Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
3. Results
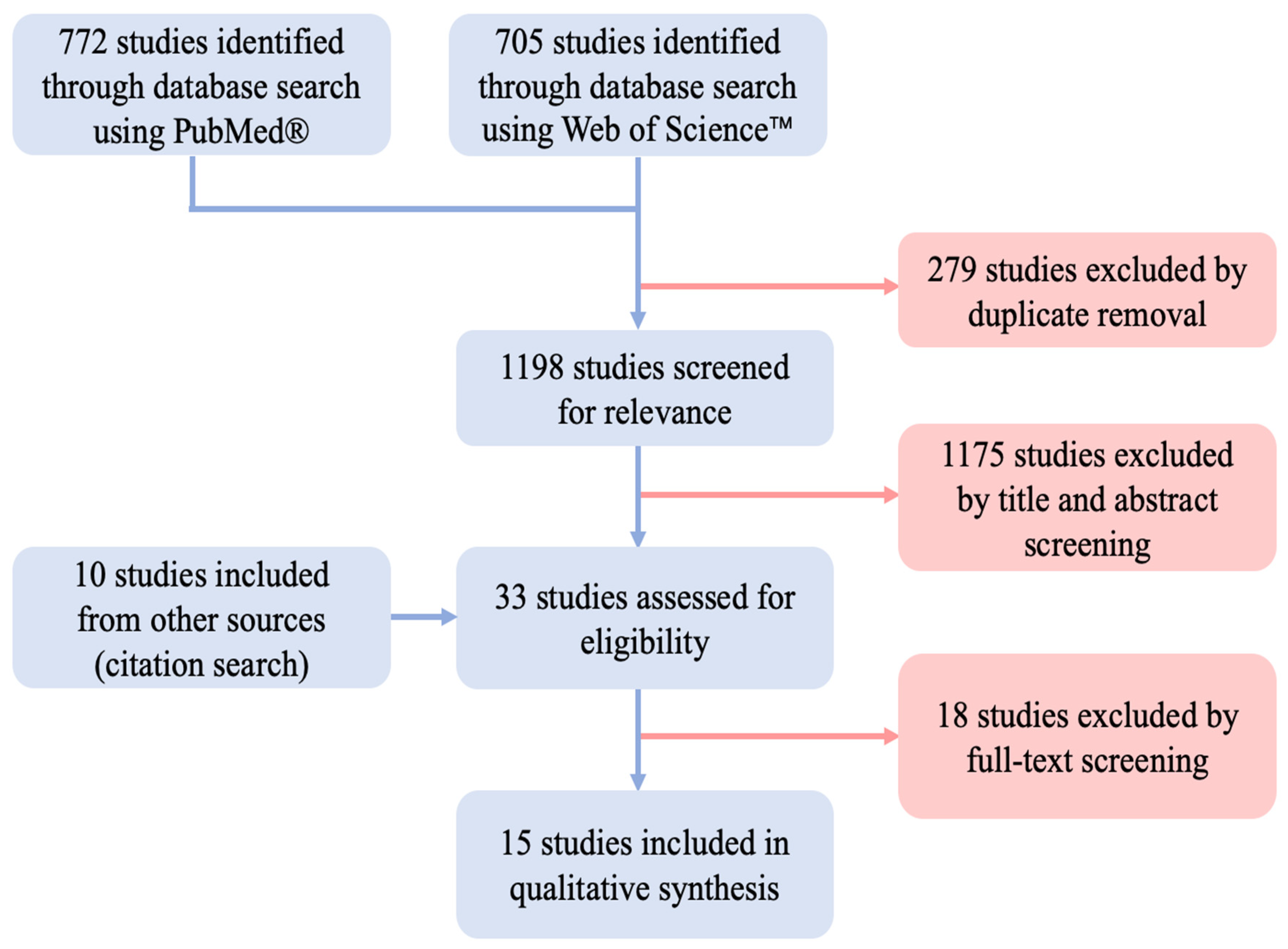
3.1. Study Selection
3.2. Study Characteristics
3.3. Scarring Assessment
3.4. Used Spacer Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aman, M.; Zimmermann, K.S.; Thielen, M.; Thomas, B.; Daeschler, S.; Boecker, A.H.; Stolle, A.; Bigdeli, A.K.; Kneser, U.; Harhaus, L. An Epidemiological and Etiological Analysis of 5026 Peripheral Nerve Lesions from a European Level I Trauma Center. J. Pers. Med. 2022, 12, 1673. [Google Scholar] [CrossRef]
- Tos, P.; Crosio, A.; Pugliese, P.; Adani, R.; Toia, F.; Artiaco, S. Painful Scar Neuropathy: Principles of Diagnosis and Treatment. Plast. Aesthet. Res. 2015, 2, 156–164. [Google Scholar] [CrossRef]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral Nerve Injury, Scarring, and Recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Millesi, H.; Zöch, G.; Rath, T. The Gliding Apparatus of Peripheral Nerve and Its Clinical Significance. Ann. Hand Up. Limb Surg. 1990, 9, 87–97. [Google Scholar] [CrossRef]
- Atkins, S.; Smith, K.G.; Loescher, A.R.; Boissonade, F.M.; O’Kane, S.; Ferguson, M.W.J.; Robinson, P.P. Scarring Impedes Regeneration at Sites of Peripheral Nerve Repair. Neuroreport 2006, 17, 1245–1249. [Google Scholar] [CrossRef]
- Clark, W.L.; Trumble, T.E.; Swiontkowski, M.F.; Tencer, A.F. Nerve Tension and Blood Flow in a Rat Model of Immediate and Delayed Repairs. J. Hand Surg. Am. 1992, 17, 677–687. [Google Scholar] [CrossRef]
- Millesi, H.; Zöch, G.; Reihsner, R. Mechanical Properties of Peripheral Nerves. Clin. Orthop. Relat. Res. 1995, 314, 76–83. [Google Scholar] [CrossRef]
- Pripotnev, S.; Mackinnon, S.E. Revision of Carpal Tunnel Surgery. J. Clin. Med. 2022, 11, 1386. [Google Scholar] [CrossRef]
- Masear, V.R.; Colgin, S. The Treatment of Epineural Scarring with Allograft Vein Wrapping. Hand Clin. 1996, 12, 773–779. [Google Scholar] [CrossRef]
- Dy, C.J.; Aunins, B.; Brogan, D.M. Barriers to Epineural Scarring: Role in Treatment of Traumatic Nerve Injury and Chronic Compressive Neuropathy. J. Hand Surg. 2018, 43, 360–367. [Google Scholar] [CrossRef]
- Crosio, A.; Ronchi, G.; Fornasari, B.E.; Odella, S.; Raimondo, S.; Tos, P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. J. Clin. Med. 2021, 10, 1613. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Dumanian, G.A.; McClinton, M.A.; Brushart, T.M. The Effects of Free Fat Grafts on the Stiffness of the Rat Sciatic Nerve and Perineural Scar. J. Hand Surg. 1999, 24, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Görgülü, A.; Imer, M.; Şimşek, O.; Sencer, A.; Kutlu, K.; Çobanoǧlu, S. The Effect of Aprotinin on Extraneural Scarring in Peripheral Nerve Surgery: An Experimental Study. Acta Neurochir. 1998, 140, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Russell, L.; Andrus, K.; MacKinnon, M.; Silver, J.; Kliot, M. Reduction of Extraneural Scarring by ADCON-T/N after Surgical Intervention. Neurosurgery 1996, 38, 976–984. [Google Scholar] [CrossRef]
- Okui, N.; Yamamoto, M.; Fukuhira, Y.; Kaneko, H.; Hirata, H. Artificial Perineurium to Enhance Nerve Recovery from Damage after Neurolysis. Muscle Nerve 2010, 42, 570–575. [Google Scholar] [CrossRef]
- Yamamoto, M.; Endo, N.; Ito, M.; Okui, N.; Koh, S.; Kaneko, H.; Hirata, H. Novel Polysaccharide-Derived Hydrogel Prevents Perineural Adhesions in a Rat Model of Sciatic Nerve Adhesion. J. Orthop. Res. 2010, 28, 284–288. [Google Scholar] [CrossRef]
- Smit, X.; Van Neck, J.W.; Afoke, A.; Hovius, S.E.R. Reduction of Neural Adhesions by Biodegradable Autocrosslinked Hyaluronic Acid Gel after Injury of Peripheral Nerves: An Experimental Study. J. Neurosurg. 2004, 101, 648–652. [Google Scholar] [CrossRef]
- Shintani, K.; Uemura, T.; Takamatsu, K.; Yokoi, T.; Onode, E.; Okada, M.; Nakamura, H. Protective Effect of Biodegradable Nerve Conduit against Peripheral Nerve Adhesion after Neurolysis. J. Neurosurg. 2018, 129, 815–824. [Google Scholar] [CrossRef]
- Özgenel, G.Y.; Fílíz, G. Combined Application of Human Amniotic Membrane Wrapping and Hyaluronic Acid Injection in Epineurectomized Rat Sciatic Nerve. J. Reconstr. Microsurg. 2004, 20, 153–157. [Google Scholar] [CrossRef]
- Ohsumi, H.; Hirata, H.; Nagakura, T.; Tsujii, M.; Sugimoio, T.; Miyamoto, K.; Horiuchi, T.; Nagao, M.; Nakashima, T.; Uchida, A. Enhancement of Perineurial Repair and Inhibition of Nerve Adhesion by Viscous Injectable Pure Alginate Sol. Plast. Reconstr. Surg. 2005, 116, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kuniyoshi, K.; Iwakura, N.; Matsuura, Y.; Suzuki, T.; Takahashi, K.; Ohtori, S. Vein Wrapping for Chronic Nerve Constriction Injury in a Rat Model: Study Showing Increases in VEGF and HGF Production and Prevention of Pain-Associated Behaviors and Nerve Damage. J. Bone Jt. Surg. 2014, 96, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, H.; Huang, H.; Bi, W.; Yan, R.; Tan, X.; Wen, W.; Wang, C.; Song, W.; Zhang, Y.; et al. Chitosan Conduit Combined with Hyaluronic Acid Prevent Sciatic Nerve Scar in a Rat Model of Peripheral Nerve Crush Injury. Mol. Med. Rep. 2018, 17, 4360–4368. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Setoyama, K.; Takada, S.; Otsuka, S.; Nakanishi, K.; Norimatsu, K.; Tani, A.; Sakakima, H.; Kawahara, K.I.; Hosokawa, K.; et al. E8002 Inhibits Peripheral Nerve Adhesion by Enhancing Fibrinolysis of L-Ascorbic Acid in a Rat Sciatic Nerve Model. Int. J. Mol. Sci. 2020, 21, 3972. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortés, P.; Peregrina, M.; Aneiros-Fernández, J.; Tassi, M.; Pajares-López, M.; Toledo, M.; O’Valle, F. Oxidized Regenerated Cellulose Does Not Prevent the Formation of Experimental Postoperative Perineural Fibrosis Assessed by Digital Analysis. Histol. Histopathol. 2010, 25, 741–747. [Google Scholar] [CrossRef]
- Finsterbush, A.; Porat, S.; Rousso, M.; Ashur, H. Prevention of Peripheral Nerve Entrapment Following Extensive Soft Tissue Injury, Using Silicone Cuffing: An Experimental Study. Clin. Orthop. Relat. Res. 1982, 162, 276–281. [Google Scholar] [CrossRef]
- Baltu, Y.; Uzun, H.; Özgenel, Y.G. The Reduction of Extraneural Scarring with Buccal Mucosa Graft Wrapping around the Sciatic Nerve: An Experimental Study in a Rat Model. J. Plast. Surg. Hand Surg. 2017, 51, 259–263. [Google Scholar] [CrossRef]
- Gärtner, A.; Pereira, T.; Simões, M.J.; Armada-da-Silva, P.A.S.; França, M.L.; Sousa, R.; Bompasso, S.; Raimondo, S.; Shirosaki, Y.; Nakamura, Y.; et al. Use of Hybrid Chitosan Membranes and Human Mesenchymal Stem Cells from the Wharton Jelly of Umbilical Cord for Promoting Nerve Regeneration in an Axonotmesis Rat Model. Neural Regen. Res. 2012, 7, 2247. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Golash, A.; Kay, A.; Wagner, J.G.; Peck, F.; Watson, J.S.; Lees, V.C. Efficacy of ADCON-T/N after Primary Flexor Tendon Repair in Zone II: A Controlled Clinical Trial. J. Hand Surg. 2003, 28, 113–115. [Google Scholar] [CrossRef]
- Merle, M.; Lee Dellon, A.; Campbell, J.N.; Chang, P.S. Complications from Silicon-Polymer Intubulation of Nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Derebaşınlıoğlu, H.; Demirkazık, A.; Çiçek Doğan, İ.; Eğilmez, H.R.; Çam, S.; Yeldir, N. The Effect of a Silicone Sheet on Sciatic Nerve Healing in Rats. Eur. J. Plast. Surg. 2023, 46, 453–463. [Google Scholar] [CrossRef]
- Koenig, Z.A.; Burns, J.C.; Hayes, J.D. Necrotic Granulomatous Inflammation after Use of Small Intestine Submucosa Matrix for Recurrent Compression Neuropathy. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4378. [Google Scholar] [CrossRef] [PubMed]
- Stang, F.; Keilhoff, G.; Fansa, H. Biocompatibility of Different Nerve Tubes. Materials 2009, 2, 1480–1507. [Google Scholar] [CrossRef]
- Giusti, G.; Shin, R.H.; Lee, J.Y.; Mattar, T.G.; Bishop, A.T.; Shin, A.Y. The Influence of Nerve Conduits Diameter in Motor Nerve Recovery after Segmental Nerve Repair. Microsurgery 2014, 34, 646–652. [Google Scholar] [CrossRef]
- Isaacs, J.; Mallu, S.; Yan, W.; Little, B. Consequences of Oversizing: Nerve-to-Nerve Tube Diameter Mismatch. J. Bone Jt. Surg.-Am. Vol. 2014, 96, 1461–1467. [Google Scholar] [CrossRef]
- Mathoulin, C.; Bahm, J.; Roukoz, S. Pedicled Hypothenar Fat Flap for Median Nerve Coverage in Recalcitrant Carpal Tunnel Syndrome. Hand Surg. 2000, 5, 33–40. [Google Scholar] [CrossRef]
- De Smet, L.; Vandeputte, G. Pedicled fat flap coverage of the median nerve after failed carpal tunnel decompression. J. Hand Surg. Br. 2002, 27, 350–353. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Lekholm, C.; Kardum, P.; Holmberg, J. Coverage of the Median Nerve with Free and Pedicled Flaps for the Treatment of Recurrent Severe Carpal Tunnel Syndrome. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 172–176. [Google Scholar] [CrossRef]
- Krzesniak, N.E.; Sarnowska, A.; Figiel-Dabrowska, A.; Osiak, K.; Domanska-Janik, K.; Noszczyk, B.H. Secondary Release of the Peripheral Nerve with Autologous Fat Derivates Benefits for Functional and Sensory Recovery. Neural Regen. Res. 2021, 16, 856–864. [Google Scholar] [CrossRef]
- Goitz, R.J.; Steichen, J.B. Microvascular Omental Transfer for the Treatment of Severe Recurrent Median Neuritis of the Wrist: A Long-Term Follow-Up. Plast. Reconstr. Surg. 2005, 115, 163–171. [Google Scholar] [CrossRef]
- Ruch, D.S.; Spinner, R.M.; Koman, L.A.; Challa, V.R.; O’Farrell, D.; Levin, L.S. The Histological Effect of Barrier Vein Wrapping of Peripheral Nerves. J. Reconstr. Microsurg. 1996, 12, 291–296. [Google Scholar] [CrossRef]
- Hallock, G.G. Vein Donor Site Morbidity after Coronary Bypass Surgery: An Overlooked but Important Issue. Plast. Surg. (Chir. Plast.) 1999, 7, 117–121. [Google Scholar] [CrossRef]
- Hirosawa, N.; Uchida, K.; Kuniyoshi, K.; Murakami, K.; Inoue, G.; Miyagi, M.; Matsuura, Y.; Orita, S.; Inage, K.; Suzuki, T.; et al. Vein Wrapping Promotes M2 Macrophage Polarization in a Rat Chronic Constriction Injury Model. J. Orthop. Res. 2018, 36, 2210–2217. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Hirosawa, N.; Murakami, K.; Inoue, G.; Miyagi, M.; Shiga, Y.; Sekiguchi, H.; Inage, K.; Orita, S.; et al. Frozen Vein Wrapping for Chronic Nerve Constriction Injury Reduces Sciatic Nerve Allodynia in a Rat Model. BMC Neurosci. 2022, 23, 37. [Google Scholar] [CrossRef]
- Xu, J.; Varitimidis, S.E.; Fisher, K.J.; Tomaino, M.M.; Sotereanos, D.G. The Effect of Wrapping Scarred Nerves with Autogenous Vein Graft to Treat Recurrent Chronic Nerve Compression. J. Hand Surg. 2000, 25, 93–103. [Google Scholar] [CrossRef]
- Mathieu, L.; Adam, C.; Legagneux, J.; Bruneval, P.; Masmejean, E. Reduction of Neural Scarring after Peripheral Nerve Suture: An Experimental Study about Collagen Membrane and Autologous Vein Wrapping. Chir. Main 2012, 31, 311–317. [Google Scholar] [CrossRef]
- Hirosawa, N.; Uchida, K.; Kuniyoshi, K.; Murakami, K.; Inoue, G.; Miyagi, M.; Matsuura, Y.; Orita, S.; Inage, K.; Suzuki, T.; et al. Vein Wrapping Facilitates Basic Fibroblast Growth Factor-Induced Heme Oxygenase-1 Expression Following Chronic Nerve Constriction Injury. J. Orthop. Res. 2018, 36, 898–905. [Google Scholar] [CrossRef]
- Xu, J.; Sotereanos, D.G.; Moller, A.R.; Jacobsohn, J.; Tomaino, M.M.; Fischer, K.J.; Herndon, J.H. Nerve Wrapping with Vein Grafts in a Rat Model: A Safe Technique for the Treatment of Recurrent Chronic Compressive Neuropathy. J. Reconstr. Microsurg. 1998, 14, 323–330. [Google Scholar] [CrossRef]
- Mirzayan, R.; Russo, F.; Yang, S.-J.T.; Lowe, N.; Shean, C.J.; Harness, N.G. Human Amniotic Membrane Wrapping of the Ulnar Nerve during Cubital Tunnel Surgery Reduces Recurrence of Symptoms. Arch. Bone Jt. Surg. 2022, 10, 969–975. [Google Scholar] [CrossRef]
- Gaspar, M.P.; Abdelfattah, H.M.; Welch, I.W.; Vosbikian, M.M.; Kane, P.M.; Rekant, M.S. Recurrent Cubital Tunnel Syndrome Treated with Revision Neurolysis and Amniotic Membrane Nerve Wrapping. J. Shoulder Elbow Surg. 2016, 25, 2057–2065. [Google Scholar] [CrossRef]
- Gao, Y.B.; Liu, Z.G.; Lin, G.D.; Guo, Y.; Chen, L.; Huang, B.T.; Yin, Y.B.; Yang, C.; Sun, L.Y.; Rong, Y.B.; et al. Safety and Efficacy of a Nerve Matrix Membrane as a Collagen Nerve Wrapping: A Randomized, Single-Blind, Multicenter Clinical Trial. Neural Regen. Res. 2021, 16, 1652–1659. [Google Scholar] [CrossRef]
- Economides, J.M.; Defazio, M.V.; Attinger, C.E.; Barbour, J.R. Prevention of Painful Neuroma and Phantom Limb Pain after Transfemoral Amputations through Concomitant Nerve Coaptation and Collagen Nerve Wrapping. Neurosurgery 2016, 79, 508–512. [Google Scholar] [CrossRef]
- Soltani, A.M.; Allan, B.J.; Best, M.J.; Mir, H.S.; Panthaki, Z.J. Revision Decompression and Collagen Nerve Wrap for Recurrent and Persistent Compression Neuropathies of the Upper Extremity. Ann. Plast. Surg. 2014, 72, 572–578. [Google Scholar] [CrossRef]
- Kokkalis, Z.T.; Mavrogenis, A.F.; Ballas, E.G.; Papagelopoulos, P.J.; Soucacos, P.N. Collagen Nerve Wrap for Median Nerve Scarring. Orthopedics 2015, 38, 117–121. [Google Scholar] [CrossRef][Green Version]
- Smith, M.J.; Smith, D.C.; Bowlin, G.L.; White, K.L. Modulation of Murine Innate and Acquired Immune Responses Following in Vitro Exposure to Electrospun Blends of Collagen and Polydioxanone. J. Biomed. Mater. Res. A 2010, 93, 793–806. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Jiang, Y.K.; Li, J.A.; Wei, W.F.; Shi, M.P.; Wang, Y.B.; Jia, G.L. The Effect of Poly(Lactic-Co-Glycolic Acid) Conduit Loading Insulin-like Growth Factor 1 Modified by a Collagen-Binding Domain on Peripheral Nerve Injury in Rats. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2100–2109. [Google Scholar] [CrossRef]
- Kim, P.D.; Hayes, A.; Amin, F.; Akelina, Y.; Hays, A.P.; Rosenwasser, M.P. Collagen Nerve Protector in Rat Sciatic Nerve Repair: A Morphometric and Histological Analysis. Microsurgery 2010, 30, 392–396. [Google Scholar] [CrossRef]
- Bozkurt, A.; Boecker, A.; Tank, J.; Altinova, H.; Deumens, R.; Dabhi, C.; Tolba, R.; Weis, J.; Brook, G.A.; Pallua, N.; et al. Efficient Bridging of 20 Mm Rat Sciatic Nerve Lesions with a Longitudinally Micro-Structured Collagen Scaffold. Biomaterials 2016, 75, 112–122. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Hirosawa, N.; Murakami, K.; Kuniyoshi, K.; Inoue, G.; Miyagi, M.; Sekiguchi, H.; Shiga, Y.; Inage, K.; et al. Wrapping with Basic Fibroblast Growth Factor-Impregnated Collagen Sheet Reduces Rat Sciatic Nerve Allodynia. J. Orthop. Res. 2019, 37, 2258–2263. [Google Scholar] [CrossRef]
- Kazanci, A.; Gurcan, O.; Gurcay, A.G.; Onder, E.; Kazanci, B.; Yaman, M.E.; Bavbek, M. Effects of Topical CovaTM, Tisseel® and Adcon® Gel Application on the Development of Spinal Peridural Fibrosis: An Experimental Study in Rats. Turk. Neurosurg. 2017, 27, 962–968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabb, C.H. Failed Back Syndrome and Epidural Fibrosis. Spine J. 2010, 10, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Temiz, A.; Ozturk, C.; Bakunov, A.; Kara, K.; Kaleli, T. A New Material for Prevention of Peritendinous Fibrotic Adhesions after Tendon Repair: Oxidised Regenerated Cellulose (Interceed), an Absorbable Adhesion Barrier. Int. Orthop. 2008, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Biçer, M.; Bayram, A.S.; Gürbüz, O.; Şenkaya, I.; Yerci, Ö.; Tok, M.; Anǧ, E.; Moǧol, E.B.; Saba, D. Assesssment of the Efficacy of the Bio-Absorbable Oxidized Regenerated Cellulose for Prevention of Post-Operative Pericardial Adhesion in the Rabbit Model. J. Int. Med. Res. 2008, 36, 1311–1318. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamauchi, D.; Tomita, K. Preliminary Study for Prevention of Neural Adhesion Using an Absorbable Oxidised Regenerated Cellulose Sheet. Hand Surg. 2002, 7, 11–14. [Google Scholar] [CrossRef]
- Towne, J.; Carter, N.; Neivandt, D.J. COMSOL Multiphysics® Modelling of Oxygen Diffusion through a Cellulose Nanofibril Conduit Employed for Peripheral Nerve Repair. Biomed. Eng. Online 2021, 20, 60. [Google Scholar] [CrossRef]
- Carter, N.; Towne, J.; Neivandt, D.J. Finite Element Analysis of Glucose Diffusivity in Cellulose Nanofibril Peripheral Nerve Conduits. Cellulose 2021, 28, 2791–2803. [Google Scholar] [CrossRef]
- Urano, H.; Iwatsuki, K.; Yamamoto, M.; Ohnisi, T.; Kurimoto, S.; Endo, N.; Hirata, H. Novel Anti-Adhesive CMC-PE Hydrogel Significantly Enhanced Morphological and Physiological Recovery after Surgical Decompression in an Animal Model of Entrapment Neuropathy. PLoS ONE 2016, 11, e0164572. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamauchi, D.; Osamura, N.; Hagiwara, N.; Tomita, K. Hyaluronic Acid Prevents Peripheral Nerve Adhesion. Br. J. Plast. Surg. 2003, 56, 342–347. [Google Scholar] [CrossRef]
- Özgenel, G.Y. Effects of Hyaluronic Acid on Peripheral Nerve Scarring and Regeneration in Rats. Microsurgery 2003, 23, 575–581. [Google Scholar] [CrossRef]
- Adanali, G.; Verdi, M.; Tuncel, A.; Erdogan, B.; Kargi, E. Effects of Hyaluronic Acid-Carboxymethylcellulose Membrane on Extraneural Adhesion Formation and Peripheral Nerve Regeneration. J. Reconstr. Microsurg. 2003, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.Y.; Manxhuka-Kerliu, S.; Morina, A.A.; Duci, S.B.; Shahini, L.; Mekaj, Y.H. Effects of Hyaluronic Acid and Tacrolimus on the Prevention of Perineural Scar Formation and on Nerve Regeneration after Sciatic Nerve Repair in a Rabbit Model. Eur. J. Trauma Emerg. Surg. 2017, 43, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, J.H.; Han, C.S.; Chung, D.W.; Kim, G.Y. Effect of Hyaluronic Acid-Carboxymethylcellulose Solution on Perineural Scar Formation after Sciatic Nerve Repair in Rats. Clin. Orthop. Surg. 2011, 3, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasset, W.K.; Jasim, S.A.; Sharma, S.K.; Margiana, R.; Bokov, D.O.; Obaid, M.A.; Hussein, B.A.; Lafta, H.A.; Jasim, S.F.; Mustafa, Y.F. Alginate-Based Hydrogels and Tubes, as Biological Macromolecule-Based Platforms for Peripheral Nerve Tissue Engineering: A Review. Ann. Biomed. Eng. 2022, 50, 628–653. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Marcol, W.; Larysz-Brysz, M.; Kucharska, M.; Niekraszewicz, A.; Slusarczyk, W.; Kotulska, K.; Wlaszczuk, P.; Wlaszczuk, A.; Jedrzejowska-Szypulka, H.; Lewin-Kowalik, J. Reduction of Post-Traumatic Neuroma and Epineural Scar Formation in Rat Sciatic Nerve by Application of Microcrystallic Chitosan. Microsurgery 2011, 31, 642–649. [Google Scholar] [CrossRef]
- Neubrech, F.; Sauerbier, M.; Moll, W.; Seegmüller, J.; Heider, S.; Harhaus, L.; Bickert, B.; Kneser, U.; Kremer, T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial. Plast. Reconstr. Surg. 2018, 142, 415–424. [Google Scholar] [CrossRef]
- Ducker, T.B.; Hayes, G.J. Experimental Improvements in the Use of Silastic Cuff for Peripheral Nerve Repair. J. Neurosurg. 1968, 28, 582–587. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Poly(Lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Fan, Z.; Wang, X.; Li, Z.; Wen, J.; Deng, J.; Tan, D.; Pan, M.; Hu, X.; et al. Ascorbic Acid Facilitates Neural Regeneration After Sciatic Nerve Crush Injury. Front. Cell. Neurosci. 2019, 13, 108. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Wang, X.; Liu, J.; Hu, X.; Tan, D.; Li, Z.; Guo, J. Ascorbic Acid Accelerates Wallerian Degeneration after Peripheral Nerve Injury. Neural. Regen. Res 2021, 16, 1078–1085. [Google Scholar] [CrossRef]
- Nicolas, C.F.; Corvi, J.J.; Zheng, Y.; Park, K.H.; Akelina, Y.; Engemann, A.; Strauch, R.J. Resorbable Nerve Wraps: Can They Be Overtightened? J. Reconstr. Microsurg. 2022, 38, 694–702. [Google Scholar] [CrossRef]
- Xue, J.W.; Jiao, J.B.; Liu, X.F.; Jiang, Y.T.; Yang, G.; Li, C.Y.; Yin, W.T.; Ling, L. Inhibition of Peripheral Nerve Scarring by Calcium Antagonists, Also Known as Calcium Channel Blockers. Artif. Organs 2016, 40, 514–520. [Google Scholar] [CrossRef]
- Görgülü, A.; Uzal, C.; Doǧanay, L.; Imer, M.; Eliuz, K.; Çobanoǧlu, S.; Loeffler, J.S.; Gerszten, P.C.; Kondziolka, D.; Kline, D.G. The Effect of Low-Dose External Beam Radiation on Extraneural Scarring after Peripheral Nerve Surgery in Rats. Neurosurgery 2003, 53, 1389–1396. [Google Scholar] [CrossRef]
- Soto, P.A.; Vence, M.; Piñero, G.M.; Coral, D.F.; Usach, V.; Muraca, D.; Cueto, A.; Roig, A.; van Raap, M.B.F.; Setton-Avruj, C.P. Sciatic Nerve Regeneration after Traumatic Injury Using Magnetic Targeted Adipose-Derived Mesenchymal Stem Cells. Acta Biomater. 2021, 130, 234–247. [Google Scholar] [CrossRef]
- Albayrak, B.S.; Ismailoglu, O.; Ilbay, K.; Yaka, U.; Tanriover, G.; Gorgulu, A.; Demir, N. Doxorubicin for Prevention of Epineurial Fibrosis in a Rat Sciatic Nerve Model: Outcome Based on Gross Postsurgical, Histopathological, and Ultrastructural Findings. J. Neurosurg. Spine 2010, 12, 327–333. [Google Scholar] [CrossRef]
- Vural, E.; Yilmaz, M.; Ilbay, K.; Ilbay, G. Prevention of Epidural Fibrosis in Rats by Local Administration of Mitomycin C or Daunorubicin. Turk. Neurosurg. 2016, 26, 291–296. [Google Scholar] [CrossRef]
- Ilbay, K.; Etus, V.; Yildiz, K.; Ilbay, G.; Ceylan, S. Topical Application of Mitomycin C Prevents Epineural Scar Formation in Rats. Neurosurg. Rev. 2005, 28, 148–153. [Google Scholar] [CrossRef]
- Fitzgerald, J.J. Suppression of Scarring in Peripheral Nerve Implants by Drug Elution. J. Neural Eng. 2016, 13, 026006. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Huang, Y.; Wu, W.; Deng, X.; Liu, H.; Li, R.; Tao, J.; Li, X.; Liu, X.; et al. A 3D-Printed Self-Adhesive Bandage with Drug Release for Peripheral Nerve Repair. Adv. Sci. 2020, 7, 2002601. [Google Scholar] [CrossRef]
- Boecker, A.H.; van Neerven, S.G.A.; Scheffel, J.; Tank, J.; Altinova, H.; Seidensticker, K.; Deumens, R.; Tolba, R.; Weis, J.; Brook, G.A.; et al. Pre-Differentiation of Mesenchymal Stromal Cells in Combination with a Microstructured Nerve Guide Supports Peripheral Nerve Regeneration in the Rat Sciatic Nerve Model. Eur. J. Neurosci. 2016, 43, 404–416. [Google Scholar] [CrossRef]
- Aman, M.; Schulte, M.; Li, Y.; Thomas, B.; Daeschler, S.; Mayrhofer-Schmid, M.; Kneser, U.; Harhaus, L.; Boecker, A. Benefit of Adjuvant Mesenchymal Stem Cell Transplantation to Critical-Sized Peripheral Nerve Defect Repair: A Systematic Review and Meta-Analysis of Preclinical Studies. J. Clin. Med. 2023, 12, 1306. [Google Scholar] [CrossRef]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A Systematic Review of Animal Models Used to Study Nerve Regeneration in Tissue-Engineered Scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The Overwhelming Use of Rat Models in Nerve Regeneration Research May Compromise Designs of Nerve Guidance Conduits for Humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef]

| Author | Year | Group | Scarring Induction | Wrapping Material | n | FU |
|---|---|---|---|---|---|---|
| Baltu et al. [27] | 2017 | A | Epineurectomy | Buccal mucosa graft | 24 | 8 |
| Dumanian et al. [13] | 1999 | A | Epineurectomy | Free fat grafts | 14 | 8 |
| Finsterbush et al. [26] | 1982 | A | Crush injury, muscle cauterization | Semirigid 15 mm silicone tube, cut longitudinally | 30 | 8 |
| Görgülü et al. [14] | 1998 | A | External neurolysis | Collagen fibers soaked with aprotinin | 22 | 6 |
| B | Abrasive injury | Collagen fibers soaked with aprotinin | 22 | 6 | ||
| C | External neurolysis | Collagen fibers soaked with PBS | 22 | 6 | ||
| D | Abrasive injury | Collagen fibers soaked with PBS | 22 | 6 | ||
| Hernandez-Cortes et al. [25] | 2010 | A | Perineurectomy + muscle cauterization | Oxidized regenerated cellulose wrap | 40 | 6 |
| Kikuchi et al. [24] | 2020 | A | Muscle cauterization | E8002 wrapping (Kawasumi Laboratories Inc., Tokyo, Japan) | 7 | 6 |
| B | Muscle cauterization | E8002 AA- wrapping (Kawasumi Laboratories Inc., Tokyo, Japan) | 7 | 6 | ||
| Li et al. [23] | 2018 | A | Crush injury | Chitosan conduit | 15 | 12 |
| B | Crush injury | HA gel | 15 | 12 | ||
| C | Crush injury | Chitosan conduit + HA gel | 15 | 12 | ||
| Murakami et al. [22] | 2014 | A | Chronic constriction injury by nerve ligation | Allogenic vein wrap | 30 | 20 |
| Ohsumi et al. [21] | 2005 | A | External/internal neurolysis + muscle cauterization | Viscous alginate sol | 8 | 6 |
| Okui et al. et al. [16] | 2010 | A | Internal neurolysis + muscle cauterization | Honeycomb poly-lactide film | 42 | 6 |
| B | Internal neurolysis + muscle cauterization | Cast poly-lactide film | 12 | 6 | ||
| Özgenel et al. [20] | 2004 | A | Epineurectomy | Human amniotic membrane | 12 | 12 |
| B | Epineurectomy | Human amniotic membrane + HA injection | 12 | 12 | ||
| Petersen et al. [15] | 1996 | A | Internal neurolysis | ADCON-T/N gel (Gliatech, Inc., Cleveland, OH, USA) | 9 | 4 |
| B | Internal neurolysis | Control gel | 9 | 4 | ||
| Shintani et al. [19] | 2018 | A | Muscle cauterization | PLA/PCL tube | 12 | 6 |
| B | Muscle cauterization | 1% HA | 8 | 6 | ||
| Smit et al. [18] | 2004 | A | External neurolysis | 1% HA | 5 | 6 |
| B | Crush injury | 1% HA | 7 | 6 | ||
| Yamamoto et al. [17] | 2010 | A | Internal neurolysis | 1% HA | 18 | 6 |
| B | Internal neurolysis | CMC-PE hydrogel, low viscosity | 18 | 6 | ||
| C | Internal neurolysis | CMC-PE hydrogel, high viscosity | 18 | 6 |
| Author | Year | Group | Scarring Induction | Wrapping Material | Scar Assessment Method | Scar Prevention Quantitative Ass. | Scar Prevention Descriptive Ass. |
|---|---|---|---|---|---|---|---|
| Baltu et al. [27] | 2017 | A | Epineurectomy | Buccal mucosa graft | Adhesion score (Petersen et al. 1996 [15]), epineural scar density score | yes | yes |
| Dumanian et al. [13] | 1999 | A | Epineurectomy | Free fat grafts | Nerve stiffness | yes | yes |
| Finsterbush et al. [26] | 1982 | A | Crush injury, muscle cauterization | Semirigid 15 mm silicone tube, cut longitudinally | Desriptive histology | not described | yes |
| Görgülü et al. [14] | 1998 | A | External neurolysis | Collagen fibers soaked with aprotinin | Adhesion score (Petersen et al. 1996 [15]) | yes | yes |
| B | Abrasive injury | Collagen fibers soaked with aprotinin | Adhesion score (Petersen et al. 1996 [15]) | yes | yes | ||
| C | External neurolysis | Collagen fibers soaked with PBS | Adhesion score (Petersen et al. 1996 [15]) | no | no | ||
| D | Abrasive injury | Collagen fibers soaked with PBS | Adhesion score (Petersen et al. 1996 [15]) | no | no | ||
| Hernandez-Cortes et al. [25] | 2010 | A | Perineurectomy + muscle cauterization | Oxidized regenerated cellulose wrap | Connective tissue measurement | no | no |
| Kikuchi et al. [24] | 2020 | A | Muscle cauterization | E8002 wrapping (Kawasumi Laboratories Inc.) | Adhesion score (Petersen et al. 1996 [15]), optical scar density | yes | yes |
| B | Muscle cauterization | E8002 AA- wrapping (Kawasumi Laboratories Inc.) | Adhesion score (Petersen et al. 1996 [15]), optical scar density | no | no | ||
| Li et al. [23] | 2018 | A | Crush injury | Chitosan conduit | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes |
| B | Crush injury | HA gel | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes | ||
| C | Crush injury | Chitosan conduit + HA gel | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes | ||
| Murakami et al. [22] | 2014 | A | Chronic constriction injury by nerve ligation | Allogenic vein wrap | Descriptive histology | not described | yes |
| Ohsumi et al. [21] | 2005 | A | External/internal neurolysis + muscle cauterization | Viscous alginate sol | Biomechanical breaking strength, descriptive histology | yes | yes |
| Okui et al. et al. [16] | 2010 | A | Internal neurolysis + muscle cauterization | Honeycomb poly-lactide film | Descriptive histology, descriptive macroscopic adhesion strength | not described | yes |
| B | Internal neurolysis + muscle cauterization | Cast poly-lactide film | Descriptive macroscopic adhesion strength | not described | no | ||
| Özgenel et al. [20] | 2004 | A | Epineurectomy | Human amniotic membrane | Adhesion score (Petersen et al. 1996 [15]), scar thickness measurement | yes | yes |
| B | Epineurectomy | Human amniotic membrane + HA injection | Adhesion score (Petersen et al. 1996 [15]), scar thickness measurement | yes | yes | ||
| Petersen et al. [15] | 1996 | A | Internal neurolysis | ADCON-T/N gel (Gliatech, Inc., Cleveland, OH, USA) | Adhesion score (Petersen et al. 1996 [15]), scar area measurement | yes | yes |
| B | Internal neurolysis | Control gel | Adhesion score (Petersen et al. 1996 [15]), scar area measurement | no | no | ||
| Shintani et al. [19] | 2018 | A | Muscle cauterization | PLA/PCL tube | Adhesion score (Petersen et al. 1996 [15]), biomechanical breaking strength, descriptive histology | yes | yes |
| B | Muscle cauterization | 1% HA | Adhesion score (Petersen et al. 1996 [15]), biomechanical breaking strength, descriptive histology | no | no | ||
| Smit et al. [18] | 2004 | A | External neurolysis | 1% HA | Biomechanical breaking strength | yes | yes |
| B | Crush injury | 1% HA | Biomechanical breaking strength | yes | yes | ||
| Yamamoto et al. [17] | 2010 | A | Internal neurolysis | 1% HA | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | no | yes |
| B | Internal neurolysis | CMC-PE hydrogel, low viscosity | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | yes | yes | ||
| C | Internal neurolysis | CMC-PE hydrogel, high viscosity | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | yes | yes |
| Biomaterial | Material Type | Origin |
|---|---|---|
| Buccal mucosa graft | Tissue-based | Natural |
| Fat graft | ||
| Vein graft | ||
| Human amniotic membrane | ||
| Collagen fibers + aprotinin | Protein-based | |
| Collagen fibers + PBS | ||
| ADCON-T/N gel (gel composed of gelatin and a carbohydrate polymer in PBS) | ||
| ADCON-T/N control gel | ||
| Oxidized regenerated cellulose wrap | Polysaccharide-based | |
| CMC-PE hydrogel | ||
| Hyaluronic acid (gel) | ||
| Alginate sol | ||
| Chitosan | ||
| Silicone | Synthetic Polymer | Synthetic |
| PLA | ||
| PLA-PCL | ||
| E8002 (PLA-based membrane with L-ascorbic acid) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayrhofer-Schmid, M.; Klemm, T.T.; Aman, M.; Kneser, U.; Eberlin, K.R.; Harhaus, L.; Boecker, A.H. Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. J. Pers. Med. 2023, 13, 1431. https://doi.org/10.3390/jpm13101431
Mayrhofer-Schmid M, Klemm TT, Aman M, Kneser U, Eberlin KR, Harhaus L, Boecker AH. Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. Journal of Personalized Medicine. 2023; 13(10):1431. https://doi.org/10.3390/jpm13101431
Chicago/Turabian StyleMayrhofer-Schmid, Maximilian, Tess T. Klemm, Martin Aman, Ulrich Kneser, Kyle R. Eberlin, Leila Harhaus, and Arne H. Boecker. 2023. "Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model" Journal of Personalized Medicine 13, no. 10: 1431. https://doi.org/10.3390/jpm13101431
APA StyleMayrhofer-Schmid, M., Klemm, T. T., Aman, M., Kneser, U., Eberlin, K. R., Harhaus, L., & Boecker, A. H. (2023). Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. Journal of Personalized Medicine, 13(10), 1431. https://doi.org/10.3390/jpm13101431








