Linking the Extended Autonomic System with the Homeostat Theory: New Perspectives about Dysautonomias
Abstract
:1. Introduction
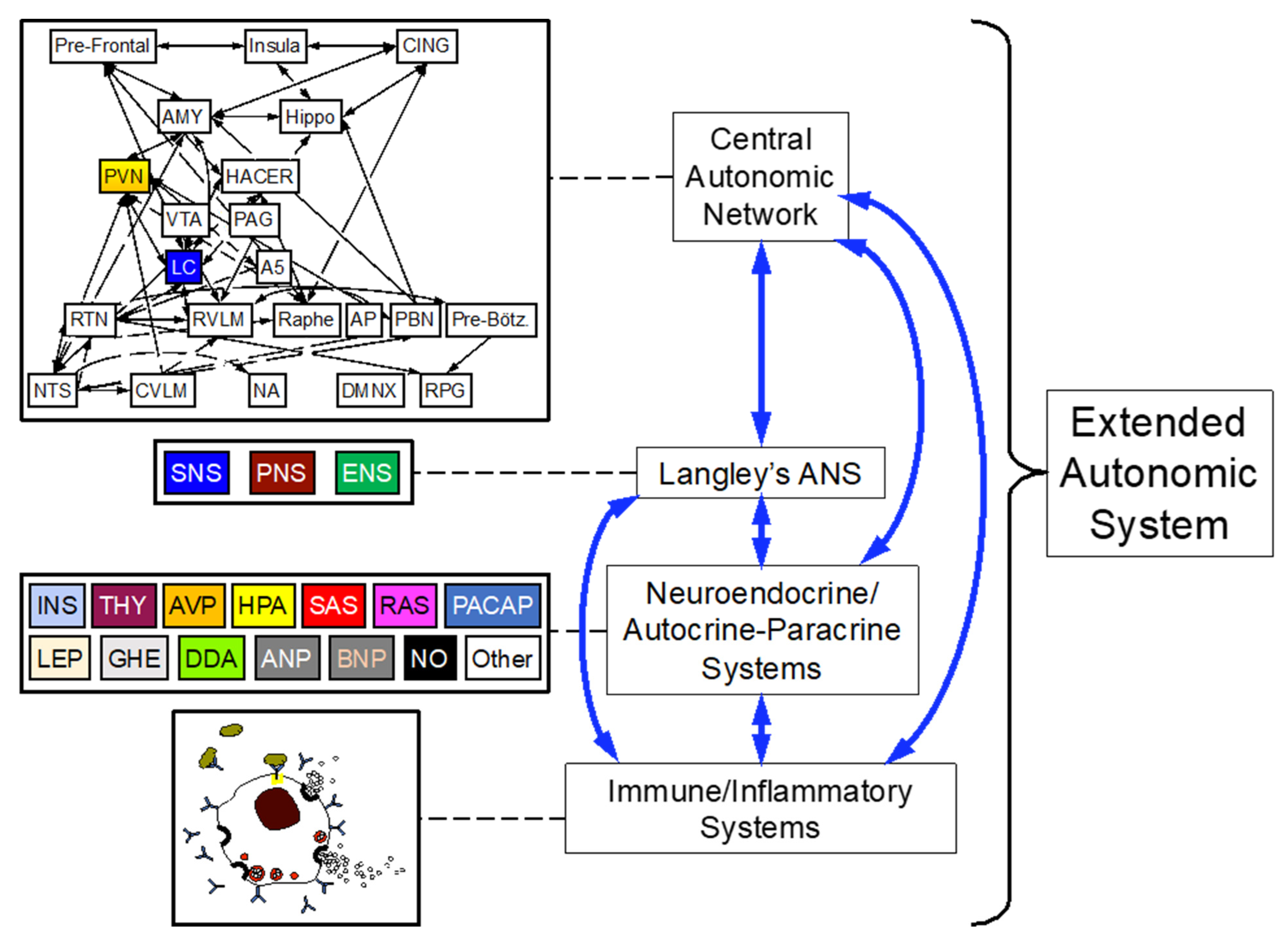
2. The “Extended” Autonomic System (EAS)
3. The Homeostat Theory
4. Allostasis and Allostatic Load
5. Principles of Homeostat Operation
5.1. Multiple Effectors
5.2. Effector Sharing
6. Homeostats at Work
6.1. Thermoregulation
6.2. Glucose
6.3. Blood Gases
6.4. Blood Flow to the Brain during Orthostasis
7. Application to Pediatric Dysautonomias: Familial Dysautonomia (FD)
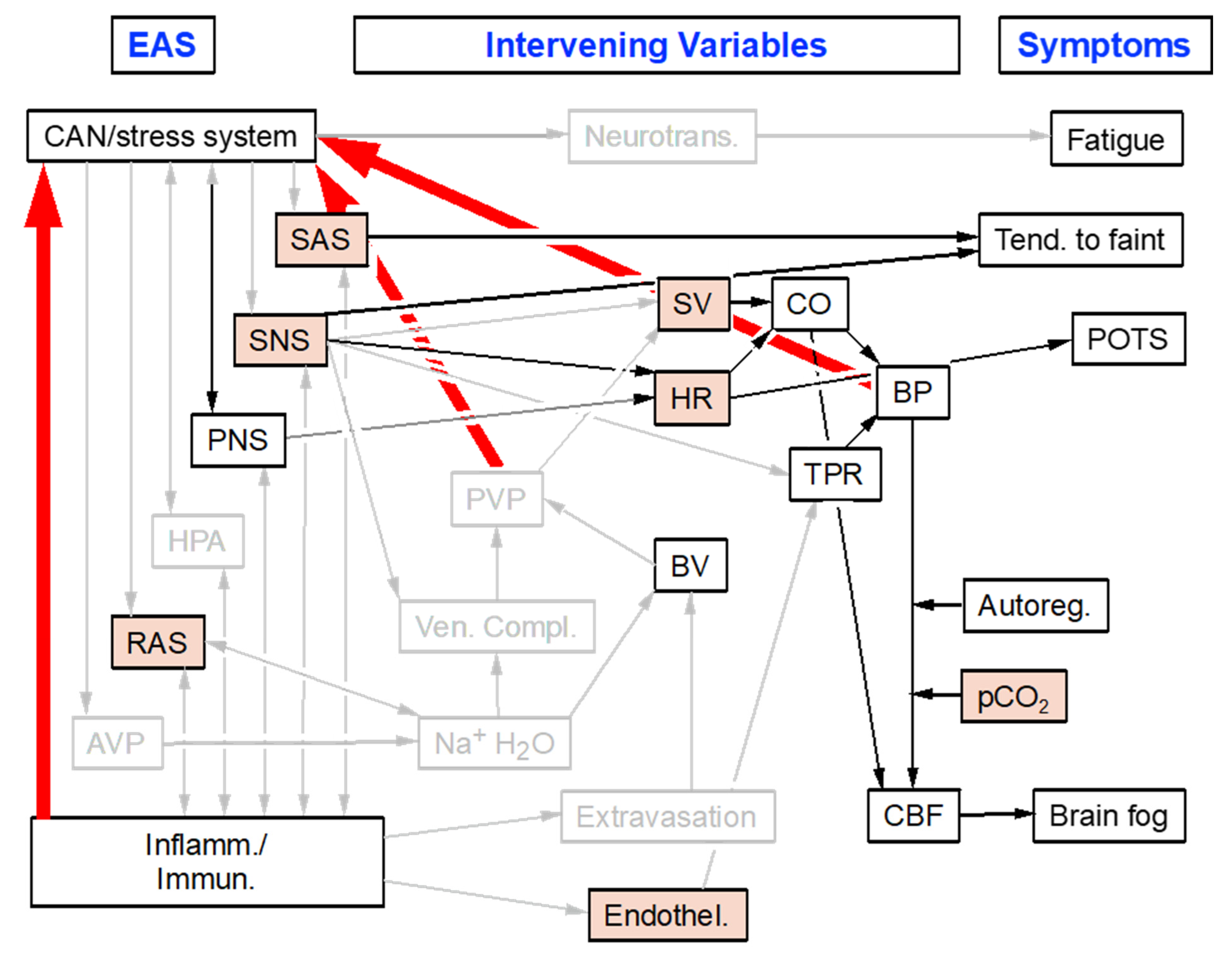
8. Application to Adult Dysautonomias: Postural Tachycardia Syndrome (POTS)
9. Application to Geriatric Dysautonomias: Central Lewy Body Diseases (LBDs)
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheshire, W.P. The grand challenge of autonomic disorders. Front. Neurol. 2022, 13, 1052137. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020, 30, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Stress and the “extended” autonomic system. Auton. Neurosci. 2021, 236, 102889. [Google Scholar] [CrossRef]
- Langley, J.N. The autonomic nervous system. Brain 1903, 26, 1–26. [Google Scholar] [CrossRef]
- Goldstein, D.S. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine; The Johns Hopkins University Press: Baltimore, MD, USA, 2006. [Google Scholar]
- Rao, Y. The First Hormone: Adrenaline. Trends Endocrinol. Metab. 2019, 30, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Udit, S.; Blake, K.; Chiu, I.M. Somatosensory and autonomic neuronal regulation of the immune response. Nat. Rev. Neurosci. 2022, 23, 157–171. [Google Scholar] [CrossRef]
- Benarroch, E.E. Physiology and Pathophysiology of the Autonomic Nervous System. Continuum 2020, 26, 12–24. [Google Scholar] [CrossRef]
- Goldstein, D.S. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R301–R317. [Google Scholar] [CrossRef]
- Schulkin, J.; Sterling, P. Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends Neurosci. 2019, 42, 740–752. [Google Scholar] [CrossRef]
- Sterling, P. What Is Health?: Allostasis and the Evolution of Human Design; MIT Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Li, A.J.; Wang, Q.; Ritter, S. Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology 2018, 159, 341–355. [Google Scholar] [CrossRef]
- Kondoh, K.; Lu, Z.; Ye, X.; Olson, D.P.; Lowell, B.B.; Buck, L.B. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature 2016, 532, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.; Palma, J.A.; Martinez, J.; Camargo, C.; Kaufmann, H. Fear conditioning as a pathogenic mechanism in the postural tachycardia syndrome. Brain 2022, 145, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.M.; Sawyer, W.B.; Platt, K.B.; Loewy, A.D. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989, 491, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.F.; Chen, L.; Shi, K.W.; Cheng, E.; Ge, S.; Xiong, Q. Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning. Nat. Commun. 2023, 14, 7231. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Computer models of stress, allostasis, and acute and chronic disease. Ann. N. Y. Acad. Sci. 2008, 1148, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, A.; Androulakis, I.P. Allostatic breakdown of cascading homeostat systems: A computational approach. Heliyon 2017, 3, e00355. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Udelsman, R.; Goldstein, D.S.; Loriaux, D.L.; Chrousos, G.P. Catecholamine-glucocorticoid interactions during surgical stress. J. Surg. Res. 1987, 43, 539–545. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endo. Regul. 2008, 42, 111–119. [Google Scholar]
- Frank, S.M.; Raja, S.N.; Bulcao, C.F.; Goldstein, D.S. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J. Appl. Physiol. 1999, 86, 1588–1593. [Google Scholar] [CrossRef]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, Y.; Goldstein, D.S.; Bentho, O.; Saleem, A.; Pechnik, S.; Geraci, M.F.; Holmes, C.; Pacak, K.; Eisenhofer, G. Sympathoadrenal function in patients with paroxysmal hypertension: Pseudopheochromocytoma. J. Hypertens. 2007, 25, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Zhang, Q.; Salehi, A.; Willems, M.; Knudsen, J.G.; Ringgaard, A.K.; Chapman, C.E.; Gonzalez-Alvarez, A.; Surdo, N.C.; Zaccolo, M.; et al. Adrenaline Stimulates Glucagon Secretion by Tpc2-Dependent Ca(2+) Mobilization From Acidic Stores in Pancreatic alpha-Cells. Diabetes 2018, 67, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Porte, D., Jr.; Graber, A.L.; Kuzuya, T.; Williams, R.H. The effect of epinephrine on immunoreactive insulin levels in man. J. Clin. Investig. 1966, 45, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, J.; Zhu, G.; Wang, Q.; Lv, Q.; Huang, Y.; Yu, Y.; Si, X.; Yi, H.; Wang, C.; et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: A retrospective cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001476. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R. Does poor glucose control increase the severity and mortality in patients with diabetes and COVID-19? Diabetes Metab. Syndr. 2020, 14, 725–727. [Google Scholar] [CrossRef]
- Scheen, A.J.; Marre, M.; Thivolet, C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020, 46, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef]
- Dziewierz, A.; Giszterowicz, D.; Siudak, Z.; Rakowski, T.; Dubiel, J.S.; Dudek, D. Admission glucose level and in-hospital outcomes in diabetic and non-diabetic patients with acute myocardial infarction. Clin. Res. Cardiol. 2010, 99, 715–721. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Bayliss, D.A.; Stornetta, R.L.; Kanbar, R.; Shi, Y.; Holloway, B.B.; Souza, G.; Basting, T.M.; Abbott, S.B.G.; Wenker, I.C. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J. Physiol. 2018, 596, 3029–3042. [Google Scholar] [CrossRef]
- Egan, B.M.; Julius, S.; Cottier, C.; Osterziel, K.J.; Ibsen, H. Role of cardiovascular receptors on the neural regulation of renin release in normal men. Hypertension 1983, 5, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Zoller, R.P.; Mark, A.L.; Abboud, F.M.; Schmid, P.G.; Heistad, D.D. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J. Clin. Investig. 1972, 51, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.C.; Goldstein, D.S.; Willemsen, J.J.; Smits, P.; Thien, T.; Lenders, J.W. Differential effects of low- and high-intensity lower body negative pressure on noradrenaline and adrenaline kinetics in humans. Clin. Sci. 1996, 90, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Picot, P.A.; Rutt, B.K.; Shoemaker, J.K.; Bondar, R.L. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000, 31, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Holmes, C.; Axelrod, F.B. Plasma catechols in familial dysautonomia: A long-term follow-up study. Neurochem. Res. 2008, 33, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, F.B.; Goldstein, D.S.; Holmes, C.; Berlin, D.; Kopin, I.J. Pattern of plasma levels of catecholamines in familial dysautonomia. Clin. Auton. Res. 1996, 6, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.; Axelrod, F.; Kaufmann, H. Afferent baroreflex failure in familial dysautonomia. Neurology 2010, 75, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.J.; Axelrod, F.B.; Kaufmann, H. Cyclic vomiting associated with excessive dopamine in Riley-day syndrome. J. Clin. Gastroenterol. 2013, 47, 136–138. [Google Scholar] [CrossRef]
- Norcliffe-Kaufmann, L.; Martinez, J.; Axelrod, F.; Kaufmann, H. Hyperdopaminergic crises in familial dysautonomia: A randomized trial of carbidopa. Neurology 2013, 80, 1611–1617. [Google Scholar] [CrossRef]
- Kopin, I.J.; Breese, G.R.; Krauss, K.R.; Weise, V.K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J. Pharmacol. Exp. Ther. 1968, 161, 271–278. [Google Scholar]
- Hatch, S.T.; Smargon, A.A.; Yeo, G.W. Engineered U1 snRNAs to modulate alternatively spliced exons. Methods 2022, 205, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kim, Y.J.; Nomakuchi, T.; Sahashi, K.; Hua, Y.; Rigo, F.; Bennett, C.F.; Krainer, A.R. Antisense oligonucleotides correct the familial dysautonomia splicing defect in IKBKAP transgenic mice. Nucleic Acids Res. 2018, 46, 4833–4844. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Cheng, S.Y.; Kirchner, E.; Costello, S.; Miettinen, H.; Chaverra, M.; King, C.; George, L.; Zhao, X.; Narasimhan, J.; et al. Reduction of retinal ganglion cell death in mouse models of familial dysautonomia using AAV-mediated gene therapy and splicing modulators. Sci. Rep. 2023, 13, 18600. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Riccardi, F.; Bussani, E.; Vodret, S.; Licastro, D.; Ragone, I.; Ronzitti, G.; Morini, E.; Slaugenhaupt, S.A.; Pagani, F. Rescue of a familial dysautonomia mouse model by AAV9-Exon-specific U1 snRNA. Am. J. Hum. Genet. 2022, 109, 1534–1548. [Google Scholar] [CrossRef]
- Singer, A.; Sagi-Dain, L. Impact of a national genetic carrier-screening program for reproductive purposes. Acta Obstet. Gynecol. Scand. 2020, 99, 802–808. [Google Scholar] [CrossRef]
- Summers, R.L.; Platts, S.; Myers, J.G.; Coleman, T.G. Theoretical analysis of the mechanisms of a gender differentiation in the propensity for orthostatic intolerance after spaceflight. Theor. Biol. Med. Model. 2010, 7, 8. [Google Scholar] [CrossRef]
- Diaz-Canestro, C.; Sehgal, A.; Pentz, B.; Montero, D. Sex specificity in orthostatic tolerance: The integration of haematological, cardiac, and endocrine factors. Eur. J. Prev. Cardiol. 2022, 29, e246–e248. [Google Scholar] [CrossRef]
- Fu, Q.; Levine, B.D. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. 2018, 215, 20–27. [Google Scholar] [CrossRef]
- Abdelazeem, B.; Abbas, K.S.; Manasrah, N.; Amin, M.A.; Mohammed, S.M.; Mostafa, M.R. Yoga as a treatment for vasovagal syncope: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2022, 48, 101579. [Google Scholar] [CrossRef]
- De Boer, S.F.; Slangen, J.L.; Van der Gugten, J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: Effects of chlordiazepoxide. Physiol. Behav. 1990, 47, 1089–1098. [Google Scholar] [CrossRef]
- Isonaka, R.; Gibbons, C.H.; Wang, N.; Freeman, R.; Goldstein, D.S. Association of innervation-adjusted alpha-synuclein in arrector pili muscles with cardiac noradrenergic deficiency in autonomic synucleinopathies. Clin. Auton. Res. 2019, 29, 587–593. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, H.E.; Oh, Y.S.; Song, I.U.; Yang, D.W.; Park, J.W.; Lee, K.S. (123)I-MIBG myocardial scintigraphy and neurocirculatory abnormalities in patients with dementia with Lewy bodies and Alzheimer’s disease. J. Neurol. Sci. 2015, 357, 173–177. [Google Scholar] [CrossRef]
- Lamotte, G.; Holmes, C.; Sullivan, P.; Lenka, A.; Goldstein, D.S. Cardioselective peripheral noradrenergic deficiency in Lewy body synucleinopathies. Ann. Clin. Transl. Neurol. 2020, 7, 2450–2460. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sullivan, P.; Lopez, G.; Gelsomino, J.; Moore, S.; Isonaka, R.; Wu, T.; Sharabi, Y. Cardiac noradrenergic deficiency revealed by 18F-dopamine positron emission tomography identifies preclinical central Lewy body diseases. J. Clin. Investig. 2024, 134, e172460. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Pekker, M.J.; Eisenhofer, G.; Sharabi, Y. Computational modeling reveals multiple abnormalities of myocardial noradrenergic function in Lewy body diseases. JCI Insight 2019, 5, e130441. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J.; Sharabi, Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 144, 268–282. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Pekker, M.J.; Sullivan, P.; Isonaka, R.; Sharabi, Y. Modeling the Progression of Cardiac Catecholamine Deficiency in Lewy Body Diseases. J. Am. Heart Assoc. 2022, 11, e024411. [Google Scholar] [CrossRef]
- Troshev, D.; Bannikova, A.; Blokhin, V.; Pavlova, E.; Kolacheva, A.; Ugrumov, M. Compensatory Processes in Striatal Neurons Expressing the Tyrosine Hydroxylase Gene in Transgenic Mice in a Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 16245. [Google Scholar] [CrossRef]
- Rao, R.; Androulakis, I.P. Allostatic adaptation and personalized physiological trade-offs in the circadian regulation of the HPA axis: A mathematical modeling approach. Sci. Rep. 2019, 9, 11212. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldstein, D.S. Linking the Extended Autonomic System with the Homeostat Theory: New Perspectives about Dysautonomias. J. Pers. Med. 2024, 14, 123. https://doi.org/10.3390/jpm14010123
Goldstein DS. Linking the Extended Autonomic System with the Homeostat Theory: New Perspectives about Dysautonomias. Journal of Personalized Medicine. 2024; 14(1):123. https://doi.org/10.3390/jpm14010123
Chicago/Turabian StyleGoldstein, David S. 2024. "Linking the Extended Autonomic System with the Homeostat Theory: New Perspectives about Dysautonomias" Journal of Personalized Medicine 14, no. 1: 123. https://doi.org/10.3390/jpm14010123
APA StyleGoldstein, D. S. (2024). Linking the Extended Autonomic System with the Homeostat Theory: New Perspectives about Dysautonomias. Journal of Personalized Medicine, 14(1), 123. https://doi.org/10.3390/jpm14010123






