Minimally Invasive Approaches to Spinal Cerebrospinal Fluid Leak Repair: Current Strategies and a Novel Technique
Abstract
1. Introduction
2. Minimally Invasive Approaches to Spinal CSF Leak Repair
2.1. Epidural Blood Patch
2.2. Epidural Fibrin Patch
2.3. Targeted Patch Repair
3. A Novel Minimally Invasive Approach to Spinal CSF Leak Repair
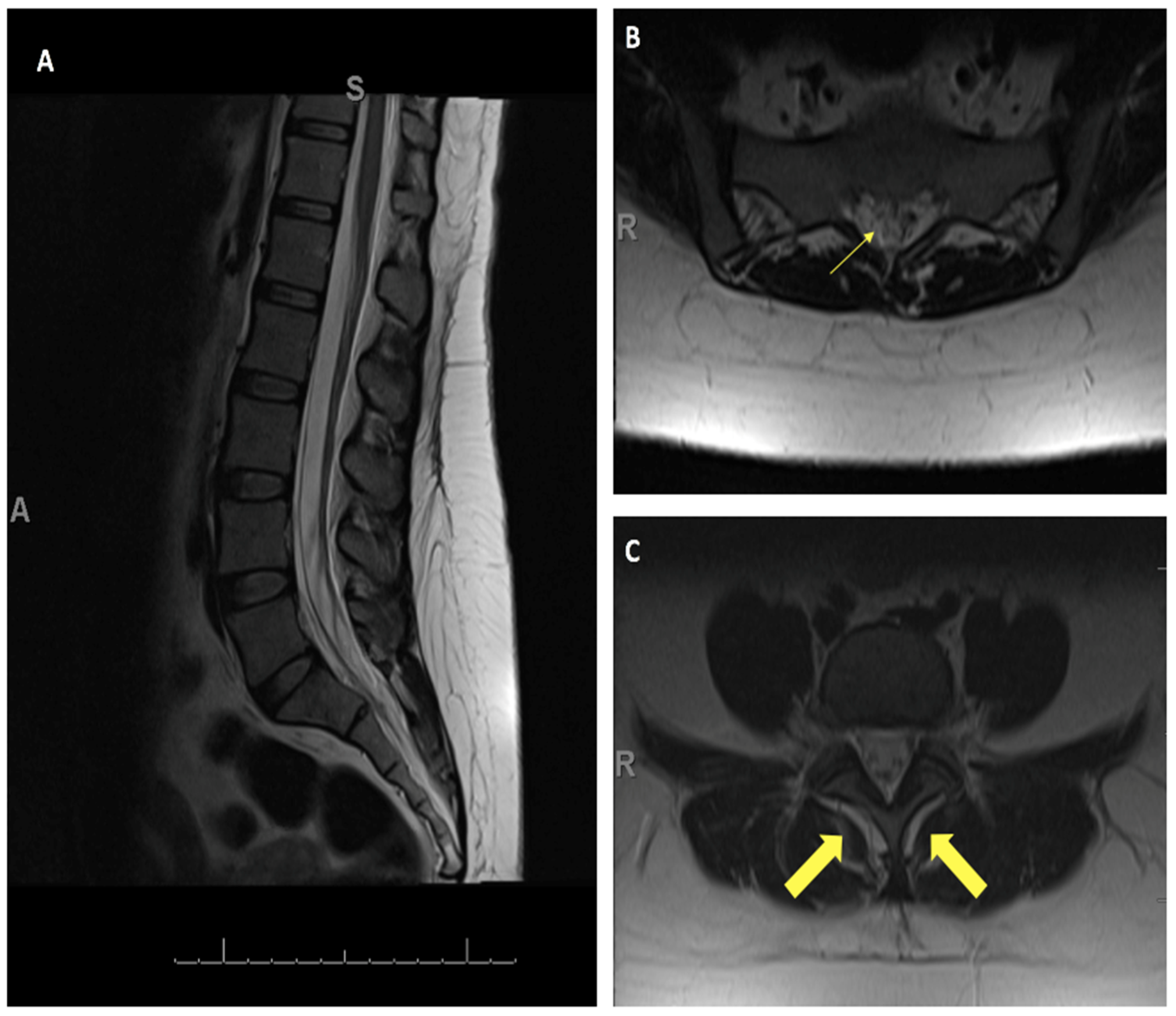
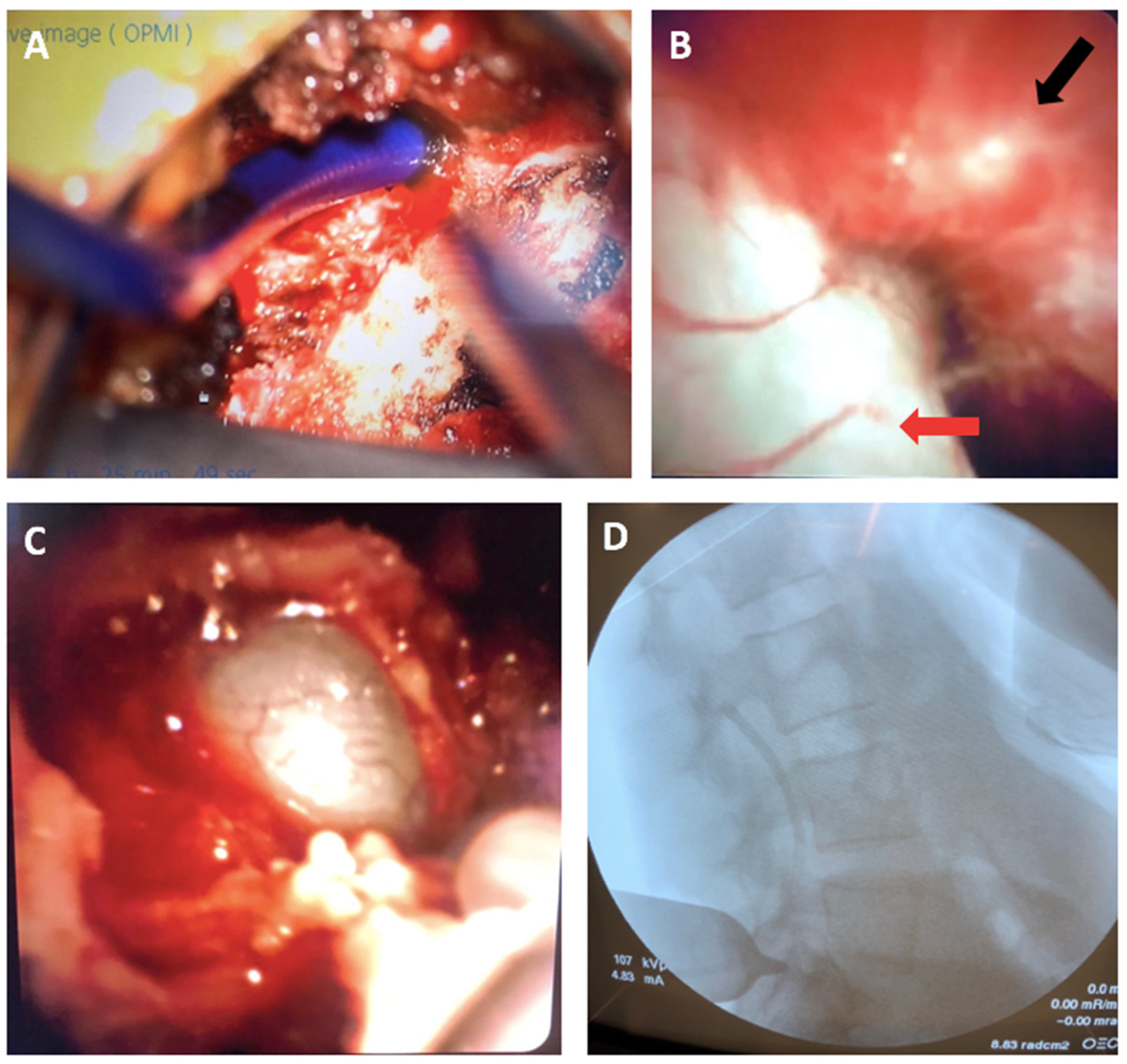
4. Illustrative Case
4.1. Patient Presentation
4.2. Novel Surgical Approach
4.3. Postoperative Outcomes
5. Flexible Endoscopy in Spine Surgery
6. Applications of wHUD with Flexible Endoscopy
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galli, J.; Morelli, F.; Rigante, M.; Paludetti, G. Management of Cerebrospinal Fluid Leak: The Importance of Multidisciplinary Approach. Acta Otorhinolaryngol. Ital. 2021, 41, S18–S29. [Google Scholar] [CrossRef] [PubMed]
- Zappi, K.; Giantini-Larsen, A.; Yan, J.; Konate, M.; Garton, A.L.A.; Knopman, J.; Stieg, P.E.; Salama, G.; Park, J.K. Innovations in the Treatment of Spinal Cerebrospinal Fluid Leaks. World Neurosurg. 2024, 187, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, L.T.; Rajkumar, S.; Liu, B.; Adil, S.M.; Wong, M.; Hodges, S.; Amrhein, T.J.; Leithe, L.G.; Parente, B.; Lee, H.-J.; et al. Treatment Patterns and Health Care Resource Utilization of Iatrogenic Spinal Cerebrospinal Fluid Leaks in the United States. Clin. Spine Surg. 2022, 35, E725–E730. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I. Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension. JAMA 2006, 295, 2286–2296. [Google Scholar] [CrossRef]
- Chae, J.K.; Rosen, K.; Zappi, K.; Giantini-Larsen, A.; Yan, J.; Sung, J.; Bander, E.; Schwartz, T.H.; Park, J.K.; Salama, G. Cranial and Spinal Cerebrospinal Fluid Leaks: Foundations of Identification and Management. World Neurosurg. 2024, 187, 288–293. [Google Scholar] [CrossRef]
- Cheema, S.; Anderson, J.; Angus-Leppan, H.; Armstrong, P.; Butteriss, D.; Carlton Jones, L.; Choi, D.; Chotai, A.; D’Antona, L.; Davagnanam, I.; et al. Multidisciplinary Consensus Guideline for the Diagnosis and Management of Spontaneous Intracranial Hypotension. J. Neurol. Neurosurg. Psychiatry 2023, 94, 835–843. [Google Scholar] [CrossRef]
- Piechowiak, E.I.; Aeschimann, B.; Häni, L.; Kaesmacher, J.; Mordasini, P.; Jesse, C.M.; Schankin, C.J.; Raabe, A.; Schär, R.T.; Gralla, J.; et al. Epidural Blood Patching in Spontaneous Intracranial Hypotension-Do We Really Seal the Leak? Clin. Neuroradiol. 2023, 33, 211–218. [Google Scholar] [CrossRef]
- Barber, S.M.; Sofoluke, N.; Reardon, T.; Mongelluzzo, G.; Weiner, G.M.; Hofstetter, C.; Telfeian, A.; Konakondla, S. Full Endoscopic Repair of Spontaneous Ventral Cerebrospinal Fluid Leaks in the Spine: Systematic Review of Surgical Treatment Options and Illustrative Case. World Neurosurg. 2022, 168, e578–e586. [Google Scholar] [CrossRef] [PubMed]
- Yuh, W.-T.; Lee, Y.-S.; Jeon, J.-H.; Choi, I. Future of Endoscopic Spine Surgery: Insights from Cutting-Edge Technology in the Industrial Field. Bioengineering 2023, 10, 1363. [Google Scholar] [CrossRef]
- Mokri, B. Spontaneous Low Pressure, Low CSF Volume Headaches: Spontaneous CSF Leaks. Headache 2013, 53, 1034–1053. [Google Scholar] [CrossRef]
- Jones, M.R.; Shlobin, N.A.; Dahdaleh, N.S. Spontaneous Spinal Cerebrospinal Fluid Leak: Review and Management Algorithm. World Neurosurg. 2021, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Angileri, F.F.; Kruse, P.; Cavallo, L.M.; Solari, D.; Esposito, V.; Tomasello, F.; Cappabianca, P. Fibrin Sealants in Dura Sealing: A Systematic Literature Review. PLoS ONE 2016, 11, e0151533. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lin, M.; Giantini-Larsen, A.; Kim, A.; Edasery, D.; Roytman, M.; Strauss, S.; Schweitzer, A.D.; Park, J.K.; Salama, G. Cerebrospinal Fluid Leaks: Challenges in Localizing Spontaneous Spinal Leak Sites and Minimally Invasive Treatment. World Neurosurg. 2024, 187, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Monroe, B.R. Successful Treatment of Postdural Puncture Headache Using Epidural Fibrin Glue Patch after Persistent Failure of Epidural Blood Patches. Pain. Pract. 2017, 17, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Hubbe, U.; Klingler, J.-H.; Roelz, R.; Kraus, L.M.; Volz, F.; Lützen, N.; Urbach, H.; Kieselbach, K.; Fung, C. Minimally Invasive Surgery for Spinal Cerebrospinal Fluid Leaks in Spontaneous Intracranial Hypotension. J. Neurosurg. Spine 2023, 38, 147–152. [Google Scholar] [CrossRef]
- Janki, S.; Mulder, E.E.A.P.; IJzermans, J.N.M.; Tran, T.C.K. Ergonomics in the Operating Room. Surg. Endosc. 2017, 31, 2457–2466. [Google Scholar] [CrossRef]
- Alleblas, C.C.J.; De Man, A.M.; Van Den Haak, L.; Vierhout, M.E.; Jansen, F.W.; Nieboer, T.E. Prevalence of Musculoskeletal Disorders Among Surgeons Performing Minimally Invasive Surgery: A Systematic Review. Ann. Surg. 2017, 266, 905–920. [Google Scholar] [CrossRef]
- Van Det, M.J.; Meijerink, W.J.H.J.; Hoff, C.; Totté, E.R.; Pierie, J.P.E.N. Optimal Ergonomics for Laparoscopic Surgery in Minimally Invasive Surgery Suites: A Review and Guidelines. Surg. Endosc. 2009, 23, 1279–1285. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chen, R.E.; Kim, E.J.; Akinduro, O.O.; Kerezoudis, P.; Han, P.K.; Si, P.; Freeman, W.D.; Diaz, R.J.; Komotar, R.J.; et al. Augmented Reality for the Surgeon: Systematic Review. Robot. Comput. Surg. 2018, 14, e1914. [Google Scholar] [CrossRef]
- Kolcun, J.P.G.; Brusko, G.D.; Basil, G.W.; Epstein, R.; Wang, M.Y. Endoscopic Transforaminal Lumbar Interbody Fusion without General Anesthesia: Operative and Clinical Outcomes in 100 Consecutive Patients with a Minimum 1-Year Follow-Up. Neurosurg. Focus. 2019, 46, E14. [Google Scholar] [CrossRef]
- Liounakos, J.I.; Urakov, T.; Wang, M.Y. Head-up Display Assisted Endoscopic Lumbar Discectomy—A Technical Note. Robot. Comput. Surg. 2020, 16, e2089. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chen, R.E.; Han, P.K.; Si, P.; Freeman, W.D.; Pirris, S.M. Technical Feasibility and Safety of an Intraoperative Head-up Display Device during Spine Instrumentation. Robot. Comput. Surg. 2017, 13, e1770. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.; Singh, S. An Introduction to Flexible Endoscopy. Surgery 2011, 29, 80–84. [Google Scholar] [CrossRef]
- Rahman, R.; Wood, M.E.; Qian, L.; Price, C.L.; Johnson, A.A.; Osgood, G.M. Head-Mounted Display Use in Surgery: A Systematic Review. Surg. Innov. 2020, 27, 88–100. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, K.; Turlip, R.; Ahmad, H.S.; Ghenbot, Y.G.; Chauhan, D.; Yoon, J.W. Virtual and Augmented Reality in Spine Surgery: A Systematic Review. World Neurosurg. 2023, 173, 96–107. [Google Scholar] [CrossRef]
- Farhat, H.I.; Hood, B.; Vanni, S.; Levi, A.D. Minimally Invasive Repair of Spontaneous Intracranial Hypotension: Report of 4 Cases. J. Neurosurg. 2011, 114, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Jitpakdee, K.; Liu, Y.; Heo, D.H.; Kotheeranurak, V.; Suvithayasiri, S.; Kim, J.-S. Minimally Invasive Endoscopy in Spine Surgery: Where Are We Now? Eur. Spine J. 2023, 32, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Gunjotikar, S.; Pestonji, M.; Tanaka, M.; Komatsubara, T.; Ekade, S.J.; Heydar, A.M.; Hieu, H.K. Evolution, Current Trends, and Latest Advances of Endoscopic Spine Surgery. J. Clin. Med. 2024, 13, 3208. [Google Scholar] [CrossRef]
- Mastorakos, P.; Pomeraniec, I.J.; Bryant, J.-P.; Chittiboina, P.; Heiss, J.D. Flexible Thecoscopy for Extensive Spinal Arachnoiditis. J. Neurosurg. Spine 2022, 36, 325–335. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Sun, Z.; Guo, Y.; Wang, G.; Wang, J.J. Ultrafine Flexible Endoscope Visualization to Assist in the Removal of a Huge Spinal Extradural Arachnoid Cyst: Case Report and Literature Review. World Neurosurg. 2022, 159, 130–133. [Google Scholar] [CrossRef]
- Ren, Y.-C.; Zhao, B.-J.; Xie, Z.-Y.; Ying, G.-Y.; Shen, F.; Zhu, Y.-J. Flexible Endoscope Visualization to Assist in the Removal of a String of 10 Schwannomas at the Cauda Equina: Technical Case Report. J. Neurosurg. Spine 2020, 33, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Luchtmann, M.; Klammer, A.; Iova, M.-A.; Roth, A.; Chanamolu, V.K.; Mawrin, C.; Warnke, J.-P. Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection. Neurol. Int. 2024, 16, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.C.H.; Vaughan, K.A.; Koeck, H. Endoscopic-Assisted Spinal Arachnoiditis Adhesiolysis and Placement of a Spinal Cysto-Subarachnoid Shunt. World Neurosurg. 2019, 131, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Torres-Corzo, J.G.; Islas-Aguilar, M.A.; Cervantes, D.S.; Chalita-Williams, J.C. The Role of Flexible Neuroendoscopy in Spinal Neurocysticercosis: Technical Note and Report of 3 Cases. World Neurosurg. 2019, 130, 77–83. [Google Scholar] [CrossRef]
- Tobita, T.; Okamoto, M.; Tomita, M.; Yamakura, T.; Fujihara, H.; Baba, H.; Uchiyama, S.; Hamann, W.; Shimoji, K. Diagnosis of Spinal Disease with Ultrafine Flexible Fiberscopes in Patients with Chronic Pain. Spine 2003, 28, 2006–2012. [Google Scholar] [CrossRef]
- Nicolau, S.; Soler, L.; Mutter, D.; Marescaux, J. Augmented Reality in Laparoscopic Surgical Oncology. Surg. Oncol. 2011, 20, 189–201. [Google Scholar] [CrossRef]
- Shah, S.Z.; Rehman, S.T.; Khan, A.; Hussain, M.M.; Ali, M.; Sarwar, S.; Abid, S. Ergonomics of Gastrointestinal Endoscopies: Musculoskeletal Injury among Endoscopy Physicians, Nurses, and Technicians. World J. Gastrointest. Endosc. 2022, 14, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, Y.; Yao, S.; Sun, G.; Xu, B.; Chen, X. A Low-Cost Multimodal Head-Mounted Display System for Neuroendoscopic Surgery. Brain Behav. 2018, 8, e00891. [Google Scholar] [CrossRef]
- McAfee, P.C.; Phillips, F.M.; Andersson, G.; Buvenenadran, A.; Kim, C.W.; Lauryssen, C.; Isaacs, R.E.; Youssef, J.A.; Brodke, D.S.; Cappuccino, A.; et al. Minimally Invasive Spine Surgery. Spine 2010, 35, S271–S273. [Google Scholar] [CrossRef]
- Stucky, C.-C.H.; Cromwell, K.D.; Voss, R.K.; Chiang, Y.-J.; Woodman, K.; Lee, J.E.; Cormier, J.N. Surgeon Symptoms, Strain, and Selections: Systematic Review and Meta-Analysis of Surgical Ergonomics. Ann. Med. Surg. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Cardenas-Trowers, O.; Kjellsson, K.; Hatch, K. Ergonomics: Making the OR a Comfortable Place. Int. Urogynecol. J. 2018, 29, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Day, J.D.; Albuquerque, F.; Schumaker, G.; Giannotta, S.L.; McComb, J.G. Heads-up Intraoperative Endoscopic Imaging: A Prospective Evaluation of Techniques and Limitations. Neurosurgery 1997, 40, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Van Lindert, E.J.; Grotenhuis, J.A.; Beems, T. The Use of a Head-Mounted Display for Visualization in Neuroendoscopy. Comput. Aided Surg. 2004, 9, 251–256. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalafallah, A.M.; Sanghera, B.S.; Kader, M.; Boddu, J.V.; Urakov, T. Minimally Invasive Approaches to Spinal Cerebrospinal Fluid Leak Repair: Current Strategies and a Novel Technique. J. Pers. Med. 2024, 14, 1090. https://doi.org/10.3390/jpm14111090
Khalafallah AM, Sanghera BS, Kader M, Boddu JV, Urakov T. Minimally Invasive Approaches to Spinal Cerebrospinal Fluid Leak Repair: Current Strategies and a Novel Technique. Journal of Personalized Medicine. 2024; 14(11):1090. https://doi.org/10.3390/jpm14111090
Chicago/Turabian StyleKhalafallah, Adham M., Bhavjeet S. Sanghera, Michael Kader, James V. Boddu, and Timur Urakov. 2024. "Minimally Invasive Approaches to Spinal Cerebrospinal Fluid Leak Repair: Current Strategies and a Novel Technique" Journal of Personalized Medicine 14, no. 11: 1090. https://doi.org/10.3390/jpm14111090
APA StyleKhalafallah, A. M., Sanghera, B. S., Kader, M., Boddu, J. V., & Urakov, T. (2024). Minimally Invasive Approaches to Spinal Cerebrospinal Fluid Leak Repair: Current Strategies and a Novel Technique. Journal of Personalized Medicine, 14(11), 1090. https://doi.org/10.3390/jpm14111090





