OCT Findings in Patients with Methamphetamine Use Disorder
Abstract
:1. Introduction
2. Method
- No DSM 5 diagnosis other than MUD (other substance use disorders, psychotic disorders, mood disorders, ADHD);
- No previous eye disease (those with intraocular pressure greater than 20 mmHg and axial sphere outside 20–24 mm length, retinal pathologies, cataract, glaucoma, optic neuritis, spherical and cylindrical refractive errors greater than +/− 1.00 diopters, uveitis, history of corneal diseases, ocular trauma, and neurological disorders were not included in the study);
- Absence of a neurological diagnosis;
- Being between the ages of 18 and 65;
- Give written consent to participate in the study.
- Absence of a psychiatric diagnosis that meets DSM 5 criteria and absence of a neurological diagnosis;
- No previous eye disease (those with intraocular pressure greater than 20 mmHg and axial sphere outside 20–24 mm length, retinal pathologies, cataract, glaucoma, optic neuritis, spherical and cylindrical refractive errors greater than +/− 1.00 diopters, uveitis, history of corneal diseases, ocular trauma, and neurological disorders were not included in the study);
- Being between the ages of 18 and 65;
- Give written consent to participate in the study.
2.1. Sociodemographic and Clinical Data Form
2.2. Addiction Profile Index (APIS) Self-Report Scale
2.3. OCT Measurements
2.4. Statistical Method
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petit, A.; Karila, L.; Chalmin, F.; Lejoyeux, M. Methamphetamine addiction: A review of the literature. J. Addict. Res. Ther. S 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Rasmussen, N. Amphetamine-type stimulants: The early history of their medical and non-medical uses. Int. Rev. Neurobiol. 2015, 120, 9–25. [Google Scholar] [PubMed]
- Rasmussen, N. Making the first anti-depressant: Amphetamine in American medicine, 1929–1950. J. Hist. Med. Allied Sci. 2006, 61, 288–323. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M.; Locatelli, I. Age-related pharmacotherapy of attention deficit hyperactivity disorder in Slovenia in children and adolescents: A population-based study. Eur. Psychiatry 2017, 42, 129–133. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Drug Report. United Nations Publication. 2020. Available online: https://wdr.unodc.org/wdr2020/en/index2020.html (accessed on 25 December 2022).
- Wainwright, J.J.; Mikre, M.; Whitley, P.; Dawson, E.; Huskey, A.; Lukowiak, A.; Giroir, B.P. Analysis of drug test results before and after the US declaration of a national emergency concerning the COVID-19 outbreak. Jama 2020, 324, 1674–1677. [Google Scholar] [CrossRef]
- Loftis, J.M.; Janowsky, A. Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol. 2014, 118, 165–197. [Google Scholar] [PubMed]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Özen, M.E.; Kalenderoğlu, A.; Karadağ, A.S.; Orum, M.H. Comparison of optic coherence tomography results in patients diagnosed with OCD: Findings in favor of neurodegeneration. Anatol. J. Psychiatry/Anadolu Psikiyatr. Derg. 2019, 20, 166–174. [Google Scholar] [CrossRef]
- Paunescu, L.A.; Schuman, J.S.; Price, L.L.; Stark, P.C.; Beaton, S.; Ishikawa, H.; Wollstein, G.; Fujimoto, J.G. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1716–1724. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, Z.; Zhang, X.; Ming, B.; Jia, J.; Wang, R.; Ma, D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: Evidence in optical coherence tomography. Neurosci. Lett. 2010, 480, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Inzelberg, R.; Ramirez, J.A.; Nisipeanu, P.; Ophir, A. Retinal nerve fiber layer thinning in Parkinson disease. Vis. Res. 2004, 44, 2793–2797. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Alim, S.; Batmaz, S.; Demir, S.; Songur, E.; Ortak, H.; Demirci, K. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: Optical coherence tomography findings in major depression. Psychiatry Res. Neuroimaging 2016, 251, 60–66. [Google Scholar] [CrossRef]
- Talebnejad, M.R.; Khazaei, P.; Jahanbani-Ardakani, H.; Saberikia, Z.; Sarani, E.M.; Khalili, M.R. Effects of chronic methamphetamine abuse on the retinal nerve fiber layer, ganglion cell layer and Bruch’s membrane opening minimum rim width. Neurotoxicology 2020, 80, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Ozsoy, F.; Kepenek, I.; Altindag, A. Examination of optical coherence tomography findings in patients with methamphetamine use disorder. J. Addict. Dis. 2022, 40, 278–284. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.; Karg, R.S.; Spitzer, R.L. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders: Clinician Version; American Psychiatric Association Publishing: Arlington, TX, USA, 2016. [Google Scholar]
- Gemelli, H.; Fidalgo, T.M.; Gracitelli, C.P.; de Andrade, E.P. Retinal nerve fiber layer analysis in cocaine users. Psychiatry Res. 2019, 271, 226–229. [Google Scholar] [CrossRef]
- McLellan, A.T.; Kushner, H.; Metzger, D.; Peters, R.; Smith, I.; Grissom, G.; Pettinati, H.; Argeriou, M. The fifth edition of the Addiction Severity Index. J. Subst. Abus. Treat. 1992, 9, 199–213. [Google Scholar] [CrossRef]
- Ögel, K.; Evren, C.; Karadağ, F.; Gürol, D.T. The development, validity, and reliability of the Addiction Profile Index (API). Turk Psikiyatr. Derg 2012, 23, 263–275. [Google Scholar]
- Schönfeldt-Lecuona, C.; Kregel, T.; Schmidt, A.; Pinkhardt, E.H.; Lauda, F.; Kassubek, J.; Connemann, B.J.; Freudenmann, R.W.; Gahr, M. From imaging the brain to imaging the retina: Optical coherence tomography (OCT) in schizophrenia. Schizophr. Bull. 2016, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, F.; Alim, S. Optical coherence tomography findings in patients with alcohol use disorder and their relationship with clinical parameters. Cutan. Ocul. Toxicol. 2020, 39, 54–60. [Google Scholar] [CrossRef]
- Özsoy, F.; Kulu, M.; Özarslan, Y.; Korkmaz, S. Relationship between optical coherence tomography findings and clinical variables in patients with opiate use disorder. Arq. Bras. De Oftalmol. 2022, 20–26. [Google Scholar]
- Şahin, T.; Karadere, M.; Yıldız, V.; Çobanoğlu, E. Evaluation of the retinal nerve fiber layer with optic coherence tomography in patients with alcohol use disorder. J. Fr. D’ophtalmol. 2021, 44, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Orum, M.H.; Kalenderoglu, A.; Karadag, A.S.; Hocaoglu, C. Retinal nerve fiber layer decrease and choroidal layer increase after four weeks of buprenorphine/naloxone maintenance treatment in opioid use disorder. Eur. J. Psychiatry 2022, 36, 260–270. [Google Scholar] [CrossRef]
- Mahjoob, M.; Maleki, A.-R.; Askarizadeh, F.; Heydarian, S.; Rakhshandadi, T. Macula and optic disk features in methamphetamine and crystal methamphetamine addicts using optical coherence tomography. Int. Ophthalmol. 2022, 42, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Zeng, H.; Zhang, C.; Wang, L.; Tso, M.O.; Lai, S. Toxic effect of methamphetamine on the retina of CD1 mice. Curr. Eye Res. 2009, 34, 785–790. [Google Scholar] [CrossRef]
- Melo, P.; Rodrigues, L.G.; Silva, M.C.; Tavares, M.A. Effects of prenatal exposure to methamphetamine on the development of the rat retina. Ann. N. Y. Acad. Sci. 2006, 1074, 590–603. [Google Scholar] [CrossRef]
- Render, A.; Jansen, P. Dopamine and sense of agency: Determinants in personality and substance use. PLoS ONE 2019, 14, e0214069. [Google Scholar] [CrossRef]
- Weiss, F.; Markou, A.; Lorang, M.T.; Koob, G.F. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992, 593, 314–318. [Google Scholar] [CrossRef]
- Kohno, M.; Dennis, L.E.; McCready, H.; Hoffman, W.F. Dopamine dysfunction in stimulant use disorders: Mechanistic comparisons and implications for treatment. Mol. Psychiatry 2022, 27, 220–229. [Google Scholar] [CrossRef]
- Wallace, T.L.; Gudelsky, G.A.; Vorhees, C.V. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J. Neurosci. 1999, 19, 9141–9148. [Google Scholar] [CrossRef]
- Little, K.Y.; Krolewski, D.M.; Zhang, L.; Cassin, B.J. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am. J. Psychiatry 2003, 160, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Demir, F.; Taşcı, B. An effective and robust approach based on R-CNN+ LSTM model and ncar feature selection for ophthalmological disease detection from fundus images. J. Pers. Med. 2021, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Taşcı, B.; Acharya, M.R.; Barua, P.D.; Yildiz, A.M.; Gun, M.V.; Keles, T.; Dogan, S.; Tuncer, T. A new lateral geniculate nucleus pattern-based environmental sound classification using a new large sound dataset. Appl. Acoust. 2022, 196, 108897. [Google Scholar] [CrossRef]
- Macin, G.; Tasci, B.; Tasci, I.; Faust, O.; Barua, P.D.; Dogan, S.; Tuncer, T.; Tan, R.-S.; Acharya, U.R. An accurate multiple sclerosis detection model based on exemplar multiple parameters local phase quantization: ExMPLPQ. Appl. Sci. 2022, 12, 4920. [Google Scholar] [CrossRef]
- Tasci, B.; Tasci, I. Deep feature extraction based brain image classification model using preprocessed images: PDRNet. Biomed. Signal Process. Control 2022, 78, 103948. [Google Scholar] [CrossRef]
- Dogan, S.; Baygin, M.; Tasci, B.; Loh, H.W.; Barua, P.D.; Tuncer, T.; Tan, R.-S.; Acharya, U.R. Primate brain pattern-based automated Alzheimer’s disease detection model using EEG signals. Cogn. Neurodyn. 2022, 1–13. [Google Scholar] [CrossRef]
- Tasci, G.; Loh, H.W.; Barua, P.D.; Baygin, M.; Tasci, B.; Dogan, S.; Tuncer, T.; Palmer, E.E.; Tan, R.-S.; Acharya, U.R. Automated accurate detection of depression using twin Pascal’s triangles lattice pattern with EEG Signals. Knowl.-Based Syst. 2023, 260, 110190. [Google Scholar] [CrossRef]
- Tasci, B. Automated ischemic acute infarction detection using pre-trained CNN models’ deep features. Biomed. Signal Process. Control 2023, 82, 104603. [Google Scholar] [CrossRef]
- Demir, F.; Akbulut, Y.; Taşcı, B.; Demir, K. Improving brain tumor classification performance with an effective approach based on new deep learning model named 3ACL from 3D MRI data. Biomed. Signal Process. Control 2023, 81, 104424. [Google Scholar] [CrossRef]
- Tasci, B.; Tasci, G.; Dogan, S.; Tuncer, T. A novel ternary pattern-based automatic psychiatric disorders classification using ECG signals. Cogn. Neurodyn. 2022, 1–14. [Google Scholar] [CrossRef]
- Tasci, B. Ön Eğitimli Evrişimsel Sinir Ağı Modellerinde Öznitelik Seçim Algoritmasını Kullanarak Cilt lezyon görüntülerinin. Fırat Üniversitesi Mühendislik Bilim. Derg. 2022, 34, 541–552. [Google Scholar] [CrossRef]
- Tasci, İ.; Tasci, B.; Doğan, S.; Tuncer, T. A new dataset for EEG abnormality detection MTOUH. Turk. J. Sci. Technol. 2022, 17, 135–141. [Google Scholar] [CrossRef]
- Tasci, B. Beyin Mr Görüntülerinden Mrmr Tabanli Beyin Tümörlerinin Siniflandirmasi. J. Sci. Rep. B 2022, 6, 1–9. [Google Scholar]
- Tasci, B. An Overview on Covid19 Detection Algorithm Using Deep Learning. In Proceedings of the 2021 Innovations in Power and Advanced Computing Technologies (i-PACT), Kuala Lumpur, Malaysia, 27–29 November 2021; pp. 1–5. [Google Scholar]
- Tasci, B. A Classification Method for Brain MRI via AlexNet. In Proceedings of the 2021 International Conference on Disruptive Technologies for Multi-Disciplinary Research and Applications (CENTCON), Bengaluru, India, 19–21 November 2021; pp. 347–350. [Google Scholar]
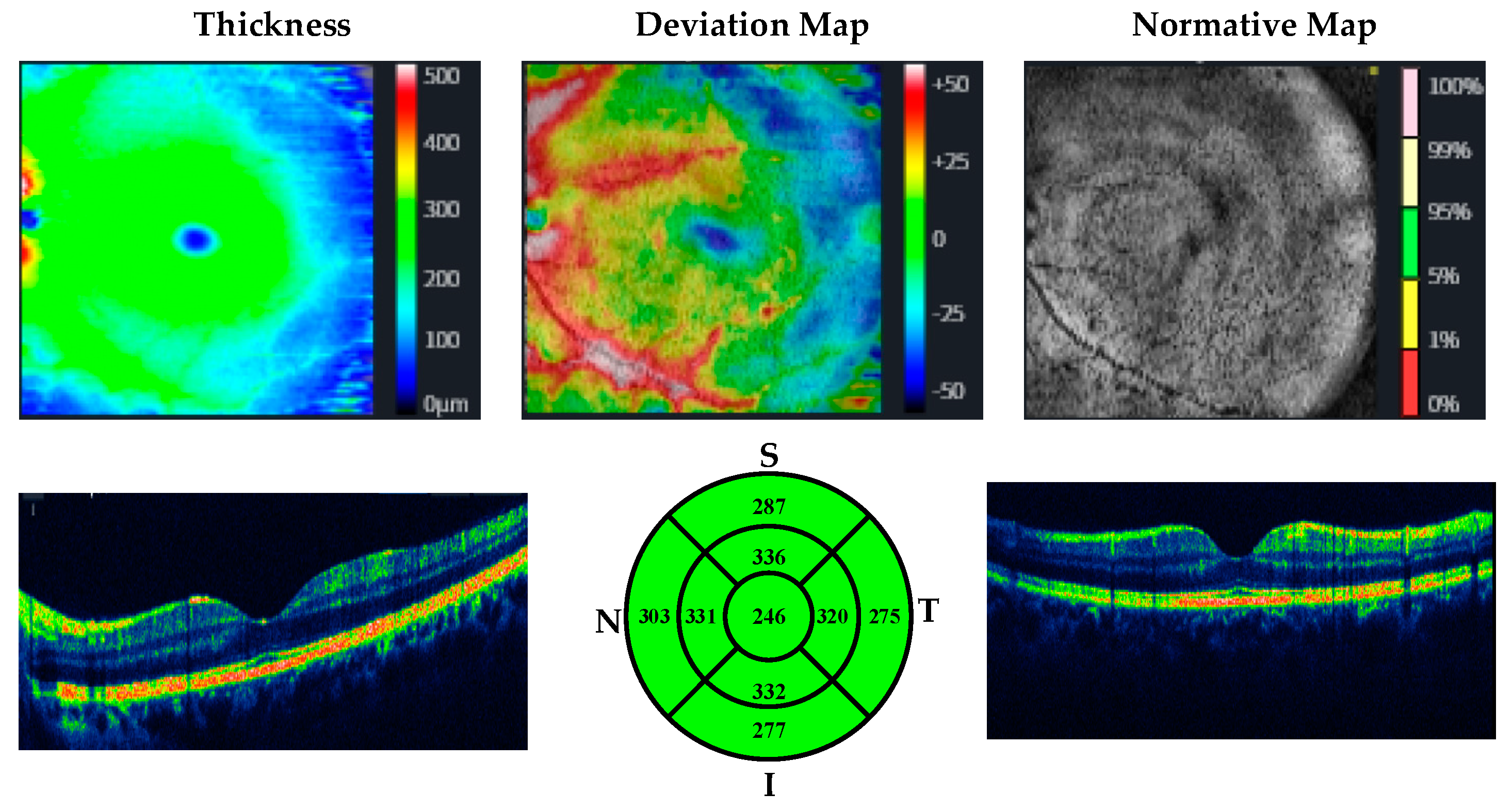
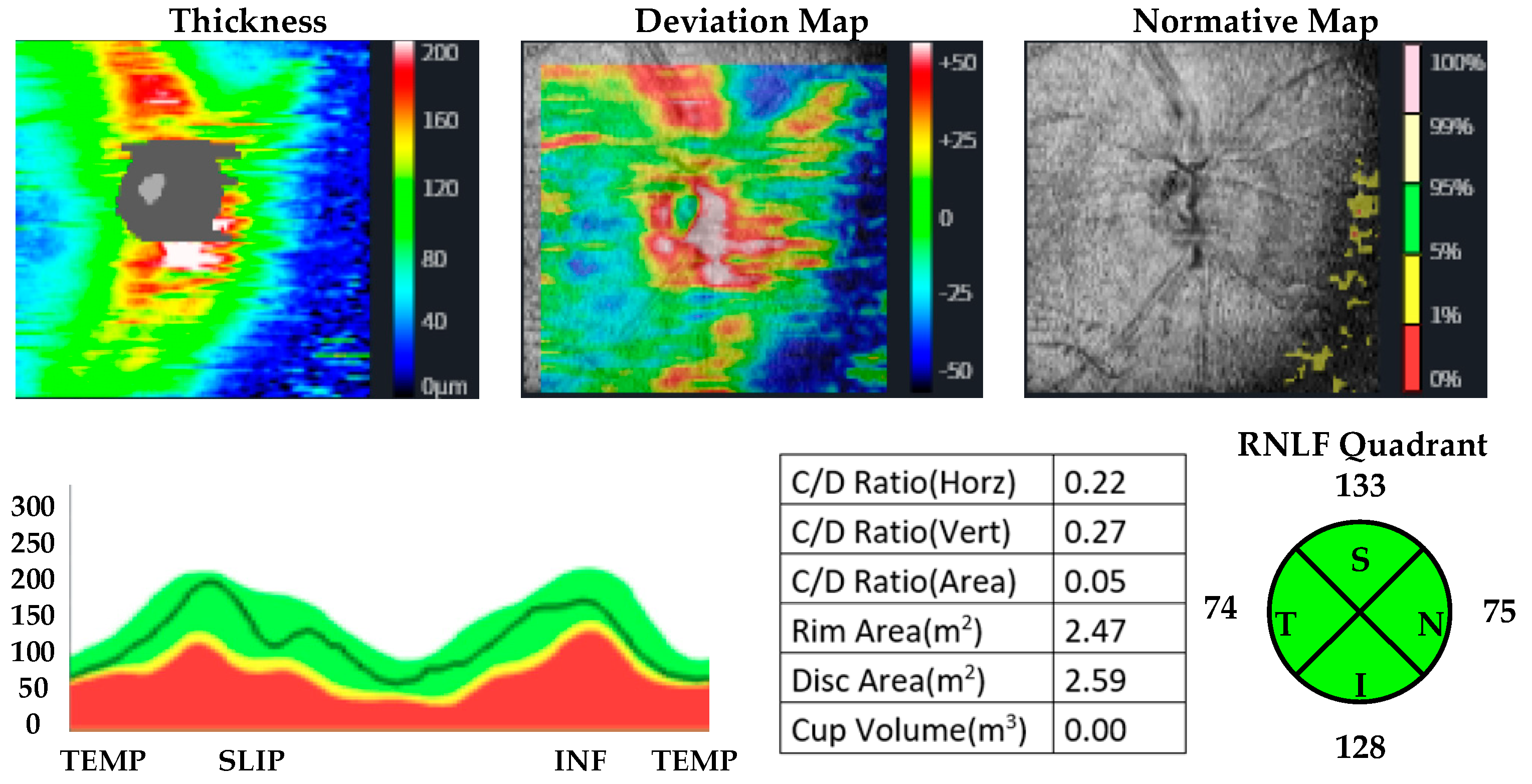

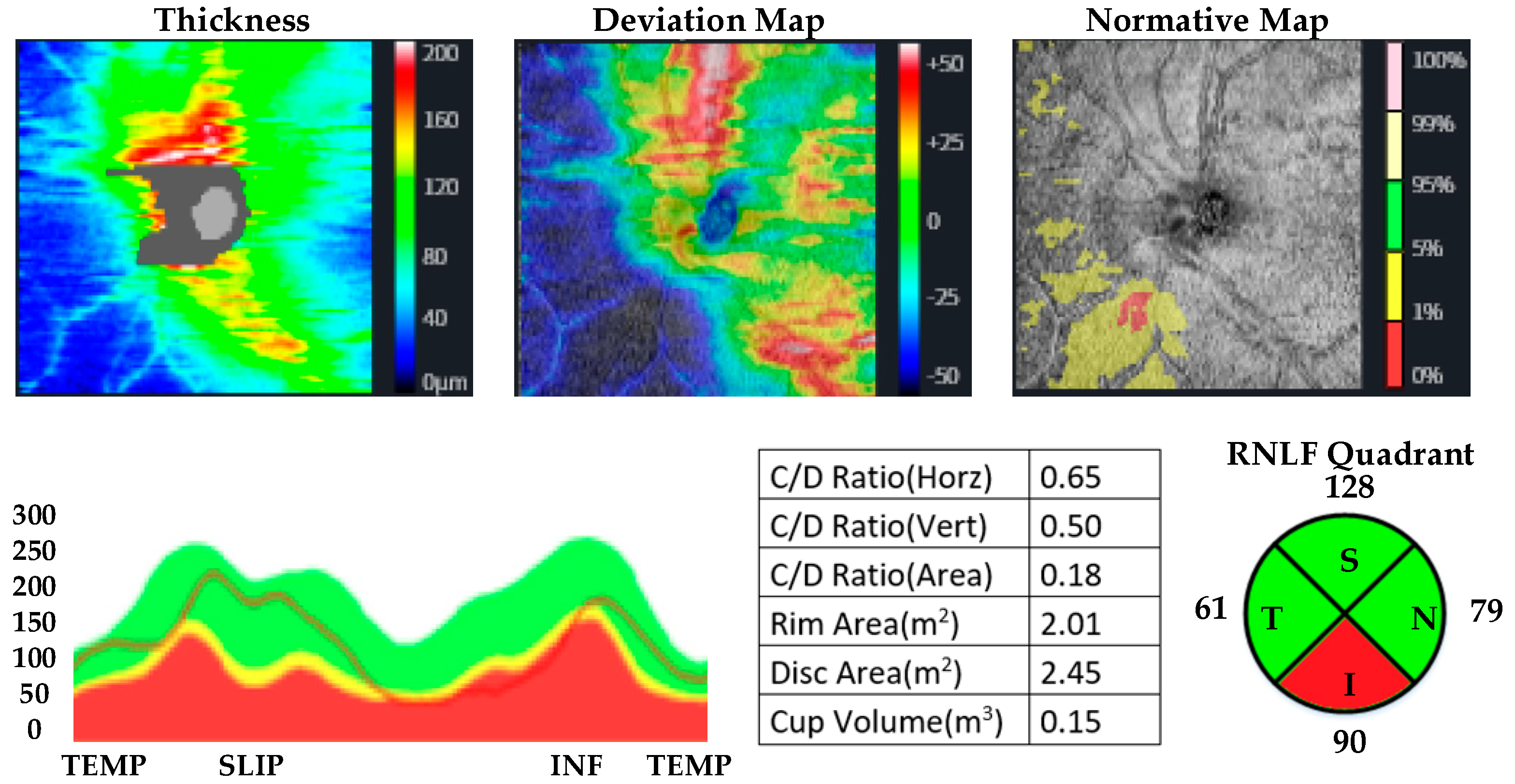
| MUD Patient Group | Healthy Control Group | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender (Male/Female) | 27/0 | 100 | 30/0 | 100 | >0.05 |
| Marital Status | |||||
| Married | 11 | 40.7 | 13 | 43.3 | >0.05 |
| Single | 14 | 51.9 | 15 | 50 | |
| Widow(er)/Divorced | 2 | 7.4 | 2 | 6.7 | |
| Educational Status | |||||
| Primary School | 5 | 18.5 | 4 | 13.3 | >0.05 |
| Secondary School | 14 | 51.9 | 16 | 53.3 | |
| High School | 5 | 18.5 | 6 | 20 | |
| University | 3 | 11.1 | 4 | 13.3 | |
| Working Status | |||||
| Part-time | 4 | 14.8 | 6 | 20 | >0.05 |
| Full-time | 10 | 37 | 13 | 43.3 | |
| Unemployed | 13 | 48.1 | 11 | 36.6 | |
| MUD Patient Group (N = 27) (Mean ± sd) | Healthy Control Group (N = 30) (Mean ± sd) | p | d | ||
|---|---|---|---|---|---|
| Retinal nerve fiber layer thickness | Superior quadrant | ||||
| Right eye | 125.70 ± 10.05 | 119.00 ± 16.98 | 0.079 a | d: 0.44, r: 0.219 | |
| Left eye | 129.85 ± 9.79 | 115.53 ± 25.89 | 0.002 b | d: 0.74, r: 0.34 | |
| Inferior quadrant | |||||
| Right eye | 130.93 ± 12.47 | 125.77 ± 16.18 | 0.187 a | d: 0.35, r: 0.17 | |
| Left eye | 134.67 ± 15.03 | 125.53 ± 16.25 | 0.032 a | d: 0.58, r: 0.27 | |
| Temporal quadrant | |||||
| Right eye | 82.67 ± 8.96 | 64.43 ± 12.10 | <0.001 a | d: 1.76, r: 0.66 | |
| Left eye | 76.70 ± 7.51 | 64.13 ± 12.17 | <0.001 b | d: 1.22, r: 0.52 | |
| Nasal quadrant | |||||
| Right eye | 84.81 ± 10.35 | 76.83 ± 13.13 | 0.012 b | d: 0.68, r: 0.32 | |
| Left eye | 82.15 ± 11.69 | 75.23 ± 13.28 | 0.019 b | d: 0.58, r: 0.27 | |
| Total value | |||||
| Right eye | 116.81 ± 9.35 | 96.50 ± 10.04 | <0.001 a | d: 2.10, r: 0.72 | |
| Left eye | 106.30 ± 7.99 | 96.53 ± 9.93 | <0.001 b | d: 1.24, r: 0.52 | |
| Macular thickness | Central Macular | ||||
| Right eye | 245.26 ± 15.54 | 249.00 ± 16.92 | 0.390 a | d: −0.25, r: −0.12 | |
| Left eye | 244.33 ± 13.49 | 247.40 ± 19.09 | 0.491 a | d: −0.18, r: −0.09 | |
| Mean Macular | |||||
| Right eye | 280.93 ± 9.61 | 286.33 ± 13.70 | 0.094 a | d: −0.53, r: −0.25 | |
| Left eye | 283.00 ± 10.21 | 283.17 ± 13.22 | 0.958 a | d: −0.08, r: −0.04 | |
| APIS | SUC | 2.7433 ± 1.66 | |||
| D | 14.68 ± 5.68 | ||||
| IOL | 29.74 ± 6.40 | ||||
| SD | 8.44 ± 4.39 | ||||
| M | 11.03 ± 1.55 | ||||
| SUC | D | IOL | SD | M | APIS | ||
|---|---|---|---|---|---|---|---|
| RNLF thickness | |||||||
| Superior | |||||||
| Right eye | r | 0.107 | 0.361 | 0.071 | −0.006 | −0.065 | 0.205 |
| Left eye | r | 0.340 | 0.108 | 0.080 | 0.178 | −0.119 | 0.247 |
| Inferior | |||||||
| Right eye | r | 0.291 | −0.028 | 0.212 | 0.234 | −0.157 | 0.120 |
| Left eye | r | −0.122 | −0.083 | 0.285 | −0.200 | −0.200 | −0.080 |
| Temporal | |||||||
| Right eye | r | −0.123 | −0.074 | 0.107 | 0.032 | 0.053 | −0.061 |
| Left eye | r | 0.475 * | 0.064 | 0.253 | 0.056 | 0.158 | 0.242 |
| Nasal | |||||||
| Right eye | r | 0.187 | −0.060 | 0.030 | 0.031 | −0.075 | −0.011 |
| Left eye | r | −0.288 | −0.180 | −0.100 | −0.300 | −0.343 | −0.394 * |
| Total value | |||||||
| Right eye | r | 0.134 | 0.059 | 0.055 | −0.114 | −0.390 * | −0.080 |
| Left eye | r | −0.245 | −0.200 | 0.040 | −0.193 | 0.060 | −0.200 |
| Macular thickness | |||||||
| Central | |||||||
| Right eye | r | −0.205 | −0.063 | −0.168 | −0.108 | 0.506 ** | −0.095 |
| Left eye | r | −0.147 | −0.015 | −0.044 | −0.028 | 0.550 ** | 0.019 |
| Mean | |||||||
| Right eye | r | −0.169 | −0.301 | −0.072 | −0.178 | 0.284 | −0.201 |
| Left eye | r | −0.105 | −0.308 | −0.082 | −0.074 | 0.338 | −0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, Ş.; Kaya, M.K. OCT Findings in Patients with Methamphetamine Use Disorder. J. Pers. Med. 2023, 13, 308. https://doi.org/10.3390/jpm13020308
Kaya Ş, Kaya MK. OCT Findings in Patients with Methamphetamine Use Disorder. Journal of Personalized Medicine. 2023; 13(2):308. https://doi.org/10.3390/jpm13020308
Chicago/Turabian StyleKaya, Şüheda, and Mehmet Kaan Kaya. 2023. "OCT Findings in Patients with Methamphetamine Use Disorder" Journal of Personalized Medicine 13, no. 2: 308. https://doi.org/10.3390/jpm13020308
APA StyleKaya, Ş., & Kaya, M. K. (2023). OCT Findings in Patients with Methamphetamine Use Disorder. Journal of Personalized Medicine, 13(2), 308. https://doi.org/10.3390/jpm13020308







