Dopaminergic Gene Dosage Reveals Distinct Biological Partitions between Autism and Developmental Delay as Revealed by Complex Network Analysis and Machine Learning Approaches
Abstract
:1. Introduction
1.1. The Putative Role of Dopaminergic Signalling in ASD
1.2. Addressing Complex Diseases with Complex Network Approaches
1.3. Prediction of ASD Diagnosis with Machine Learning
1.4. Study Approach and Aims
2. Materials and Methods
2.1. Data Source and Participant Selection
2.2. Building Networks of Participant’s Genomic Features and Diagnostics
2.3. Network Analysis Methods
2.4. Applying Machine Learning in Differential Diagnosis
3. Results
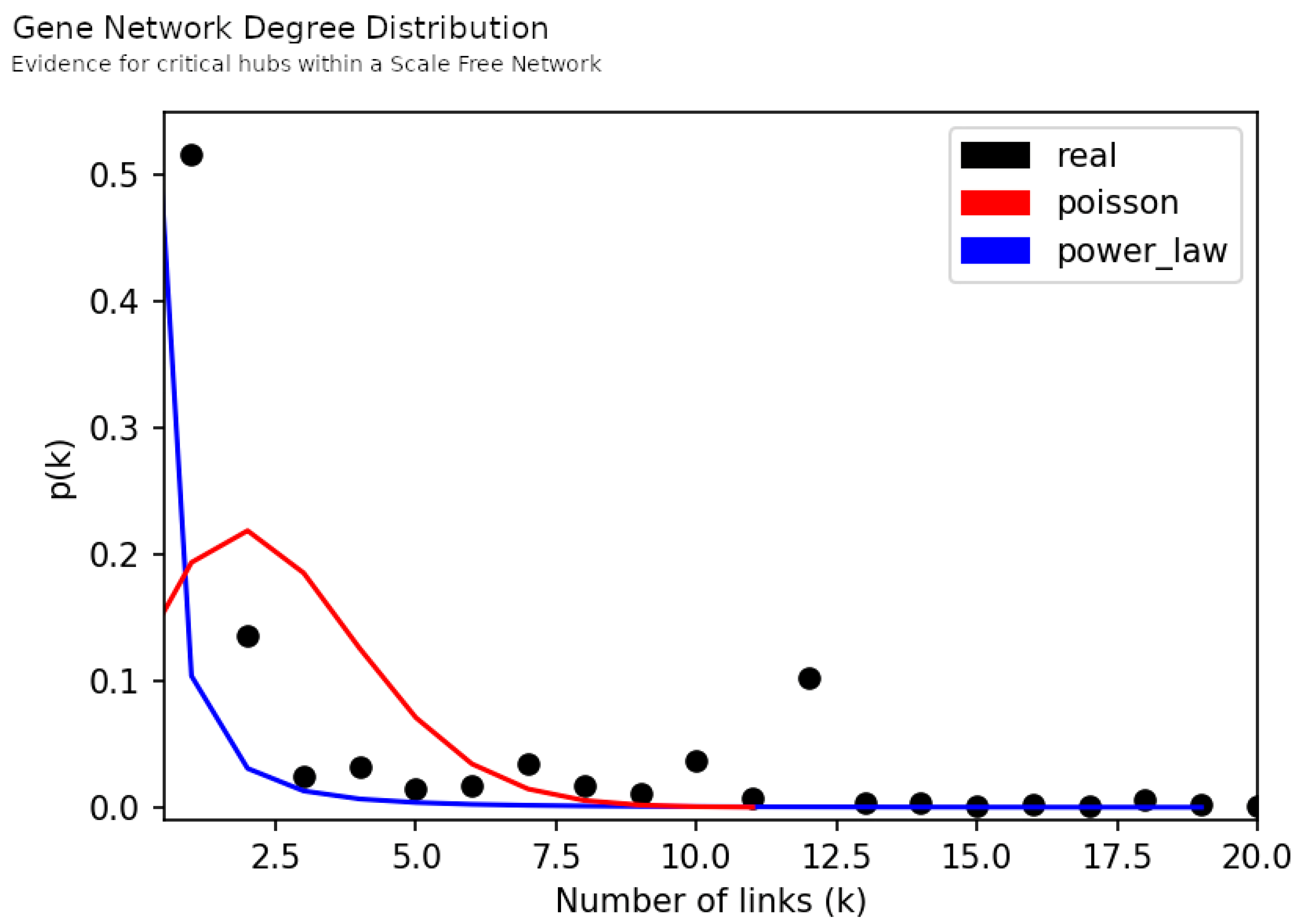
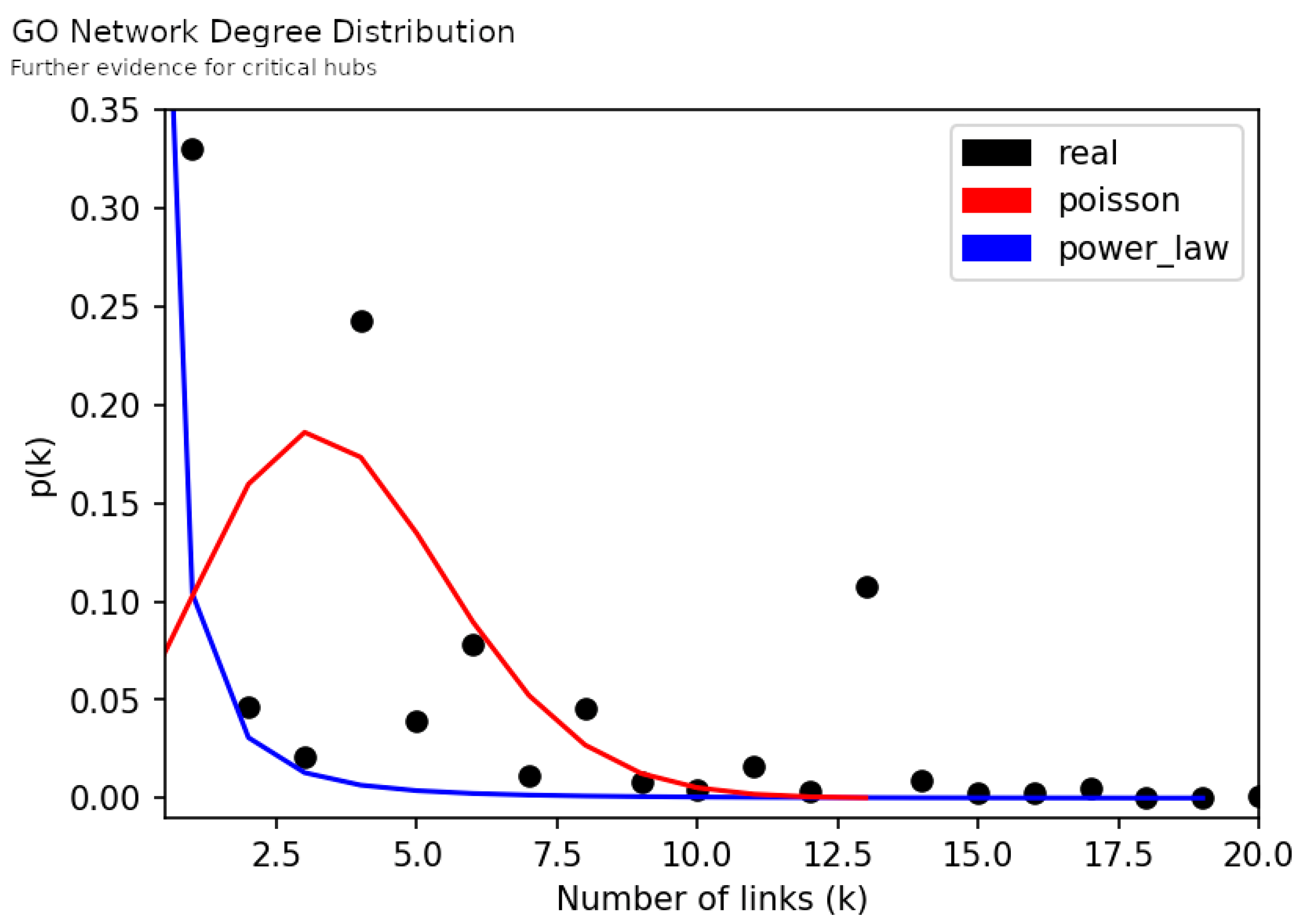
3.1. Macroscopic Network Features
3.2. Network Analysis
3.2.1. Hubs and Neighborhood Analysis
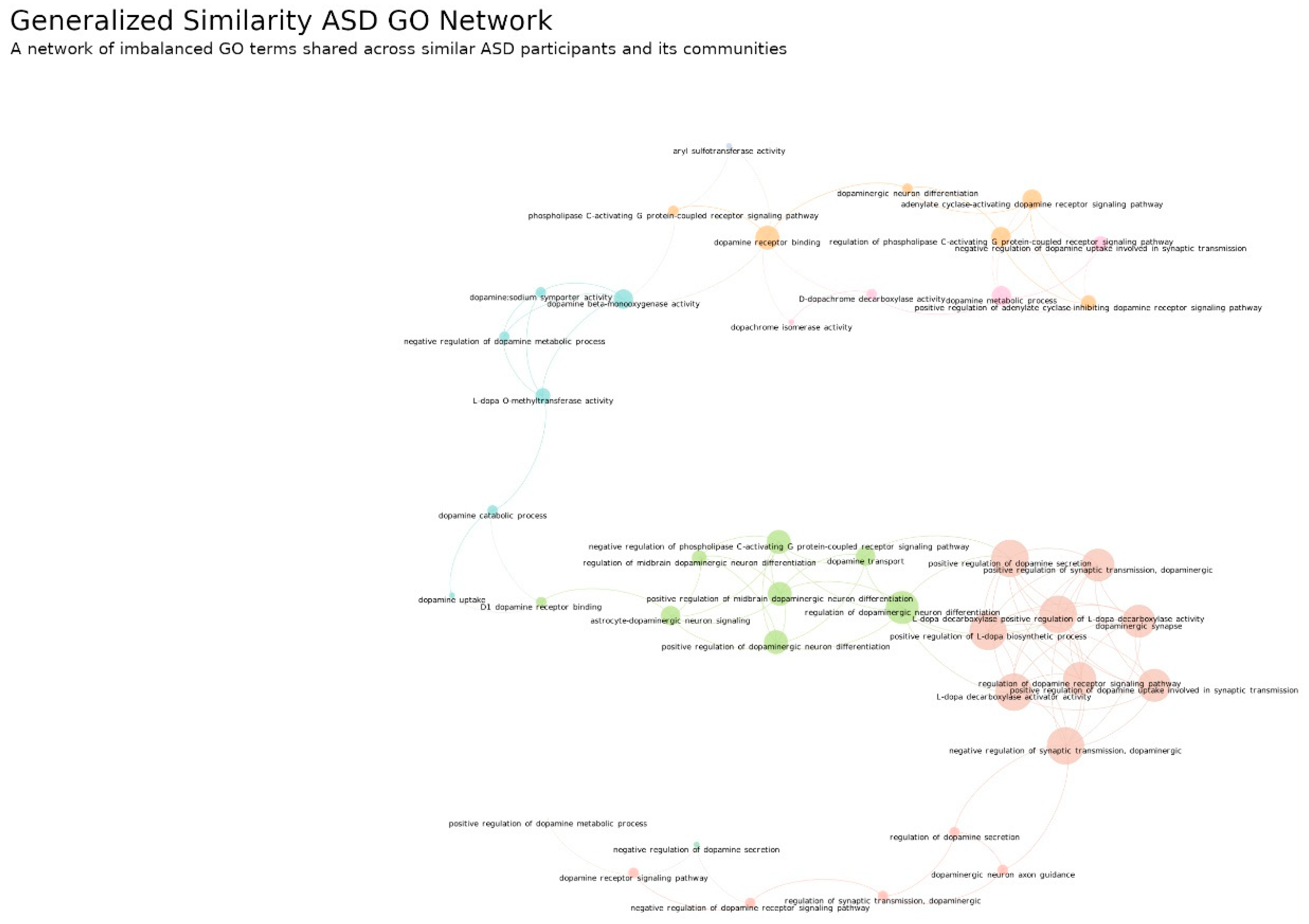
3.2.2. Generalized Similarity within ASD and DD
3.3. Statistical Classification between ASD and DD
Impact of Feature-Type in Classifier’s Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, S.J. First glimpses of the neurobiology of autism spectrum disorder. Curr. Opin. Genet. Dev. 2015, 33, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.V.; Lai, M.C.; Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry 2019, 24, 1435–1450. [Google Scholar] [CrossRef]
- Ecker, C.; Marquand, A.; Mourão-Miranda, J.; Johnston, P.; Daly, E.M.; Brammer, M.J.; Maltezos, S.; Murphy, C.M.; Robertson, D.; Williams, S.C.; et al. Describing the brain in autism in five dimensions—Magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J. Neurosci. 2010, 30, 10612–10623. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Sun, N.; Floris, D.L.; Zhang, X.; Di Martino, A.; Yeo, B.T.T. Reconciling Dimensional and Categorical Models of Autism Heterogeneity: A Brain Connectomics and Behavioral Study. Biol. Psychiatry 2020, 87, 697–707. [Google Scholar] [CrossRef]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Schumann, C.M.; Nordahl, C.W. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef]
- Pavăl, D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Greene, R.K.; Walsh, E.; Mosner, M.G.; Dichter, G.S. A Potential Mechanistic Role for Neuroinflammation in Reward Processing Impairments in Autism Spectrum Disorder. Biol. Psychol. 2018, 142, 1–12. [Google Scholar] [CrossRef]
- Ayano, G. Dopamine: Receptors, Functions, Synthesis, Pathways, Locations and Mental Disorders: Review of Literatures. J. Ment. Disord. Treat. 2016, 2, 2–5. [Google Scholar] [CrossRef]
- Supekar, K.; Kochalka, J.; Schaer, M.; Wakeman, H.; Qin, S.; Padmanabhan, A.; Menon, V. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain 2018, 141, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Mollinedo-Gajate, I.; Peñagarikano, O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience 2018, 370, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Veenstra-VanderWeele, J.; Christian, S.L.; Cook, E., Jr. H. Autism as a paradigmatic complex genetic disorder. Annu. Rev. Genomics Hum. Genet. 2004, 5, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar] [CrossRef]
- Schadt, E.E. Molecular networks as sensors and drivers of common human diseases. Nature 2009, 461, 218–223. [Google Scholar] [CrossRef]
- Albert, R. Scale-free networks in cell biology. J. Cell Sci. 2005, 118, 4947–4957. [Google Scholar] [CrossRef]
- Jonsson, P.F.; Bates, P.A. Global topological features of cancer proteins in the human interactome. Bioinformatics 2006, 22, 2291–2297. [Google Scholar] [CrossRef]
- Zotenko, E.; Mestre, J.; O’Leary, D.P.; Przytycka, T.M. Why do hubs in the yeast protein interaction network tend to be essential: Reexamining the connection between the network topology and essentiality. PLoS Comput. Biol. 2008, 4, e1000140. [Google Scholar] [CrossRef]
- Zhang, Z.; Sejdić, E. Radiological images and machine learning: Trends, perspectives, and prospects. Comput. Biol. Med. 2019, 108, 354–370. [Google Scholar] [CrossRef]
- Wolfers, T.; Floris, D.L.; Dinga, R.; van Rooij, D.; Isakoglou, C.; Kia, S.M.; Zabihi, M.; Llera, A.; Chowdanayaka, R.; Kumar, V.J.; et al. From pattern classification to stratification: Towards conceptualizing the heterogeneity of Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2019, 104, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Taheri, M.; Omrani, M.D.; Daaee, A.; Mohammad-Rahimi, H.; Kazazi, H. Application of Single-Nucleotide Polymorphisms in the Diagnosis of Autism Spectrum Disorders: A Preliminary Study with Artificial Neural Networks. J. Mol. Neurosci. 2019, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Vicari, S.; Napoli, E.; Cordeddu, V.; Menghini, D.; Alesi, V.; Loddo, S.; Novelli, A.; Tartaglia, M. Copy number variants in autism spectrum disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2019, 92, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.; Hudgins, L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet. Med. 2010, 12, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Riggs, E.R.; Andersen, E.F.; Cherry, A.M.; Kantarci, S.; Kearney, H.; Patel, A.; Raca, G.; Ritter, D.I.; South, S.T.; Thorland, E.C.; et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med. 2020, 22, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Binns, D.; Dimmer, E.; Huntley, R.; Barrell, D.; O’Donovan, C.; Apweiler, R. QuickGO: A web-based tool for Gene Ontology searching. Bioinformatics 2009, 25, 3045–3046. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Carbon, S.; Douglass, E.; Dunn, N.; Good, B.; Harris, N.L.; Lewis, S.E.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; et al. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Fischbach, G.D.; Lord, C. The simons simplex collection: A resource for identification of autism genetic risk factors. Neuron 2010, 68, 192–195. [Google Scholar] [CrossRef]
- Conte, F.; Fiscon, G.; Licursi, V.; Bizzarri, D.; D’Antò, T.; Farina, L.; Paci, P. A paradigm shift in medicine: A comprehensive review of network-based approaches. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1780, 194416. [Google Scholar] [CrossRef]
- Persico, A.M.; Napolioni, V. Autism genetics. Behav. Brain Res. 2013, 251, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.K. Random Decision Forests. In Proceedings of the International Conference on Document Analysis and Recognition, ICDAR, Montreal, QC, Canada,, 14–16 August 1995; IEEE Computer Society: Washington, DC, USA, 1995; Volume 1, pp. 278–282. [Google Scholar]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; De Moor, B.; Brazma, A.; Huber, W. BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef] [PubMed]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Santos, A.; Caramelo, F.; Melo, J.B.; Castelo-Branco, M. Study Repository: A Relational Database of SFARI Gene CNVs Data Integrated with Associated Genes and GO Terms for the Study of Genetics in Neurodevelopmental Disorders—Autism Imaging Genetics Dataverse. 2022. Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/HO1JLJ (accessed on 8 March 2022).
- Goh, K.I.; Cusick, M.E.; Valle, D.; Childs, B.; Vidal, M.; Barabási, A.L. The human disease network. Proc. Natl. Acad. Sci. USA 2007, 104, 8685–8690. [Google Scholar] [CrossRef]
- Hagberg, A.A.; Schult, D.A.; Swart, P.J. Exploring Network Structure, Dynamics, and Function Using NetworkX. In Proceedings of the 7th Python in Science Conference (SciPy 2008), Pasadena, CA, USA, 19–24 August 2008; pp. 11–15. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Bollobás, B. Random Graphs; Cambridge University Press: Cambridge, UK, 2001; ISBN 0521797225. [Google Scholar]
- Newman, M. Networks; Oxford University Press: Oxford, UK, 2018; Volume 1, ISBN 9780198805090. [Google Scholar]
- Barabási, A.L.; Albert, R. Emergence of scaling in random networks. Science 1999, 286, 509–512. [Google Scholar] [CrossRef]
- Barrat, A.; Barthélemy, M.; Pastor-Satorras, R.; Vespignani, A. The architecture of complex weighted networks. Proc. Natl. Acad. Sci. USA 2004, 101, 3747–3752. [Google Scholar] [CrossRef]
- Kovács, B. A generalized model of relational similarity. Soc. Networks 2010, 32, 197–211. [Google Scholar] [CrossRef]
- Hastie, T.T. The Elements of Statistical Learning Second Edition. Math. Intell. 2017, 27, 83–85. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An introduction to Statistical Learning; Springer: New York, NY, USA, 2000; Volume 7, p. 18. ISBN 978-1-4614-7137-0. [Google Scholar]
- Varoquaux, G.; Buitinck, L.; Louppe, G.; Grisel, O.; Pedregosa, F.; Mueller, A. Scikit-learn. GetMobile Mob. Comput. Commun. 2015, 19, 29–33. [Google Scholar] [CrossRef]
- Saeys, Y.; Inza, I.; Larrañaga, P. A review of feature selection techniques in bioinformatics. Bioinformatics 2007, 23, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Boldi, P.; Vigna, S. Four Degrees of Separation. In Proceedings of the IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining, ASONAM, San Francisco, CA, USA, 18–21 August 2012; pp. 1222–1227. [Google Scholar] [CrossRef]
- De Sola Pool, I.; Kochen, M. Contacts and influence. Soc. Networks 1978, 1, 5–51. [Google Scholar] [CrossRef]
- Travers, J.; Milgram, S. An Experimental Study of the Small World Problem. Sociometry 1969, 32, 425. [Google Scholar] [CrossRef]
- Faloutsos, M.; Faloutsos, P.; Faloutsos, C. On power-law relationships of the internet topology. Proc. SIGCOMM. Comput. Commun. Rev. 1999, 1, 251–262. [Google Scholar] [CrossRef]
- Newman, M.E.J. Power laws, Pareto distributions and Zipf’s law. Contemp. Phys. 2005, 46, 323–351. [Google Scholar] [CrossRef]
- Albert, R.; Jeong, H.; Barabási, A.L. Error and attack tolerance of complex networks. Nature 2000, 406, 378–382. [Google Scholar] [CrossRef]
- Callaway, D.S.; Newman, M.E.J.; Strogatz, S.H.; Watts, D.J. Network robustness and fragility: Percolation on random graphs. Phys. Rev. Lett. 2000, 85, 5468–5471. [Google Scholar] [CrossRef]
- Bollobás, B.; Riordan, O. Robustness and Vulnerability of scale-free random graphs. Internet Math. 2004, 1, 335–380. [Google Scholar] [CrossRef]
- Bousquet, O.; Elisseeff, A. Stability and Generalization. J. Mach. Learn. Res. 2002, 2, 499–526. [Google Scholar] [CrossRef]
- Bousquet, O.; von Luxburg, U.; Rätsch, G. Subseries of Lecture Notes in Computer Science. In Advanced Lectures on Machine Learning; Carbonell, J.G., Siekmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 3540231226. [Google Scholar]
- Duffy, F.H.; Als, H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls—A large case control study. BMC Med. 2012, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Subbaraju, V.; Suresh, M.B.; Sundaram, S.; Narasimhan, S. Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Med. Image Anal. 2017, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Ghiassian, S.; Greiner, R.; Jin, P.; Brown, M.R.G. Using Functional or Structural Magnetic Resonance Images and Personal Characteristic Data to Identify ADHD and Autism. PLoS ONE 2016, 11, e0166934. [Google Scholar] [CrossRef]
- Abraham, A.; Milham, M.P.; Di Martino, A.; Craddock, R.C.; Samaras, D.; Thirion, B.; Varoquaux, G. Deriving reproducible biomarkers from multi-site resting-state data: An Autism-based example. Neuroimage 2017, 147, 736–745. [Google Scholar] [CrossRef]
- Heinsfeld, A.S.; Franco, A.R.; Craddock, R.C.; Buchweitz, A.; Meneguzzi, F. Identification of autism spectrum disorder using deep learning and the ABIDE dataset. NeuroImage Clin. 2018, 17, 16–23. [Google Scholar] [CrossRef]
- Parikh, M.N.; Li, H.; He, L. Enhancing Diagnosis of Autism With Optimized Machine Learning Models and Personal Characteristic Data. Front. Comput. Neurosci. 2019, 13, 9. [Google Scholar] [CrossRef]
- Aluko, O.M.; Lawal, S.A.; Ijomone, O.M.; Aschner, M. Perturbed MAPK signaling in ASD: Impact of metal neurotoxicity. Curr. Opin. Toxicol. 2021, 26, 155. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Yar, M.S.; Grover, G.; Nath, R. Neurological and psychiatric management using COMT inhibitors: A review. Bioorg. Chem. 2020, 94, 103418. [Google Scholar] [CrossRef]
- Esmaiel, N.N.; Ashaat, E.A.; Mosaad, R.; Fayez, A.; Ibrahim, M.; Abdallah, Z.Y.; Issa, M.Y.; Salem, S.; Ramadan, A.; El Wakeel, M.A.; et al. The potential impact of COMT gene variants on dopamine regulation and phenotypic traits of ASD patients. Behav. Brain Res. 2020, 378, 112272. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.K.; Hou, Z.S.; Tao, Y.X. Biased signaling in naturally occurring mutations of G protein-coupled receptors associated with diverse human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165973. [Google Scholar] [CrossRef] [PubMed]
- Rokach, L. Ensemble-based classifiers. Artif. Intell. Rev. 2010, 33, 100538. [Google Scholar] [CrossRef]

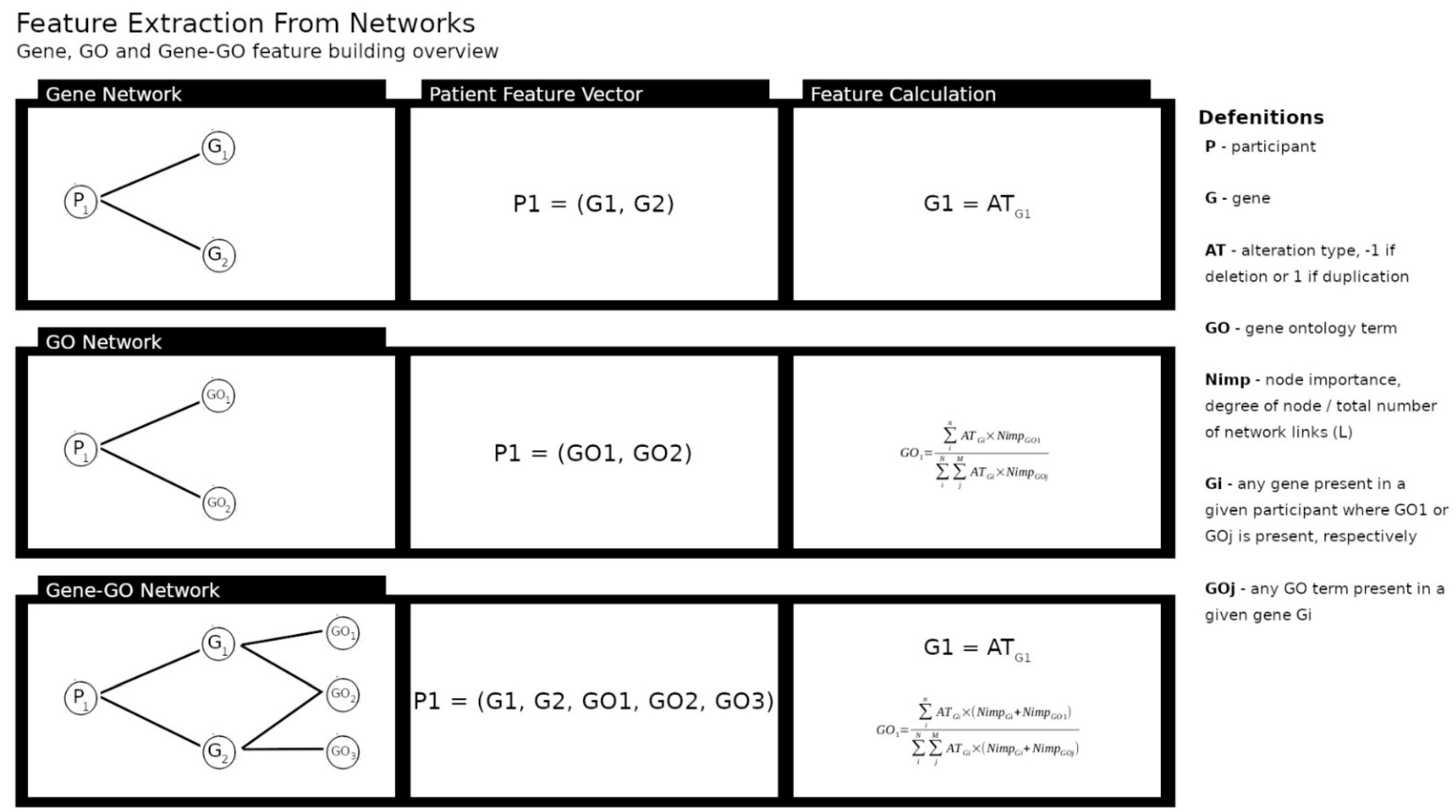
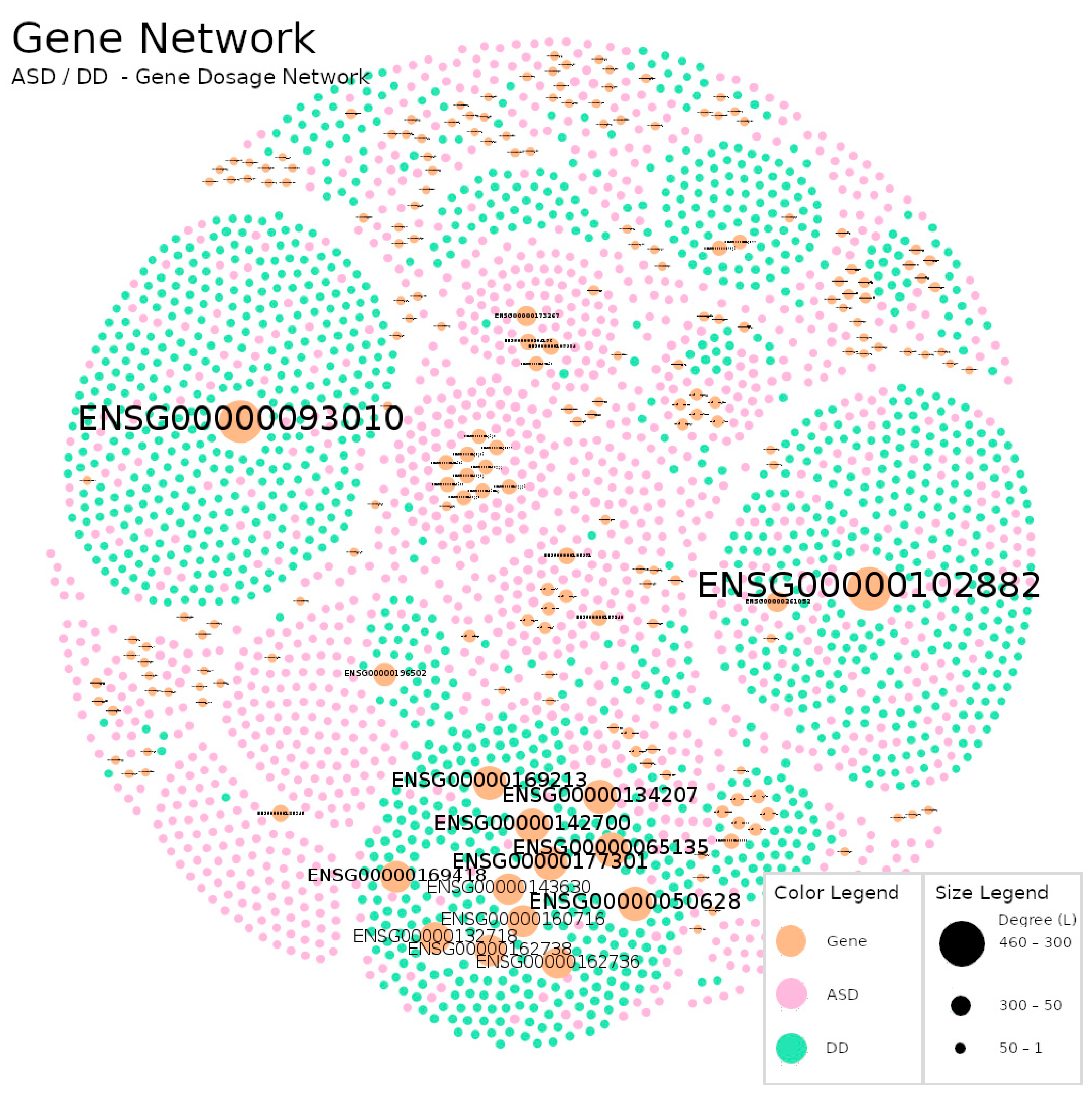
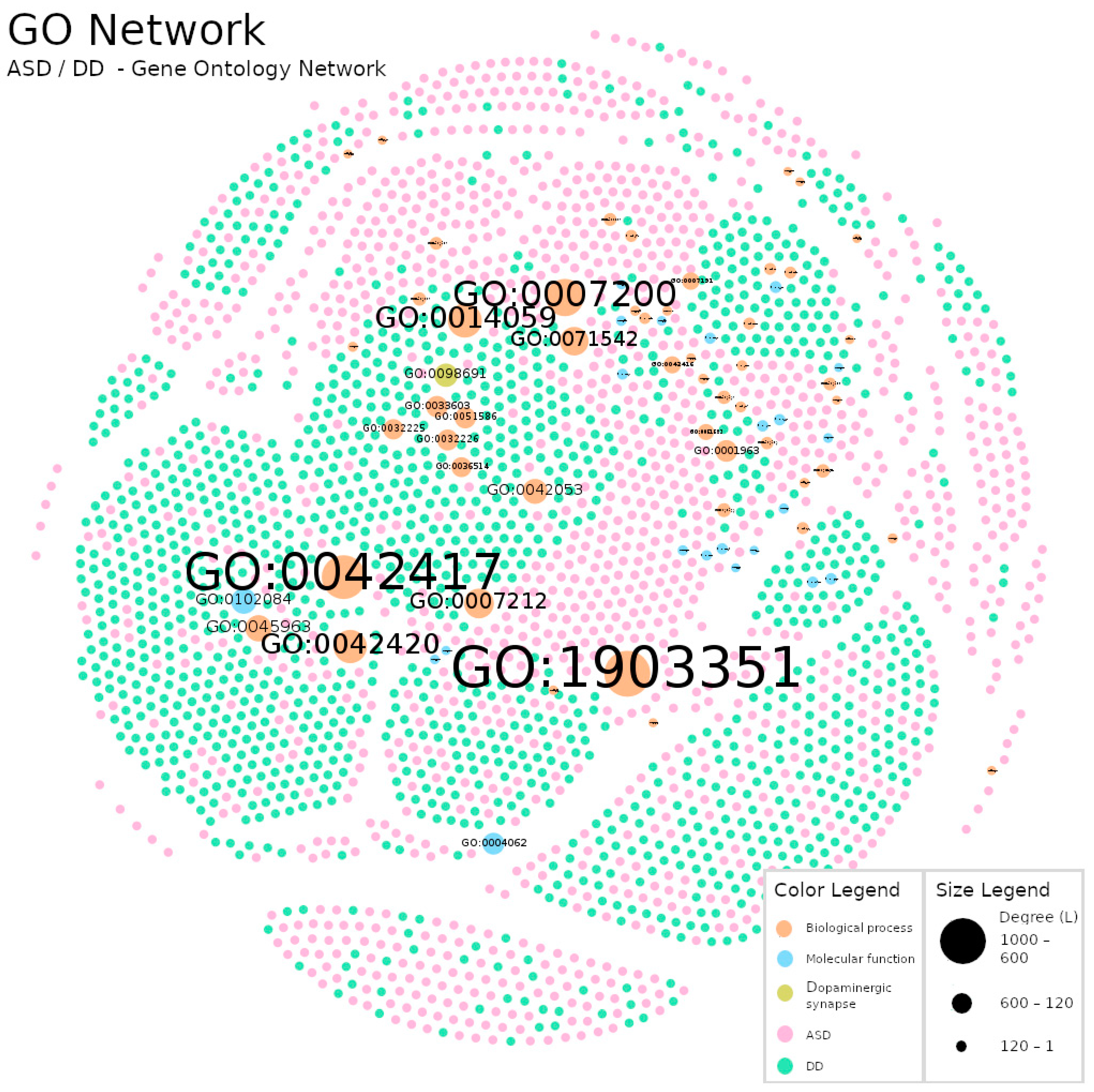
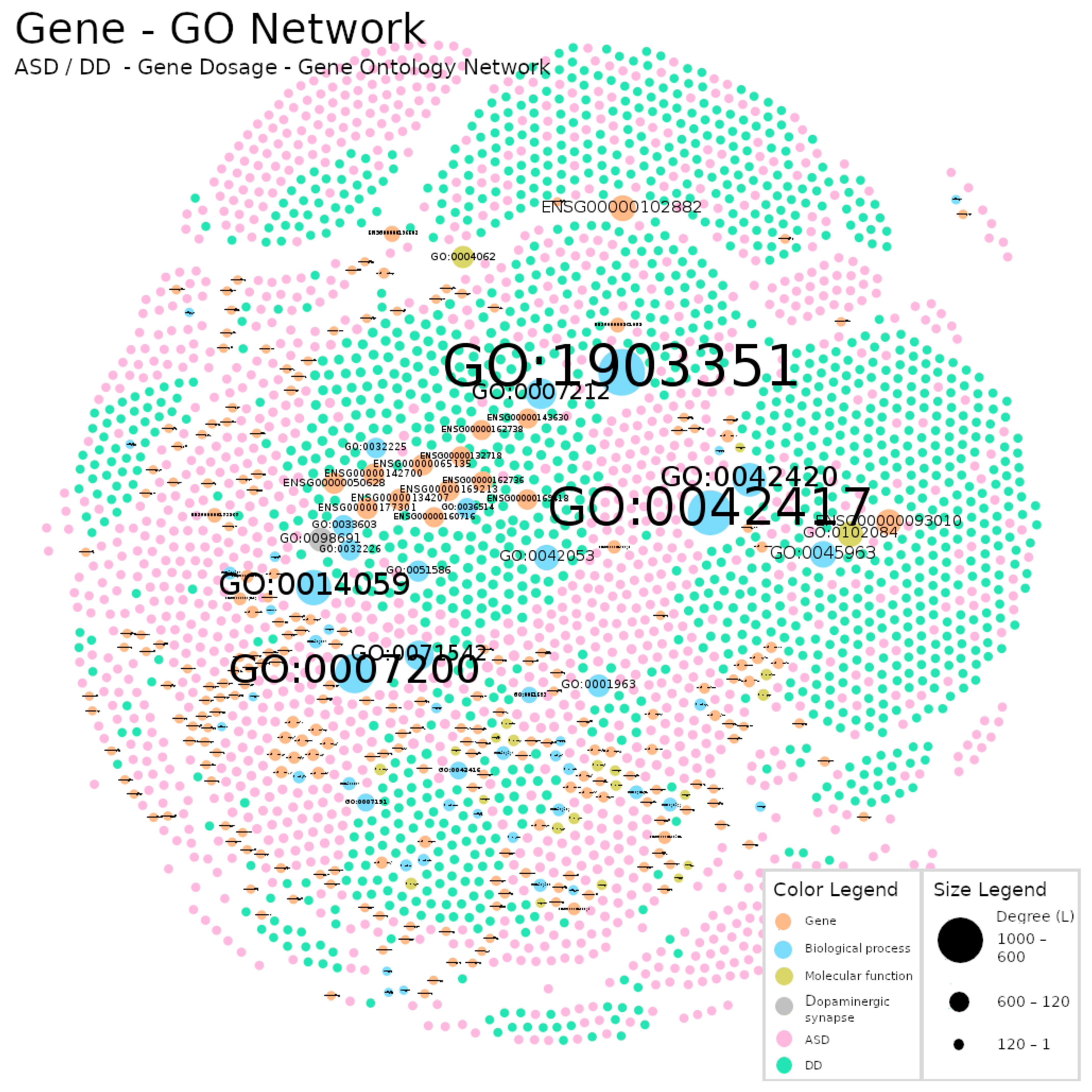



| Diagnosis | Total (N) | Sex(M/F/NS) |
|---|---|---|
| Developmental Delay (DD) | 1327 * | 81/67/1179 |
| Autism Spectrum Disorder (ASD) | 1318 * | 699/134/485 |
| Schizophrenia | 50 | 18/10/22 |
| Intellectual Delay (ID) | 41 | 20/18/3 |
| Middle Cerebral Artery Syndrome (MCA) | 23 | 8/11/4 |
| Epilepsy | 17 | 11/6/0 |
| Childhood Apraxia of Speech (CAS) | 13 | 6/3/4 |
| Polymicrogyria (PMG) | 10 | 0/0/10 |
| Attention Deficit and Hyperactivity Disorder (ADHD) | 6 | 4/0/2 |
| Bipolar Disorder | 3 | 1/2/0 |
| Schizoaffective Disorder | 3 | 0/3/0 |
| Borderline Personality Disorder (BPD) | 1 | 0/1/0 |
| Congenital Heart Disease (CHD) | 1 | 0/1/0 |
| Microcephaly | 1 | 0/1/0 |
| Angelman Syndrome | 1 | 0/1/0 |
| Network | N | L | <k> | Density | Diameter | Radius |
|---|---|---|---|---|---|---|
| Gene Dosage | 2770 | 9387 | 3.389 | 0.001 | 16 | 8 |
| GO | 2719 | 12669 | 4.659 | 0.002 | 7 | 4 |
| Gene Dosage–GO | 2952 | 22568 | 7.645 | 0.003 | 7 | 4 |
| Network | Hub | Name | k | Average Neighbors Degree |
|---|---|---|---|---|
| Gene Dosage | ENSG00000102882 | MAPK3 | 463 | 1.6 |
| ENSG00000093010 | COMT | 450 | 1.2 | |
| ENSG00000050628 | PTGER3 | 339 | 11.8 | |
| GO | GO:1903351 | Cellular response to dopamine | 1029 | 6.3 |
| GO:0042417 | Dopamine metabolic process | 968 | 7.5 | |
| GO:0007200 | Phospholipase C-activating G protein-coupled receptor signaling pathway | 788 | 8.9 |
| Dataset | Tn | Fp | Fn | Tp | N Features | Acc Train (%) | Acc Test(%) | AUC | DD Precision (%) | DD Recall (%) | ASD Precision (%) | ASD Recall (%) | DD f1 Score (%) | ASD f1 Score(%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Dosage | mean | 369.6 | 25.4 | 91.7 | 303.3 | 117.4 | 88.59 | 85.18 | 0.85 | 80.15 | 93.57 | 92.30 | 76.79 | 86.33 | 83.82 |
| sd | 5.3 | 5.3 | 7.7 | 7.7 | 3.9 | 0.41 | 1.11 | 0.01 | 1.35 | 1.35 | 1.47 | 1.96 | 0.98 | 1.31 | |
| GO | mean | 368.0 | 27.0 | 105.5 | 289.5 | 62.0 | 86.25 | 83.22 | 0.83 | 77.73 | 93.15 | 91.50 | 73.28 | 84.73 | 81.36 |
| sd | 6.6 | 6.6 | 7.4 | 7.4 | 1.5 | 0.41 | 1.09 | 0.01 | 1.18 | 1.68 | 1.81 | 1.87 | 0.99 | 1.28 | |
| Gene Dosage–GO | mean | 371.8 | 23.2 | 94.3 | 300.7 | 58.6 | 88.59 | 85.13 | 0.85 | 79.79 | 94.13 | 92.87 | 76.13 | 86.36 | 83.66 |
| sd | 5.0 | 5.0 | 7.2 | 7.2 | 1.6 | 0.37 | 1.06 | 0.01 | 1.24 | 1.27 | 1.42 | 1.83 | 0.94 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.; Caramelo, F.; Melo, J.B.; Castelo-Branco, M. Dopaminergic Gene Dosage Reveals Distinct Biological Partitions between Autism and Developmental Delay as Revealed by Complex Network Analysis and Machine Learning Approaches. J. Pers. Med. 2022, 12, 1579. https://doi.org/10.3390/jpm12101579
Santos A, Caramelo F, Melo JB, Castelo-Branco M. Dopaminergic Gene Dosage Reveals Distinct Biological Partitions between Autism and Developmental Delay as Revealed by Complex Network Analysis and Machine Learning Approaches. Journal of Personalized Medicine. 2022; 12(10):1579. https://doi.org/10.3390/jpm12101579
Chicago/Turabian StyleSantos, André, Francisco Caramelo, Joana Barbosa Melo, and Miguel Castelo-Branco. 2022. "Dopaminergic Gene Dosage Reveals Distinct Biological Partitions between Autism and Developmental Delay as Revealed by Complex Network Analysis and Machine Learning Approaches" Journal of Personalized Medicine 12, no. 10: 1579. https://doi.org/10.3390/jpm12101579





