Longitudinal Follow-Up Using the Heel Enthesitis Magnetic Resonance Imaging Scoring System (HEMRIS) Shows Minimal Changes in Heel Enthesitis Assessed in Spondyloarthritis and Psoriasis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Ankle-MRI Protocol and Scoring
2.3. Clinical Assessments
2.4. Data Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Change in Disease Activity during Follow-Up
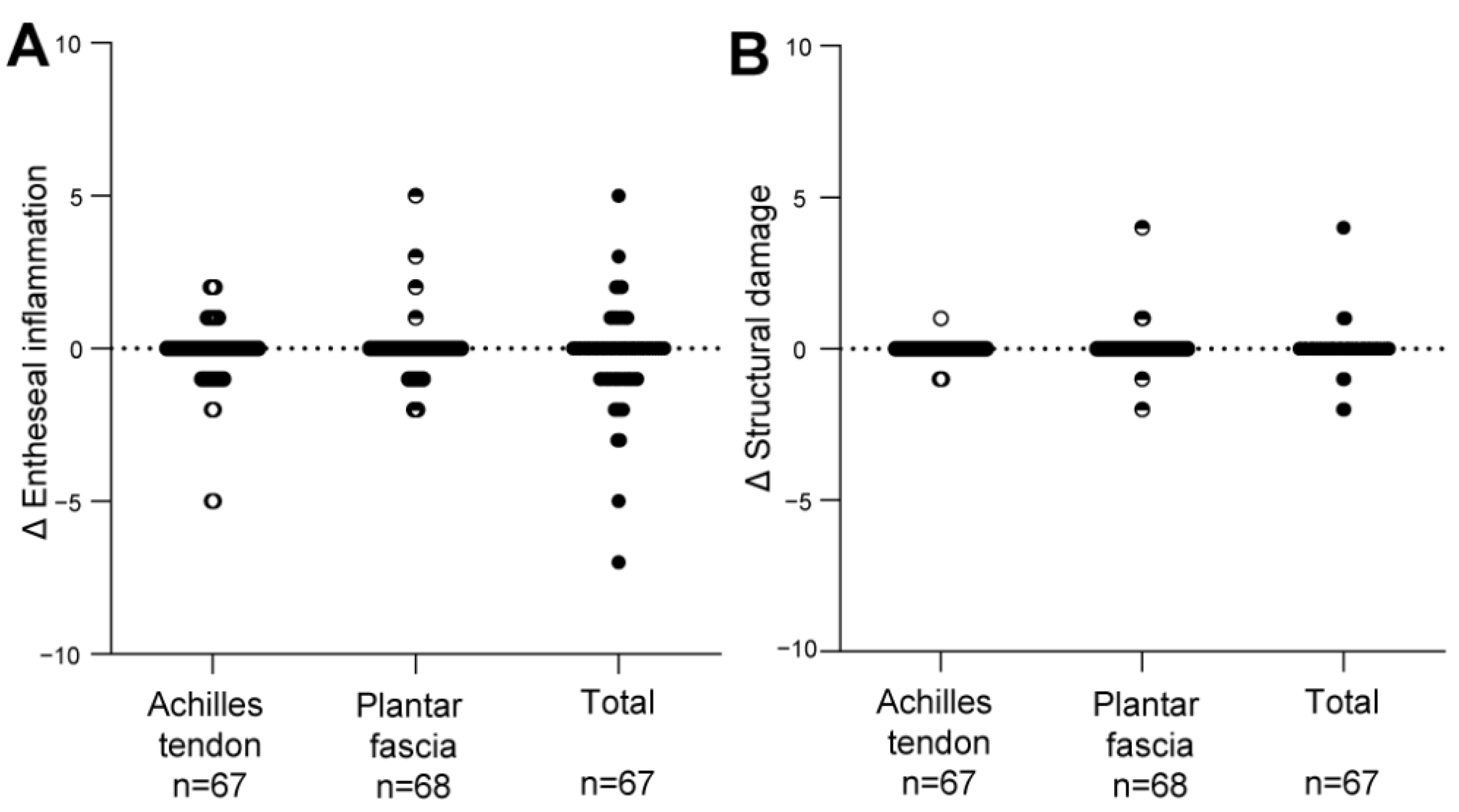
3.3. Change in the HEMRIS during Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Stockwin, L.; Isaacs, J.; Emery, P. An enthesitis based model for the pathogenesis of spondyloarthropathy. Additive effects of microbial adjuvant and biomechanical factors at disease sites. J. Rheumatol. 2001, 28, 2155–2159. [Google Scholar] [PubMed]
- Coates, L.C.; Ávila, D.G.F.; Fitzgerald, O.; Garg, A.; Gladman, D.D.; Lindsay, C. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijde, D.; Ramiro, S.; Landewé, R.; Baraliakos, X.; Van Den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; Dagfinrud, H.; Dougados, M.; et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 978–991. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Tan, A.L. The enthesis in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, 36–39. [Google Scholar]
- Kehl, A.S.; Corr, M.; Weisman, M.H. Review: Enthesitis: New Insights Into Pathogenesis, Diagnostic Modalities, and Treatment. Arthritis Rheumatol. 2016, 68, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P. Enthesitis in psoriatic arthritis (Part 3): Clinical assessment and management. Rheumatology 2020, 59, i21–i28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakewell, C.; Aydin, S.Z.; Ranganath, V.K.; Eder, L.; Kaeley, G.S. Imaging Techniques: Options for the Diagnosis and Monitoring of Treatment of Enthesitis in Psoriatic Arthritis. J. Rheumatol. 2019, 47, 973–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.J.; Krabbe, S.; Eshed, I.; Gandjbakhch, F.; Bird, P.; Pedersen, S.J.; Stoenoiu, M.S.; Foltz, V.; Glinatsi, D.; Lambert, R.G.; et al. The OMERACT MRI in Enthesitis Initiative: Definitions of Key Pathologies, Suggested MRI Sequences and Novel Heel Enthesitis Scoring System (HEMRIS). J. Rheumatol. 2019, 46, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.J.; Krabbe, S.; Eshed, I.; Lambert, R.G.; Laredo, J.-D.; Maksymowych, W.P.; Gandjbakhch, F.; Emad, Y.; Stoenoiu, M.S.; Foltz, V.; et al. Atlas of the OMERACT Heel Enthesitis MRI Scoring System (HEMRIS). RMD Open 2020, 6, e001150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraliakos, X.; Sewerin, P.; de Miguel, E.; Pournara, E.; Kleinmond, C.; Shekhawat, A.; Jentzsch, C.; Wiedon, A.; Behrens, F. Magnetic resonance imaging characteristics in patients with spondyloarthritis and clinical diagnosis of heel enthesitis: Post hoc analysis from the phase 3 ACHILLES trial. Arthritis Res. Ther. 2022, 24, 111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinrensink, N.J.; Foppen, W.; Ten Katen, I.; Van Der Veen, P.H.; De Klerk, B.; Diepstraten, S.C.E.; Radstake, T.R.D.J.; Lafeber, F.P.J.G.; De Jong, P.A.; Leijten, E.F.A.A. Comparison of the Heel Enthesitis MRI Scoring System (HEMRIS) with clinical enthesitis and local metabolic activity on PET-CT. RMD Open 2020, 6, e001424. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, T.; Pettersson, U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, L.C.; Fransen, J.; Helliwell, P.S. Defining minimal disease activity in psoriatic arthritis: A proposed objective target for treatment. Ann. Rheum. Dis. 2010, 69, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Healy, P.J.; Helliwell, P.S. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Care Res. 2008, 59, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Krabbe, S.; Eshed, I.; Gandjbakhch, F.; Pedersen, S.J.; Bird, P.; Mathew, A.J.; Lambert, R.G.; Maksymowych, W.P.; Glinatsi, D.; Stoenoiu, M.S.; et al. Development and validation of an OMERACT MRI whole-body score for inflammation in peripheral joints and entheses in inflammatory arthritis (MRI-WIPE). J. Rheumatol. 2019, 46, 1215–1221. [Google Scholar] [CrossRef] [PubMed]





| Disease Category | ||||
|---|---|---|---|---|
| Pso | PsA | AS | All | |
| Total one year intervals, N | 24 | 24 | 24 | 72 |
| Demographics | ||||
| Male gender, n (%): | 12 (50.0) | 18 (75.0) | 18 (75.0) | 48 (66.7) |
| Age, median (IQR): | 42.2 (34.7–53.4) | 50.9 (40.6–52.9) | 49.1 (38.8–52.2) | 49.2 (36.8–52.8) |
| Disease duration in years, median (IQR) | 22.5 (14.4–42.2) | 7.8 (0.9–12.6) | 8.7 (3.4–17.2) | NA |
| General disease activity: | ||||
| Pso: moderate-severe psoriasis, n (%) | 5 (20.8) | NA | NA | NA |
| PsA: MDA, n (%) | NA | 11 (45.8) | NA | NA |
| Missing, n (%) | NA | 3 (12.5) | NA | NA |
| AS: BASDAI score ≥ 4, n (%) | NA | NA | 22 (91.7) | NA |
| Medication: | ||||
| Current DMARD use, n (%): | 0 | 4 (16.7) | 1 (4.2) | 5 (6.6) |
| Current NSAID use, n (%): | 2 (8.3) | 7(29.2) | 16 (66.7) | 25 (34.7) |
| Missing, n (%) | 1 (4.2) | 0 | 0 | 1 (1.4) |
| Inflammatory markers: | ||||
| ESR, median (IQR): | 4.0 (2.0–10.0) | 4.0 (2.0–6.0) | 5.0 (3.0–6.0) | 4.0 (2.0–6.5) |
| Missing, n (%) | 2 (8.0) | 0 | 1 | 2 (2.8) |
| CRP, median (IQR): | 2.5 (0.9–5.7) | 3.0 (1.6–4.6) | 1.6 (0.9–4.4) | 2.8 (1.2–4.5) |
| Missing, n (%) | 2 (8.0) | 0 | 1 | 3 (4.2) |
| Local disease activity at the enthesis: | ||||
| Achilles tendon, N enthesis | 48 | 48 | 48 | 144 |
| Clinical enthesitis, n (%) | 2 (4.2) | 1 (2.1) | 3 (6.3) | 6 (4.2) |
| Missing, n (%) | 0 | 4 (8.3) | 6 (12.5) | 10 (6.9) |
| Plantar fascia, N enthesis | 48 | 48 | 48 | 144 |
| Clinical enthesitis, n (%) | 1 (2.1) | 4 (8.3) | 2 (4.2) | 7 (4.9) |
| Missing, n (%) | 0 | 4 (8.3) | 6 (12.5) | 10 (6.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinrensink, N.J.; Foppen, W.; Katen, I.t.; Leijten, E.F.A.; de Jong, P.A.; Spierings, J. Longitudinal Follow-Up Using the Heel Enthesitis Magnetic Resonance Imaging Scoring System (HEMRIS) Shows Minimal Changes in Heel Enthesitis Assessed in Spondyloarthritis and Psoriasis Patients. J. Pers. Med. 2022, 12, 1765. https://doi.org/10.3390/jpm12111765
Kleinrensink NJ, Foppen W, Katen It, Leijten EFA, de Jong PA, Spierings J. Longitudinal Follow-Up Using the Heel Enthesitis Magnetic Resonance Imaging Scoring System (HEMRIS) Shows Minimal Changes in Heel Enthesitis Assessed in Spondyloarthritis and Psoriasis Patients. Journal of Personalized Medicine. 2022; 12(11):1765. https://doi.org/10.3390/jpm12111765
Chicago/Turabian StyleKleinrensink, Nienke J., Wouter Foppen, Iris ten Katen, Emmerik F. A. Leijten, Pim A. de Jong, and Julia Spierings. 2022. "Longitudinal Follow-Up Using the Heel Enthesitis Magnetic Resonance Imaging Scoring System (HEMRIS) Shows Minimal Changes in Heel Enthesitis Assessed in Spondyloarthritis and Psoriasis Patients" Journal of Personalized Medicine 12, no. 11: 1765. https://doi.org/10.3390/jpm12111765





