpm11070680
Metta V, Batzu L, Leta V, Trivedi D, Powdleska A, Mridula KR, Kukle P, Goyal V, Borgohain R, Chung-Faye G, et al. Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors. Journal of Personalized Medicine. 2021; 11(7):680. https://doi.org/10.3390/jpm11070680
Chicago/Turabian StyleMetta, Vinod, Lucia Batzu, Valentina Leta, Dhaval Trivedi, Aleksandra Powdleska, Kandadai Rukmini Mridula, Prashanth Kukle, Vinay Goyal, Rupam Borgohain, Guy Chung-Faye, and et al. 2021. "Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors" Journal of Personalized Medicine 11, no. 7: 680. https://doi.org/10.3390/jpm11070680
APA StyleMetta, V., Batzu, L., Leta, V., Trivedi, D., Powdleska, A., Mridula, K. R., Kukle, P., Goyal, V., Borgohain, R., Chung-Faye, G., & Chaudhuri, K. R. (2021). Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors. Journal of Personalized Medicine, 11(7), 680. https://doi.org/10.3390/jpm11070680




_Chaudhuri_also_Ray-Chaudhuri.png)

