Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Outcomes of Interest
2.3.1. Primary Outcomes
Experimental Studies
Clinical Studies
2.3.2. Secondary Outcomes
Experimental Studies
Clinical Studies
2.4. Search Strategy
2.5. Data Extraction
2.6. Assessment of Methodological Quality
2.7. Data Analysis and Synthesis
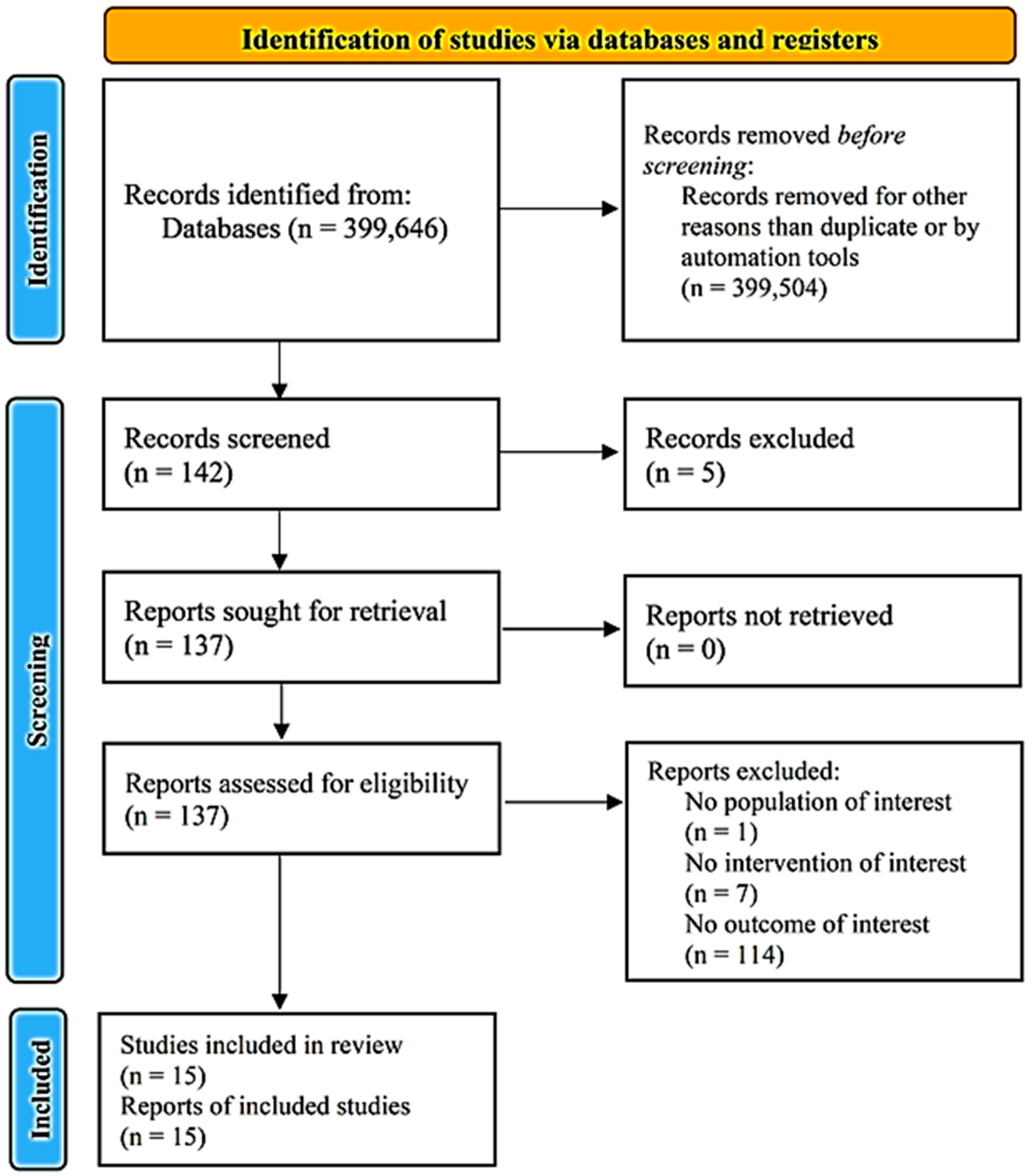
3. Results
3.1. Risk of Bias, Quality of Evidence
3.2. Synthesis including All Data
Relationship of Vasopressin Receptor Agonists with Hemodynamic Parameters
Characteristics of Experimental (Animal) Studies
Characteristics of Clinical Studies
3.3. Secondary Outcomes
3.3.1. Experimental (Animal) Studies
3.3.2. Clinical Studies
4. Discussion
5. Limitations
6. Conclusions
7. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galbraith, C.M.; Wagener, B.M.; Chalkias, A.; Siddiqui, S.; Douin, D.J. Massive Trauma and Resuscitation Strategies. Anesthesiol. Clin. 2023, 41, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Garg, N.; Ramachandran, R. Vasopressors: Do they have any role in hemorrhagic shock? J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fecher, A.; Stimpson, A.; Ferrigno, L.; Pohlman, T.H. The Pathophysiology and Management of Hemorrhagic Shock in the Polytrauma Patient. J. Clin. Med. 2021, 10, 4793. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef]
- Sperry, J.L.; Minei, J.P.; Frankel, H.L.; West, M.A.; Harbrecht, B.G.; Moore, E.E.; Maier, R.V.; Nirula, R. Early use of vasopressors after injury: Caution before constriction. J. Trauma 2008, 64, 9–14. [Google Scholar] [CrossRef]
- Plurad, D.S.; Talving, P.; Lam, L.; Inaba, K.; Green, D.; Demetriades, D. Early vasopressor use in critical injury is associated with mortality independent from volume status. J. Trauma 2011, 71, 562–565. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef]
- Fangio, P.; Asehnoune, K.; Edouard, A.; Smail, N.; Benhamou, D. Early embolization and vasopressor administration for management of life-threatening hemorrhage from pelvic fracture. J. Trauma 2005, 58, 978–984. [Google Scholar] [CrossRef]
- Richards, J.E.; Harris, T.; Dünser, M.W.; Bouzat, P.; Gauss, T. Vasopressors in Trauma: A Never Event? Anesth. Analg. 2021, 133, 68–79. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Liu, L.; Tian, K.; Xue, M.; Zhu, Y.; Lan, D.; Peng, X.; Wu, Y.; Li, T. Small doses of arginine vasopressin in combination with norepinephrine “buy” time for definitive treatment for uncontrolled hemorrhagic shock in rats. Shock 2013, 40, 398–406. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, K.H.; Wagner-Berger, H.G.; Raedler, C.; Voelckel, W.G.; Wenzel, V.; Krismer, A.C.; Klima, G.; Rheinberger, K.; Nussbaumer, W.; Pressmar, D.; et al. Vasopressin, but not fluid resuscitation, enhances survival in a liver trauma model with uncontrolled and otherwise lethal hemorrhagic shock in pigs. Anesthesiology 2003, 98, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Jochem, J. Central histamine-induced reversal of critical haemorrhagic hypotension in rats—A comparison with the pressor effect of arginine vasopressin. Inflamm. Res. 2004, 53 (Suppl. S1), S61–S62. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Kim, M.S.; Park, H.M. Hemodynamic characteristics of vasopressin in dogs with severe hemorrhagic shock. J. Vet. Med. Sci. 2006, 68, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Pearce, F.J.; Jeffreys, N.; McJames, S.W.; Cluff, M. Impact of vasopressin on hemodynamic and metabolic function in the decompensatory phase of hemorrhagic shock. J. Cardiothorac. Vasc. Anesth. 2006, 20, 167–172. [Google Scholar] [CrossRef]
- Meybohm, P.; Cavus, E.; Bein, B.; Steinfath, M.; Weber, B.; Hamann, C.; Scholz, J.; Dörges, V. Small volume resuscitation: A randomized controlled trial with either norepinephrine or vasopressin during severe hemorrhage. J. Trauma 2007, 62, 640–646. [Google Scholar] [CrossRef]
- Stadlbauer, K.H.; Wagner-Berger, H.G.; Krismer, A.C.; Voelckel, W.G.; Konigsrainer, A.; Lindner, K.H.; Wenzel, V. Vasopressin improves survival in a porcine model of abdominal vascular injury. Crit. Care 2007, 11, R81. [Google Scholar] [CrossRef]
- Li, T.; Fang, Y.; Zhu, Y.; Fan, X.; Liao, Z.; Chen, F.; Liu, L. A small dose of arginine vasopressin in combination with norepinephrine is a good early treatment for uncontrolled hemorrhagic shock after hemostasis. J. Surg. Res. 2011, 169, 76–84. [Google Scholar] [CrossRef]
- Lima, R.; Villela, N.R.; Bouskela, E. Microcirculatory effects of selective receptor blockade during hemorrhagic shock treatment with vasopressin: Experimental study in the hamster dorsal chamber. Shock 2012, 38, 493–498. [Google Scholar] [CrossRef]
- Sims, C.A.; Yuxia, G.; Singh, K.; Werlin, E.C.; Reilly, P.M.; Baur, J.A. Supplemental arginine vasopressin during the resuscitation of severe hemorrhagic shock preserves renal mitochondrial function. PLoS One 2017, 12, e0186339. [Google Scholar] [CrossRef]
- Truse, R.; Grewe, S.; Herminghaus, A.; Schulz, J.; Weber, A.P.M.; Mettler-Altmann, T.; Bauer, I.; Picker, O.; Vollmer, C. Exogenous vasopressin dose-dependently modulates gastric microcirculatory oxygenation in dogs via V1A receptor. Crit. Care 2019, 23, 353. [Google Scholar] [CrossRef]
- Gil-Anton, J.; Mielgo, V.E.; Rey-Santano, C.; Galbarriatu, L.; Santos, C.; Unceta, M.; López-Fernández, Y.; Redondo, S.; Morteruel, E. Addition of terlipressin to initial volume resuscitation in a pediatric model of hemorrhagic shock improves hemodynamics and cerebral perfusion. PLoS ONE 2020, 15, e0235084. [Google Scholar] [CrossRef]
- Dickson, J.M.; Wang, X.; St John, A.E.; Lim, E.B.; Stern, S.A.; White, N.J. Damage Control Resuscitation Supplemented with Vasopressin in a Severe Polytrauma Model with Traumatic Brain Injury and Uncontrolled Internal Hemorrhage. Mil. Med. 2018, 183, e460–e466. [Google Scholar] [CrossRef]
- Cohn, S.M.; McCarthy, J.; Stewart, R.M.; Jonas, R.B.; Dent, D.L.; Michalek, J.E. Impact of low-dose vasopressin on trauma outcome: Prospective randomized study. World J. Surg. 2011, 35, 430–439. [Google Scholar] [CrossRef]
- Sims, C.A.; Holena, D.; Kim, P.; Pascual, J.; Smith, B.; Martin, N.; Seamon, M.; Shiroff, A.; Raza, S.; Kaplan, L.; et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients With Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019, 154, 994–1003. [Google Scholar] [CrossRef]
- Uchida, K.; Nishimura, T.; Hagawa, N.; Kaga, S.; Noda, T.; Shinyama, N.; Yamamoto, H.; Mizobata, Y. The impact of early administration of vasopressor agents for the resuscitation of severe hemorrhagic shock following blunt trauma. BMC Emerg Med. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Chighine, A.; Noto, A.; Ferino, G.; Baldi, A.; Varvarousis, D.; Xanthos, T.; De-Giorgio, F.; Stocchero, M.; d’Aloja, E. Metabolomics improves the histopathological diagnosis of asphyxial deaths: An animal proof-of-concept model. Sci. Rep. 2021, 11, 10102. [Google Scholar] [CrossRef] [PubMed]
- Barmparas, G.; Dhillon, N.K.; Smith, E.J.; Mason, R.; Melo, N.; Thomsen, G.M.; Margulies, D.R.; Ley, E.J. Patterns of vasopressor utilization during the resuscitation of massively transfused trauma patients. Injury 2018, 49, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Abe, T.; Saitoh, D.; Hagiwara, S.; Oshima, K. Use of vasopressor increases the risk of mortality in traumatic haemorrhagic shock: A nationwide cohort study in Japan. Crit. Care Med. 2018, 46, e1145–e1151. [Google Scholar] [CrossRef]
- Fisher, A.D.; April, M.D.; Cunningham, C.; Schauer, S.G. Prehospital vasopressor use is associated with worse mortality in combat wounded. Prehospital Emerg. Care 2021, 25, 268–273. [Google Scholar] [CrossRef]
- Gauss, T.; Gayat, E.; Harrois, A.; Raux, M.; Follin, A.; Daban, J.L.; Cook, F.; Hamada, S. TraumaBase group, prehospital traumabase group Ile de France SAMU=service d’Aide Médicale Urgente: Effect of early use of noradrenaline on in-hospital mortality in haemorrhagic shock after major trauma: A propensity-score analysis. Br. J. Anaesth. 2018, 120, 1237–1244. [Google Scholar] [CrossRef]
- Beloncle, F.; Meziani, F.; Lerolle, N.; Radermacher, P.; Asfar, P. Does vasopressor therapy have an indication in hemorrhagic shock? Ann. Intensive Care 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.W.; Levin, H.R.; Gallant, E.M.; Ashton, R.C., Jr.; Seo, S.; D’Alessandro, D.; Oz, M.C.; Oliver, J.A. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 1997, 95, 1122–1125. [Google Scholar] [CrossRef]
- Cohn, S.M.; DeRosa, M.; McCarthy, J.; Song, J.; White, C.; Louden, C.; Ehler, B.; Michalek, J.; Landry, D.W. Characterizing vasopressin and other vasoactive mediators released during resuscitation of trauma patients. J. Trauma Acute Care Surg. 2013, 75, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Treschan, T.A.; Peters, J. The vasopressin system: Physiology and clinical strategies. Anesthesiology 2006, 105, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Madigan, J.; Cullinane, S.; Chen, J.; Heath, M.; Oz, M.; Oliver, J.A.; Landry, D.W. Reversal by vasopressin of intractable hypotension in the late phase of hemorrhagic shock. Circulation 1999, 100, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Da Costa, D.; Mathias, C.J.; Bannister, R.; Lightman, S.L. Pressor effect of arginine vasopressin in progressive autonomic failure. Clin. Sci. 1986, 71, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sanui, M.; King, D.R.; Feinstein, A.J.; Varon, A.J.; Cohn, S.M.; Proctor, K.G. Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit. Care Med. 2006, 34, 433–438. [Google Scholar] [CrossRef]
- Cossu, A.P.; Mura, P.; De Giudici, L.M.; Puddu, D.; Pasin, L.; Evangelista, M.; Xanthos, T.; Musu, M.; Finco, G. Vasopressin in hemorrhagic shock: A systematic review and meta-analysis of randomized animal trials. Biomed. Res. Int. 2014, 2014, 421291. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.; Turmaine, M.; Boulos, P.B.; Burnstock, G. Sympathetic innervation of human mesenteric artery and vein. J. Vasc. Res. 2008, 45, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Nemergut, E.C.; Lynch, C., 3rd. The clinical implications of isolated alpha1 adrenergic stimulation. Anesth. Analg. 2011, 113, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Rutlen, D.; Supple, E.W.; Powell, P.W., Jr. Adrenergic regulation of total systemic distensibility. Venous distensibility effects of norepinephrine and isoproterenol before and after selective adrenergic blockade. Am. J. Cardiol. 1981, 47, 579–588. [Google Scholar] [CrossRef]
- Gelman, S. Venous circulation: A few challenging concepts in goal-directed hemodynamic therapy (GDHT). In Perioperative Fluid Management; Farag, E., Kurz, A., Troianos, C., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 365–385. [Google Scholar]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Spyropoulos, V.; Kouskouni, E.; Theodoraki, K.; Xanthos, T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. J. Pers. Med. 2022, 12, 724. [Google Scholar] [CrossRef]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Varvarousi, G.; Ragias, D.; Koutsovasilis, A.; Makris, D.; Varvarousis, D.; Iacovidou, N.; Pantazopoulos, I.; et al. Determinants of venous return in steady-state physiology and asphyxia-induced circulatory shock and arrest: An experimental study. Intensive Care Med. Exp. 2022, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Koutsovasilis, A.; Laou, E.; Papalois, A.; Xanthos, T. Measurement of mean systemic filling pressure after severe hemorrhagic shock in swine anesthetized with propofol-based total intravenous anesthesia: Implications for vasopressor-free resuscitation. Acute Crit. Care 2020, 35, 93–101. [Google Scholar] [CrossRef]
- Hartmann, C.; Radermacher, P.; Wepler, M.; Nußbaum, B. Non-Hemodynamic Effects of Catecholamines. Shock 2017, 48, 390–400. [Google Scholar] [CrossRef]
- Pang, C.C.Y.; Tabrizchi, R. The effects of noradrenaline, B-HT 920, methoxamine, angiotensin II and vasopressin on mean circulatory filling pressure in conscious rats. Br. J. Pharmacol. 1986, 89, 389–394. [Google Scholar] [CrossRef]
- Martin, D.S.; McNeill, J.R. Whole body vascular capacitance response to vasopressin is mediated by autonomic function. Am. J. Physiol. Heart Circ. Physiol. 1991, 261, H493–H499. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.C.; Jain, K.M.; Swan, K.G.; Rocko, J.M. Effects of vasopressin on cardiac output and its distribution in the subhuman primate. J. Vasc. Surg. 1985, 2, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Quail, A.W.; Woods, R.L.; Korner, P.I. Cardiac and arterial baroreceptor influences in release of vasopressin and renin during hemorrhage. Am. J. Physiol. 1987, 252, H1120–H1126. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.P.; Thompson, C.J.; Keil, L.C.; Thrasher, T.N. Renin and vasopressin responses to graded reductions in atrial pressure in conscious dogs. Am. J. Physiol. 1994, 266, R714–R721. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, V.; Raab, H.; Dünser, M.W. Arginine vasopressin: A promising rescue drug in the treatment of uncontrolled haemorrhagic shock. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and its analogues in shock states: A review. Ann. Intensive Care 2020, 10, 9. [Google Scholar] [CrossRef]
- Fage, N.; Asfar, P.; Radermacher, P.; Demiselle, J. Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. Int. J. Mol. Sci. 2023, 24, 4103. [Google Scholar] [CrossRef]
- Fox, A.W.; May, R.E.; Mitch, W.E. Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J. Cardiovasc. Pharmacol. 1992, 20, 282–289. [Google Scholar] [CrossRef]
- Ida, K.K.; Chisholm, K.I.; Malbouisson, L.M.S.; Papkovsky, D.B.; Dyson, A.; Singer, M.; Duchen, M.R.; Smith, K.J. Protection of cerebral microcirculation, mitochondrial function, and electrocortical activity by small-volume resuscitation with terlipressin in a rat model of haemorrhagic shock. Br. J. Anaesth. 2018, 120, 1245–1254. [Google Scholar] [CrossRef]
- Gelman, S.; Ernst, E.A. Nitroprusside prevents adverse hemodynamic effects of vasopressin. Arch. Surg. 1978, 113, 1465–1471. [Google Scholar] [CrossRef]


| Author Name, Year | Species | VRA/ Comparator | Dosage | Variable | ||||
|---|---|---|---|---|---|---|---|---|
| CO/CI | SVR/SVRI | SV | CVP/RAP | MAP | ||||
| Stadlbauer et al., 2003 [13] | Swine | AVP/Ringer’s and 3% gelatine solution | 0.4 IU kg−1 + infusion 0.08 IU kg−1 min−1 | NA | NA | NA | NA | 72 (26) vs. 38 (16) |
| Jochem et al., 2004 [14] | Rat | AVP/Saline | 0.25 nmol kg−1 | 11.5 (2.19) vs. 5 (0.44) | 5.01 (0.22) vs. 4.02 (0.25) * | NA | NA | 57.7 (6.1) vs. 20.1 (2.8) |
| Yoo et al., 2006 [15] | Dog | Vasopressin/ Saline | 0.4 IU kg−1 | 5.33 (0.44) vs. 6.67 (0.86) | 1485 (174) vs. 1502 (228) | NA | NA | NA |
| Johnson et al., 2006 [16] | Rat | AVP/Ringer’s | 0.05 IU kg−1 min−1 | NA | 2386 (295) vs. 1362 (316) | NA | 4 (1) vs. 7 (2) | NA |
| Meybohm et al., 2007 [17] | Swine | AVP/Norepinephrine | 0.4 IU kg−1 | NA | NA | NA | NA | 49 (16) vs. 39 (19) |
| Stadlbauer et al., 2007 [18] | Swine | AVP/Ringer’s and 3% gelatine solution | 0.4 IU kg−1 + infusion 0.08 IU kg−1 min−1 | NA | NA | NA | NA | 60 vs. 55 # |
| Li et al., 2011 [19] | Rat | AVP/Ringer’s | 0.4 IU kg−1 | NA | NA | NA | NA | 39.8 (2) vs. 41.3 (1.1) |
| Lima et al., 2012 [20] | Hamster | AVP/Saline | 0.4 IU kg−1 | NA | NA | NA | NA | 73.56 (12.51) vs. 72.5 (15.07) |
| Liu et al., 2013 [11] | Rat | AVP/Ringer’s | NA | NA | NA | NA | NA | 36.25 (3.34) vs. 37.94 (2.74) |
| Sims et al., 2017 [21] | Rat | AVP/Ringer’s | 0.5 IU kg−1 + 0.03 IU kg−1 min−1 | NA | NA | NA | NA | 125 vs. 70 |
| Dickson et al. 2018 [24] | Swine | AVP/No fluids | 0.4 IU kg−1 + 0.4 IU kg−1 after 40 min | NA | NA | NA | NA | 44.1 (17.4) vs. 28.6 (10.6) |
| Truse et al., 2019 [22] | Dog | AVP/Saline | 0.001 ng kg−1 min−1 | ↓ CO # | NA | 23 (3) vs. 23 (2) | NA | NA |
| Gil-Anton et al., 2020 [23] | Swine | Terlipressin/ Saline | 20 mg kg−1 | 3.4 (0.5) vs. 3.2 (0.4) | 1361 (227) vs. 1157 (217) | NA | 6 (1) vs. 6 (1) | 65 (8) vs. 53 (5) |
| Author, Year | Type of Study | Type of Injury | VAR/Comparator | Dosage | End-Organ Damage/MOF | Mortality at 30 Days |
|---|---|---|---|---|---|---|
| Cohn et al., 2011 [25] | Double-blind, randomized, parallel-group, controlled trial | Acute traumatic injury | Vasopressin/Saline | 4 IU bolus + 2.4 IU h −1 for 5 h | No significant differences | 13/38 vs. 11/40, p = 0.52 |
| Sims et al., 2019 [26] | Randomized, double-blind placebo-controlled clinical trial | Acute traumatic injury | AVP/Saline placebo | 4 IU bolus or placebo + ≤0.04 IU min−1 or placebo for 48 h | No significant differences | 6/49 vs. 6/51, p = 0.94 |
| Parameter | Number of Studies | N (Total) | Estimate (SMD) | p-Value | 95% CI | I2 | Q | p (Q) |
|---|---|---|---|---|---|---|---|---|
| Mortality at 30 days | 2 | 179 | 0.17 | 0.562 | 0.67 to 2.08 | 50% | 1.06 | 0.586 |
| Author, Year | Species | VRA/ Comparator | Dosage | Variable | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | PaO2 | PaCO2 | HCO3 | BD | Lactate | SaO2 | ||||
| Stadlbauer et al., 2003 [13] | Swine | AVP/Ringer’s and 3% gelatine solution | 0.4 IU kg−1 + infusion 0.08 IU kg−1 min−1 | 7.44 (0.11) vs. 7.27 (0.05) | 199 (161) vs. 179 (37) | 26 (7) vs. 36 (8) | NA | NA | 9.5 (3.1) vs. 9.0 (0.7) | NA |
| Meybohm et al., 2007 [17] | Swine | AVP/Norepinephrine | 10 IU bolus + 2 IU kg−1 h−1 | 7.24 (0.06) vs. 7.25 (0.05) | NA | NA | NA | −7.5 (3.5) vs. −5.9 (4.9) | NA | NA |
| Stadlbauer et al., 2007 [18] | Swine | AVP/Ringer’s and 3% gelatine solution | 0.4 IU kg−1 + infusion 0.08 IU kg−1 min−1 | 7.15 (0.05) vs. 7.51 (0.01) | 312 (134) vs. 239 (131) | 30 (3) vs. 24 (7) | NA | −8.8 (5.8) vs. −8.9 (3.3) | 11.1 (3.11) vs. 8.44 (2.66) | NA |
| Lima et al., 2012 [20] | Hamster | AVP/Saline | 0.0001 IU kg−1 min−1 | 7.40 (0.05) vs. 7.38 (0.11) | 84.3 (19.41) vs. 113.4 (28.6) | 42.31 (7.85) vs. 41.2 (8.81) | 26.1 (3.8) vs. 24.7 (4.8) | 1.20 (3.99) vs. −0.30 (5.98) | 1.85 (1.04) vs. 3.75 (3.08) | NA |
| Liu et al., 2013 [11] | Rat | AVP/Ringer’s | 0.4 U kg−1 | 7.39 (0.032) vs. 7.39 (0.05) | 118.4 (9.7) vs. 121.1 (10.1) | 32 (2.9) vs. 34.1 (1.9) | NA | −3.81 (2.81) vs. −4.43 (2.08) | NA | NA |
| Sims et al., 2017 [21] | Rat | AVP/Ringer’s | 0.5 IU kg−1 + 0.03 IU kg−1 min−1 | 7.37 (0.05) vs. 7.35 (0.10) | 107 (11) vs. 118 (35) | 34 (4) vs. 31 (9) | NA | NA | 25 (5) vs. 25 (5) | NA |
| Truse et al., 2019 [22] | Dog | AVP/Saline | 0.001–1 ng kg−1 min−1 | 7.39 (0.01) vs. 7.38 (0.02) | NA | 36 (1) vs. 36 (2) | 21 (0.6) vs. 20.2 (0.6) | NA | 1.2 (0.3) vs. 1.9 (0.6) | 99 (0.2) vs. 98 (0.2) |
| Author, Year | Type of Study | Type of Injury | VAR/Comparator | Dosage | Fluid | |
|---|---|---|---|---|---|---|
| Type | Dose | |||||
| Cohn et al., 2011 [25] | Double-blind, randomized, parallel-group, controlled trial | Acute traumatic injury | Vasopressin/Saline | 4 IU bolus + 2.4 IU h −1 for 5 h | Crystalloids | 13.2 ± 9.8 L vs. 16 ± 12.8 L * |
| Blood and blood products | 3.8 ± 5 L vs. 5.4 ± 6.6 L | |||||
| Sims et al., 2019 [26] | Randomized, double-blind placebo-controlledclinical trial | Acute traumatic injury | AVP/Saline placebo | 4 IU bolus or placebo + ≤0.04 IU min−1 or placebo for 48 h | Crystalloids | 5.0 [IQR, 2.5–7.0] vs. 6.7 [IQR, 4.0–11.4] L, p = 0.03 |
| Blood and blood products | 1.4 [IQR, 0.5–2.6] vs. 2.9 [IQR, 1.1–4.8] L; p = 0.01 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laou, E.; Papagiannakis, N.; Papadopoulou, A.; Choratta, T.; Sakellakis, M.; Ippolito, M.; Pantazopoulos, I.; Cortegiani, A.; Chalkias, A. Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies. J. Pers. Med. 2023, 13, 1143. https://doi.org/10.3390/jpm13071143
Laou E, Papagiannakis N, Papadopoulou A, Choratta T, Sakellakis M, Ippolito M, Pantazopoulos I, Cortegiani A, Chalkias A. Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies. Journal of Personalized Medicine. 2023; 13(7):1143. https://doi.org/10.3390/jpm13071143
Chicago/Turabian StyleLaou, Eleni, Nikolaos Papagiannakis, Androniki Papadopoulou, Theodora Choratta, Minas Sakellakis, Mariachiara Ippolito, Ioannis Pantazopoulos, Andrea Cortegiani, and Athanasios Chalkias. 2023. "Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies" Journal of Personalized Medicine 13, no. 7: 1143. https://doi.org/10.3390/jpm13071143
APA StyleLaou, E., Papagiannakis, N., Papadopoulou, A., Choratta, T., Sakellakis, M., Ippolito, M., Pantazopoulos, I., Cortegiani, A., & Chalkias, A. (2023). Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies. Journal of Personalized Medicine, 13(7), 1143. https://doi.org/10.3390/jpm13071143









