Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition
2.3. Data Pre-Processing
2.4. Feature Selection
2.5. Support Vector Machines
3. Results
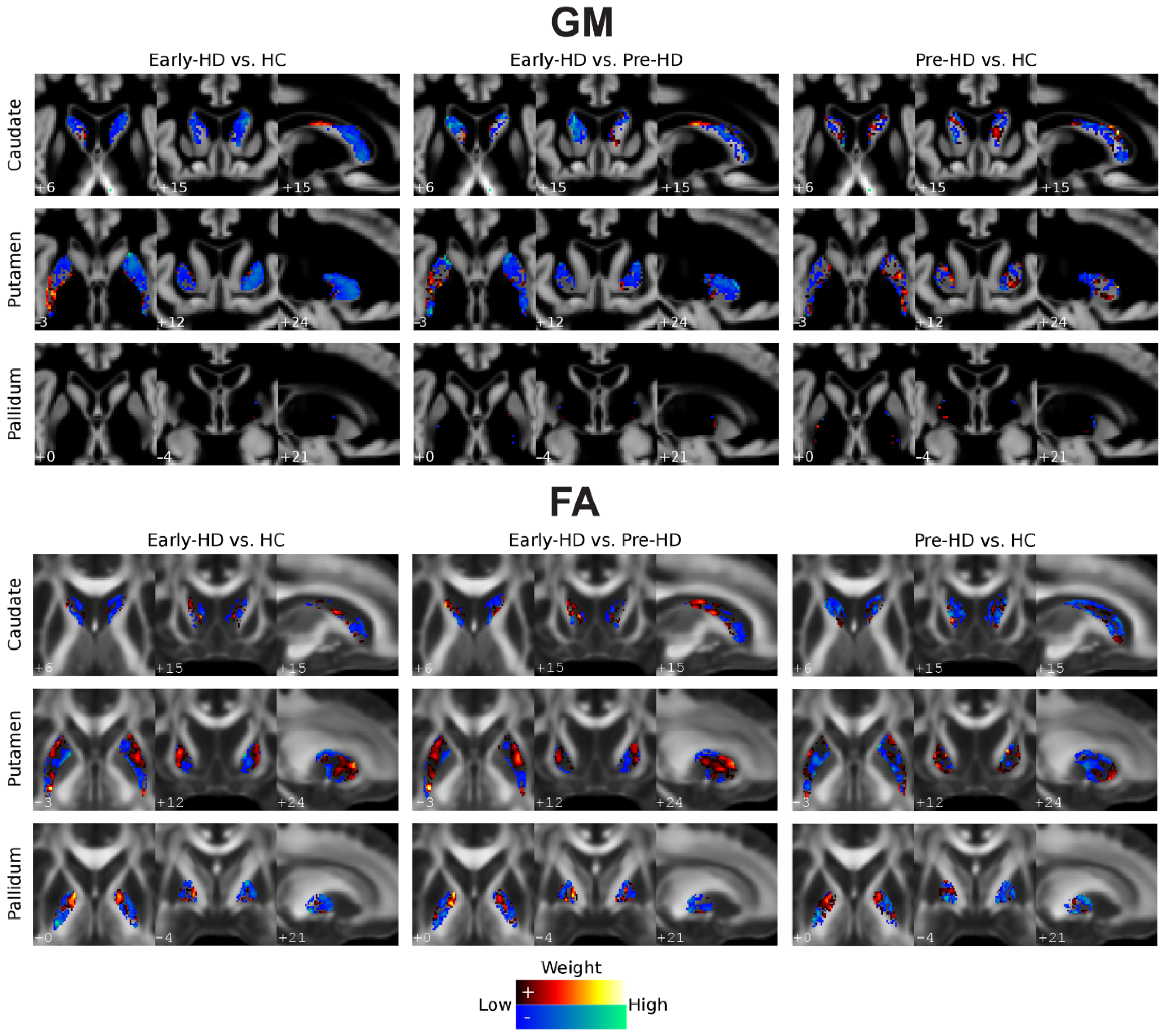
3.1. Subcortical Volumes
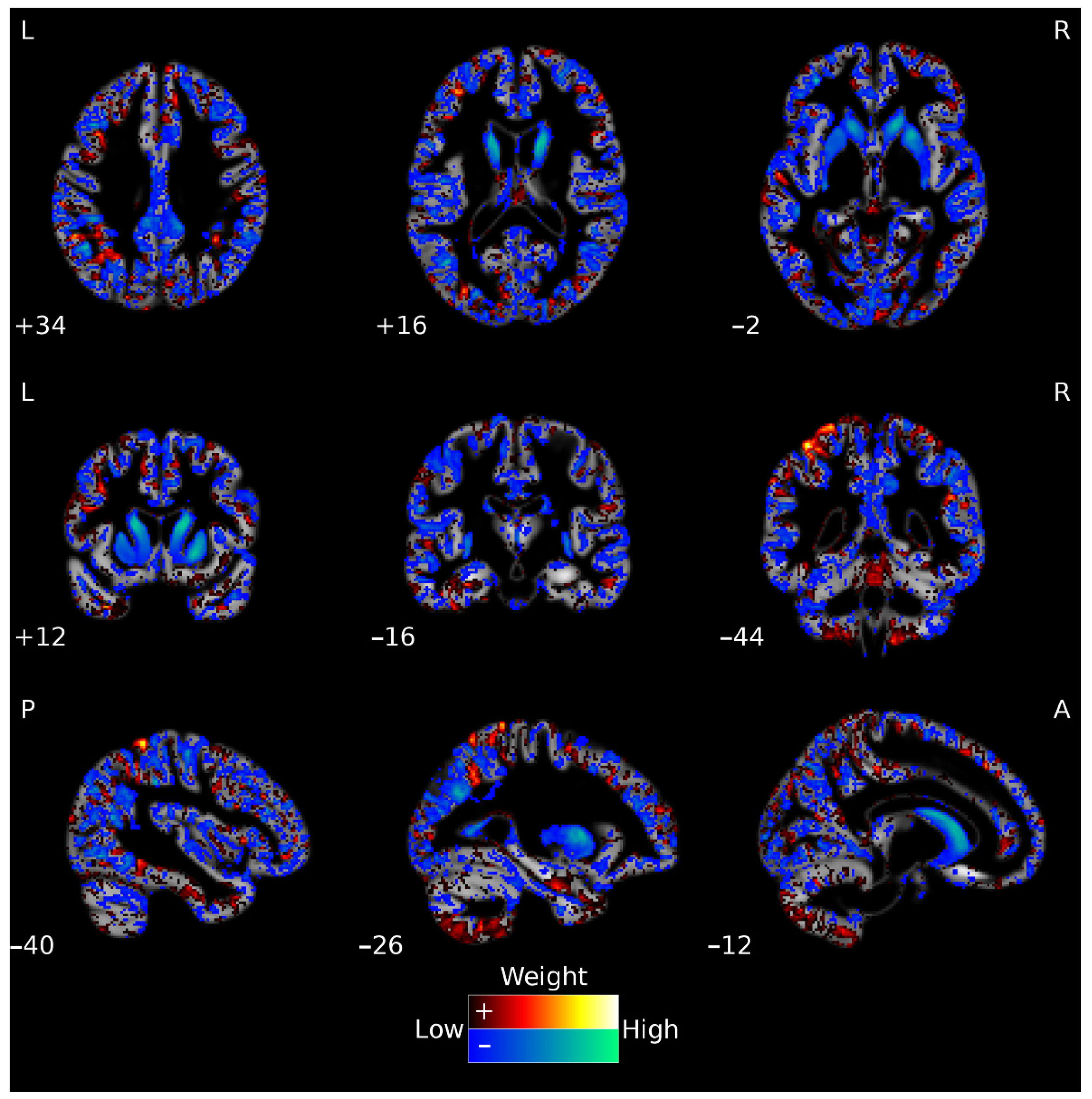
3.2. GM Classification
3.3. FA Classification
3.4. Multimodal Neuroimaging Classification
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aylward, E.H. Change in MRI striatal volumes as a biomarker in preclinical Huntington’s disease. Brain Res. Bull. 2007, 72, 152–158. [Google Scholar] [CrossRef]
- Aylward, E.H.; Sparks, B.F.; Field, K.M.; Yallapragada, V.; Shpritz, B.D.; Rosenblatt, A.; Brandt, J.; Gourley, L.M.; Liang, K.; Zhou, H.; et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology 2004, 63, 66–72. [Google Scholar] [CrossRef]
- Kipps, C.M.; Duggins, A.J.; Mahant, N.; Gomes, L.; Ashburner, J.; McCusker, E.A. Progression of structural neuropathology in preclinical Huntington’s disease: A tensor based morphometry study. J. Clin. Exp. Neuropsychol. 2005, 76, 650–655. [Google Scholar] [CrossRef]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P. Neuropathological Classification of Huntingtons-Disease. J. Neuropath. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef]
- Bohanna, I.; Georgiou-Karistianis, N.; Hannan, A.J.; Egan, G.F. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington’s disease. Brain Res. Rev. 2008, 58, 209–225. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Magnotta, V.A.; Mikos, A.E.; Paulson, H.L.; Penziner, E.; Andreasen, N.C.; Nopoulos, P.C. Brain structure in preclinical Huntington’s disease. Biol. Psychiatry 2006, 59, 57–63. [Google Scholar] [CrossRef]
- Aylward, E.H.; Codori, A.M.; Barta, P.E.; Pearlson, G.D.; Harris, G.J.; Brandt, J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch. Neurol. 1996, 53, 1293–1296. [Google Scholar] [CrossRef]
- Aylward, E.H.; Nopoulos, P.C.; Ross, C.A.; Langbehn, D.R.; Pierson, R.K.; Mills, J.A.; Johnson, H.J.; Magnotta, V.A.; Juhl, A.R.; Paulsen, J.S.; et al. Longitudinal change in regional brain volumes in prodromal Huntington disease. J. Neurol. Neurosurg. Psychiatry 2011, 82, 405–410. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Brinkman, R.R.; Falush, D.; Paulsen, J.S.; Hayden, M.R.; International Huntington’s Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004, 65, 267–277. [Google Scholar] [CrossRef]
- Gómez-Ansón, B.; Alegret, M.; Munoz, E.; Monte, G.C.; Alayrach, E.; Sanchez, A.; Boada, M.; Tolosa, E. Prefrontal cortex volume reduction on MRI in preclinical Huntington’s disease relates to visuomotor performance and CAG number. Parkinsonism Relat. Disord. 2009, 15, 213–219. [Google Scholar] [CrossRef]
- Reading, S.A.; Yassa, M.A.; Bakker, A.; Dziorny, A.C.; Gourley, L.M.; Yallapragada, V.; Rosenblatt, A.; Margolis, R.L.; Aylward, E.H.; Brandt, J.; et al. Regional white matter change in pre-symptomatic Huntington’s disease: A diffusion tensor imaging study. Psychiat. Res.-Neuroim. 2005, 140, 55–62. [Google Scholar] [CrossRef]
- Rosas, H.D.; Hevelone, N.D.; Zaleta, A.K.; Greve, D.N.; Salat, D.H.; Fischl, B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology 2005, 65, 745–747. [Google Scholar] [CrossRef]
- Rosas, H.D.; Tuch, D.S.; Hevelone, N.D.; Zaleta, A.K.; Vangel, M.; Hersch, S.M.; Salat, D.H. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov. Disord. 2006, 21, 1317–1325. [Google Scholar] [CrossRef]
- Stoffers, D.; Sheldon, S.; Kuperman, J.M.; Goldstein, J.; Corey-Bloom, J.; Aron, A.R. Contrasting gray and white matter changes in preclinical Huntington disease: An MRI study. Neurology 2010, 74, 1208–1216. [Google Scholar] [CrossRef]
- Thieben, M.J.; Duggins, A.J.; Good, C.D.; Gomes, L.; Mahant, N.; Richards, F.; McCusker, E.; Frackowiak, R.S. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain 2002, 125, 1815–1828. [Google Scholar] [CrossRef]
- Aylward, E.H.; Brandt, J.; Codori, A.M.; Mangus, R.S.; Barta, P.E.; Harris, G.J. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology 1994, 44, 823–828. [Google Scholar] [CrossRef]
- Klöppel, S.; Draganski, B.; Golding, C.V.; Chu, C.; Nagy, Z.; Cook, P.A.; Hicks, S.L.; Kennard, C.; Alexander, D.C.; Parker, G.J.; et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain 2008, 131, 196–204. [Google Scholar] [CrossRef][Green Version]
- Klöppel, S.; Chu, C.; Tan, G.C.; Draganski, B.; Johnson, H.; Paulsen, J.S.; Kienzle, W.; Tabrizi, S.J.; Ashburner, J.; Frackowiak, R.S.; et al. Automatic detection of preclinical neurodegeneration: Presymptomatic Huntington disease. Neurology 2009, 72, 426–431. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Hayden, M.R.; Paulsen, J.S.; PREDICT-HD Investigators of the Huntington Study Group. CAG-repeat length and the age of onset in Huntington disease (HD): A review and validation study of statistical approaches. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 397–408. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Langbehn, D.R.; Stout, J.C.; Aylward, E.; Ross, C.A.; Nance, M.; Guttman, M.; Johnson, S.; MacDonald, M.; Beglinger, L.J.; et al. Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 874–880. [Google Scholar] [CrossRef]
- Rizk-Jackson, A.; Stoffers, D.; Sheldon, S.; Kuperman, J.; Dale, A.; Goldstein, J.; Corey-Bloom, J.; Poldrack, R.A.; Aron, A.R. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington’s disease using machine learning techniques. Neuroimage 2011, 56, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Rosas, H.D.; Liu, A.K.; Hersch, S.; Glessner, M.; Ferrante, R.J.; Salat, D.H.; van der Kouwe, A.; Jenkins, B.G.; Dale, A.M.; Fischl, B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002, 58, 695–701. [Google Scholar] [CrossRef]
- Rosas, H.D.; Salat, D.H.; Lee, S.Y.; Zaleta, A.K.; Pappu, V.; Fischl, B.; Greve, D.; Hevelone, N.; Hersch, S.M. Cerebral cortex and the clinical expression of Huntington’s disease: Complexity and heterogeneity. Brain 2008, 131, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Bohanna, I.; Georgiou-Karistianis, N.; Sritharan, A.; Asadi, H.; Johnston, L.; Churchyard, A.; Egan, G. Diffusion tensor imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 2011, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Lolli, F.; Della Nave, R.; Tessa, C.; Petralli, R.; Gavazzi, C.; Politi, L.S.; Macucci, M.; Filippi, M.; Piacentini, S. Huntington disease: Volumetric, diffusion-weighted, and magnetization transfer MR imaging of brain. Radiology 2004, 232, 867–873. [Google Scholar] [CrossRef]
- Sánchez-Castañeda, C.; Cherubini, A.; Elifani, F.; Peran, P.; Orobello, S.; Capelli, G.; Sabatini, U.; Squitieri, F. Seeking Huntington disease biomarkers by multimodal, cross-sectional basal ganglia imaging. Hum. Brain Mapp. 2013, 34, 1625–1635. [Google Scholar] [CrossRef]
- Seppi, K.; Schocke, M.F.; Mair, K.J.; Esterhammer, R.; Weirich-Schwaiger, H.; Utermann, B.; Egger, K.; Brenneis, C.; Granata, R.; Boesch, S.; et al. Diffusion-weighted imaging in Huntington’s disease. Mov. Disord. 2006, 21, 1043–1047. [Google Scholar] [CrossRef]
- Douaud, G.; Behrens, T.E.; Poupon, C.; Cointepas, Y.; Jbabdi, S.; Gaura, V.; Golestani, N.; Krystkowiak, P.; Verny, C.; Damier, P.; et al. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage 2009, 46, 958–966. [Google Scholar] [CrossRef]
- Dumas, E.M.; van den Bogaard, S.J.; Ruber, M.E.; Reilman, R.R.; Stout, J.C.; Craufurd, D.; Hicks, S.L.; Kennard, C.; Tabrizi, S.J.; van Buchem, M.A.; et al. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Hum. Brain Mapp. 2012, 33, 203–212. [Google Scholar] [CrossRef]
- Marrakchi-Kacem, L.; Delmaire, C.; Guevara, P.; Poupon, F.; Lecomte, S.; Tucholka, A.; Roca, P.; Yelnik, J.; Durr, A.; Mangin, J.F.; et al. Mapping cortico-striatal connectivity onto the cortical surface: A new tractography-based approach to study Huntington disease. PLoS ONE 2013, 8, e53135. [Google Scholar] [CrossRef] [PubMed]
- Della Nave, R.; Ginestroni, A.; Tessa, C.; Giannelli, M.; Piacentini, S.; Filippi, M.; Mascalchi, M. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: A tract-based spatial statistics study. AJNR Am. J. Neuroradiol. 2010, 31, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Georgiou-Karistianis, N.; Scahill, R.; Tabrizi, S.J.; Squitieri, F.; Aylward, E. Structural MRI in Huntington’s disease and recommendations for its potential use in clinical trials. Neurosci. Biobehav. Rev. 2013, 37, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.I.; Zeun, P.; Osborne-Crowley, K.; Johnson, E.B.; Gregory, S.; Parker, C.; Lowe, J.; Nair, A.; O’Callaghan, C.; Langley, C.; et al. Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington’s disease Young Adult Study (HD-YAS): A cross-sectional analysis. Lancet Neurol. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Orrù, G.; Pettersson-Yeo, W.; Marquand, A.F.; Sartori, G.; Mechelli, A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012, 36, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Georgiou-Karistianis, N.; Gray, M.A.; Dominguez, J.F.; Dymowski, A.R.; Bohanna, I.; Johnston, L.A.; Churchyard, A.; Chua, P.; Stout, J.C.; Egan, G.F. Automated differentiation of pre-diagnosis Huntington’s disease from healthy control individuals based on quadratic discriminant analysis of the basal ganglia: The IMAGE-HD study. Neurobiol. Dis. 2013, 51, 82–92. [Google Scholar] [CrossRef]
- Mason, S.L.; Daws, R.E.; Soreq, E.; Johnson, E.B.; Scahill, R.I.; Tabrizi, S.J.; Barker, R.A.; Hampshire, A. Predicting clinical diagnosis in Huntington’s disease: An imaging polymarker. Ann. Neurol. 2018, 83, 532–543. [Google Scholar] [CrossRef]
- Polosecki, P.; Castro, E.; Rish, I.; Pustina, D.; Warner, J.H.; Wood, A.; Sampaio, C.; Cecchi, G.A. Resting-state connectivity stratifies premanifest Huntington’s disease by longitudinal cognitive decline rate. Sci. Rep. 2020, 10, 1252. [Google Scholar] [CrossRef]
- Huntington-Study-Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Mov. Disord. 1996, 11, 136–142. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Kononenko, I.; Šimec, E.; Robnik-Šikonja, M. Overcoming the Myopia of Inductive Learning Algorithms with RELIEFF. Appl. Intell. 1997, 7, 39–55. [Google Scholar] [CrossRef]
- Haller, S.; Missonnier, P.; Herrmann, F.R.; Rodriguez, C.; Deiber, M.P.; Nguyen, D.; Gold, G.; Lovblad, K.O.; Giannakopoulos, P. Individual classification of mild cognitive impairment subtypes by support vector machine analysis of white matter DTI. AJNR Am. J. Neuroradiol. 2013, 34, 283–291. [Google Scholar] [CrossRef]
- Schrouff, J.; Rosa, M.J.; Rondina, J.M.; Marquand, A.F.; Chu, C.; Ashburner, J.; Phillips, C.; Richiardi, J.; Mourao-Miranda, J. PRoNTo: Pattern recognition for neuroimaging toolbox. Neuroinformatics 2013, 11, 319–337. [Google Scholar] [CrossRef]
- Schrouff, J.; Cremers, J.; Garraux, G.; Baldassarre, L.; Mourão-Miranda, J.; Phillips, C. Localizing and Comparing Weight Maps Generated from Linear Kernel Machine Learning Models. In Proceedings of the 2013 International Workshop on Pattern Recognition in Neuroimaging, Philadelphia, PA, USA, 22–24 June 2013; pp. 124–127. [Google Scholar]
- Rakotomamonjy, A.; Bach, F.R.; Canu, S.; Grandvalet, Y. SimpleMKL. J. Mach. Learn. Res. 2008, 9, 2491–2521. [Google Scholar]
- Estevez-Fraga, C.; Scahill, R.; Rees, G.; Tabrizi, S.J.; Gregory, S. Diffusion imaging in Huntington’s disease: Comprehensive review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 62. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Burgunder, J.; Cheng, B.; Shang, H. Diffusion imaging studies of Huntington’s disease: A meta-analysis. Parkinsonism Relat. Disord. 2016, 32, 94–101. [Google Scholar] [CrossRef]
- Shaffer, J.J.; Ghayoor, A.; Long, J.D.; Kim, R.E.Y.; Lourens, S.; O’Donnell, L.J.; Westin, C.F.; Rathi, Y.; Magnotta, V.; Paulsen, J.S.; et al. Longitudinal diffusion changes in prodromal and early HD: Evidence of white-matter tract deterioration. Hum. Brain Mapp. 2017, 38, 1460–1477. [Google Scholar] [CrossRef]
- Novak, M.J.U.; Seunarine, K.K.; Gibbard, C.R.; Hobbs, N.Z.; Scahill, R.I.; Clark, C.A.; Tabrizi, S.J. White matter integrity in premanifest and early Huntington’s disease is related to caudate loss and disease progression. Cortex 2014, 52, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.P.; Gorges, M.; Grön, G.; Kassubek, J.; Landwehrmeyer, G.B.; Süßmuth, S.D.; Wolf, R.C.; Orth, M. Motor network structure and function are associated with motor performance in Huntington’s disease. J. Neurol. 2016, 263, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Bohanna, I.; Georgiou-Karistianis, N.; Egan, G.F. Connectivity-based segmentation of the striatum in Huntington’s disease: Vulnerability of motor pathways. Neurobiol. Dis. 2011, 42, 475–481. [Google Scholar] [CrossRef]
- Hobbs, N.Z.; Cole, J.H.; Farmer, R.E.; Rees, E.M.; Crawford, H.E.; Malone, I.B.; Roos, R.A.C.; Sprengelmeyer, R.; Durr, A.; Landwehrmeyer, B.; et al. Evaluation of multi-modal, multi-site neuroimaging measures in Huntington’s disease: Baseline results from the PADDINGTON study. Neuroimage Clin. 2012, 2, 204–211. [Google Scholar] [CrossRef]
- Chu, C.; Hsu, A.L.; Chou, K.H.; Bandettini, P.; Lin, C.; Alzheimer’s Disease Neuroimaging Initiative. Does feature selection improve classification accuracy? Impact of sample size and feature selection on classification using anatomical magnetic resonance images. Neuroimage 2012, 60, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.; Singh, V.; Xu, G.; Johnson, S.C.; Alzheimers Disease Neuroimaging Initiative. Predictive markers for AD in a multi-modality framework: An analysis of MCI progression in the ADNI population. Neuroimage 2011, 55, 574–589. [Google Scholar] [CrossRef]
- Zu, C.; Jie, B.; Liu, M.; Chen, S.; Shen, D.; Zhang, D.; Alzheimer’s Disease Neuroimaging, I. Label-aligned multi-task feature learning for multimodal classification of Alzheimer’s disease and mild cognitive impairment. Brain Imaging Behav. 2016, 10, 1148–1159. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Stout, J.C.; Gregory, S.; Mills, J.A.; Durr, A.; Leavitt, B.R.; Roos, R.A.C.; Long, J.D.; Owen, G.; Johnson, H.J.; et al. Association of CAG Repeats With Long-term Progression in Huntington Disease. JAMA Neurol. 2019, 76, 1375–1385. [Google Scholar] [CrossRef]

| GM and FA | GM | FA | |||
|---|---|---|---|---|---|
| HC | Pre-HD | Early-HD | Pre-HD | Early-HD | |
| N | 18 | 14 | 11 | 12 | 10 |
| Sex, M/F | 6/12 | 5/9 | 4/7 | 4/8 | 4/6 |
| Age (range) | 36.4 ± 11.3 (18–62) | 35.8 ± 9.5 (19–58) | 45.1 ± 14.2 (26–71) | 34.6 ± 7.6 (19–48) | 45.7 ± 14.7 (26–71) |
| CAG (range) | - | 41.4 ± 1.8 (39–45) | 44.0 ± 2.7 (39–48) | 41.4 ± 2.0 (39–45) | 43.8 ± 2.7 (39–48) |
| YTO (range) | - | 20.3 ± 10.5 * (−0.9–43.1) | - | 21.7 ± 9.4 (10.2–43.1) | - |
| Disease duration (range) | - | - | 5.4 ± 2.5 (2–12) | - | 5.6 ± 2.5 (2–12) |
| GM Features | |||||||||
| Early-HD vs. HC | Early-HD vs. Pre-HD | Pre-HD vs. HC | |||||||
| ROIs | Acc. | Sen. | Spe. | Acc. | Sen. | Spe. | Acc. | Sen. | Spe. |
| Whole-brain | 88.6 ± 3.8 ** | 84.4 ± 4.4 | 92.9 ± 4.9 | 78.2 ± 6.8 * | 72.9 ± 8.7 | 83.5 ± 7.0 | 40.5 ± 6.6 | 30.7 ± 7.4 | 50.3 ± 8.7 |
| Caudate | 91.6 ± 2.6 ** | 91.3 ± 1.7 | 92.0 ± 4.4 | 83.0 ± 3.7 ** | 83.8 ± 3.8 | 82.1 ± 5.6 | 59.5 ± 6.6 | 57.8 ± 7.4 | 61.2 ± 7.6 |
| Putamen | 94.8 ± 2.8 ** | 98.3 ± 3.6 | 91.3 ± 6.8 | 86.3 ± 4.2 ** | 84.7 ± 4.3 | 87.8 ± 5.5 | 56.7 ± 6.0 | 60.1 ± 6.1 | 53.4 ± 9.0 |
| Pallidum | 77.5 ± 8.1 ** | 75.9 ± 14.9 | 79.2 ± 9.6 | 68.1 ± 3.2 ** | 38.3 ± 6.9 | 98.0 ± 4.2 | 51.9 ± 2.6 | 91.9 ± 21.8 | 11.9 ± 18.6 |
| Relief-F 100 | 94.8 ± 2.5 ** | 100.0 ± 0.0 | 89.5 ± 5.1 | 83.1 ± 5.4 ** | 85.2 ± 4.4 | 81.0 ± 7.4 | 45.9 ± 9.6 | 47.3 ± 12.3 | 44.4 ± 9.7 |
| Relief-F 500 | 93.3 ± 4.3 ** | 96.5 ± 4.4 | 90.1 ± 6.4 | 80.7 ± 5.3 * | 82.5 ± 2.5 | 78.8 ± 8.9 | 48.2 ± 7.6 | 43.6 ± 6.9 | 52.9 ± 11.4 |
| Relief-F 1000 | 93.1 ± 4.8 ** | 94.9 ± 4.5 | 91.3 ± 6.0 | 83.5 ± 3.6 ** | 82.0 ± 1.8 | 84.9 ± 6.6 | 51.6 ± 7.0 | 46.7 ± 7.7 | 56.5 ± 9.3 |
| Relief-F 10,000 | 95.0 ± 3.8 ** | 96.3 ± 4.5 | 93.7 ± 4.2 | 89.8 ± 2.2 ** | 81.8 ± 0.0 | 97.8 ± 4.3 | 57.7 ± 6.4 | 48.7 ± 7.3 | 66.7 ± 7.7 |
| Relief-F 100,000 | 92.3 ± 2.2 ** | 90.8 ± 0.9 | 93.8 ± 4.2 | 87.1 ± 1.8 ** | 82.0 ± 1.3 | 92.2 ± 3.2 | 51.3 ± 6.3 | 44.1 ± 6.2 | 58.5 ± 8.6 |
| FA Features | |||||||||
| Early-HD vs. HC | Early-HD vs. Pre-HD | Pre-HD vs. HC | |||||||
| ROIs | Acc. | Sen. | Spe. | Acc. | Sen. | Spe. | Acc. | Sen. | Spe. |
| Whole-brain | 92.7 ± 4.6 ** | 92.4 ± 6.6 | 93.0 ± 4.8 | 86.9 ± 2.8 ** | 80.7 ± 3.1 | 93.1 ± 4.6 | 40.4 ±7.3 | 34.0 ± 7.8 | 46.7 ± 9.7 |
| Thr. 0.2 | 86.4 ± 5.7 ** | 80.4 ± 8.9 | 92.4 ± 4.4 | 87.2 ± 2.7 ** | 78.0 ± 4.1 | 96.4 ± 5.2 | 40.1 ± 8.0 | 35.1 ± 8.0 | 45.0 ± 11.0 |
| Caudate | 85.9 ± 4.5 ** | 89.3 ± 2.6 | 82.5 ± 8.0 | 86.4 ± 3.5 ** | 89.2 ± 2.8 | 83.6 ± 7.1 | 74.0 ± 6.4 * | 73.5 ± 7.3 | 74.4 ± 8.8 |
| Putamen | 88.2 ± 5.0 ** | 82.6 ± 7.9 | 93.8 ± 7.0 | 87.5 ± 3.4 ** | 89.8 ± 1.3 | 85.1 ± 6.8 | 61.1 ± 8.0 | 69.4 ± 9.9 | 52.8 ± 10.6 |
| Pallidum | 88.3 ± 5.7 ** | 83.1 ± 5.4 | 93.5 ± 8.1 | 84.1 ± 4.4 ** | 75.8 ± 5.0 | 92.4 ± 5.7 | 46.9 ± 8.9 | 46.7 ± 11.0 | 47.2 ± 11.3 |
| Relief-F 100 | 90.4 ± 1.5 ** | 80.9 ± 2.9 | 100.0 ± 0.6 | 73.6 ± 6.5 * | 66.3 ± 9.1 | 80.8 ± 12.2 | 46.7 ± 9.9 | 45.7 ± 12.0 | 47.6 ± 13.2 |
| Relief-F 500 | 91.2 ± 4.4 ** | 87.9 ± 5.0 | 94.6 ± 5.0 | 93.2 ± 2.4 ** | 90.0 ± 0.0 | 96.4 ± 4.8 | 61.7 ± 8.6 | 60.6 ± 8.7 | 62.8 ± 12.1 |
| Relief-F 1000 | 99.0 ± 2.0 ** | 100.0 ± 0.4 | 98.0 ± 4.0 | 94.4 ± 1.6 ** | 90.0 ± 0.0 | 98.8 ± 3.3 | 56.3 ± 8.8 | 55.9 ± 9.2 | 56.7 ± 11.9 |
| Relief-F 10,000 | 99.6 ± 1.4 ** | 100.0 ± 0.0 | 99.2 ± 2.8 | 98.3 ± 2.4 ** | 96.6 ± 4.8 | 100.0 ± 0.0 | 64.4 ± 7.2 | 59.5 ± 8.4 | 69.2 ± 9.5 |
| Relief-F 100,000 | 95.6 ± 3.1 ** | 99.4 ± 2.3 | 91.7 ± 6.3 | 94.5 ± 2.0 ** | 89.0 ± 4.0 | 100.0 ± 0.0 | 60.3 ± 9.1 | 51.7 ± 11.4 | 68.8 ± 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrador, R.; Júlio, F.; Januário, C.; Castelo-Branco, M.; Caetano, G. Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging. J. Pers. Med. 2022, 12, 704. https://doi.org/10.3390/jpm12050704
Lavrador R, Júlio F, Januário C, Castelo-Branco M, Caetano G. Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging. Journal of Personalized Medicine. 2022; 12(5):704. https://doi.org/10.3390/jpm12050704
Chicago/Turabian StyleLavrador, Rui, Filipa Júlio, Cristina Januário, Miguel Castelo-Branco, and Gina Caetano. 2022. "Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging" Journal of Personalized Medicine 12, no. 5: 704. https://doi.org/10.3390/jpm12050704
APA StyleLavrador, R., Júlio, F., Januário, C., Castelo-Branco, M., & Caetano, G. (2022). Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging. Journal of Personalized Medicine, 12(5), 704. https://doi.org/10.3390/jpm12050704







