Immunotherapy Applications for Thymine Dimers and WT1 Antigen in Renal Cancers: A Comparative Statistical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conceptual Design and Study Cohort
2.2. Procedures and Definitions
- WT1 proteins—ready-to-use, monoclonal, N-terminus targeted, clone WT49 from Leica Biosystem, Newcastle Ltd., UK. Requires predilution at room temperature (30 min). Incubation time: 20 min [34];
- Nuclear TDs—monoclonal anti-human TD mouse antibody, clone KTM53 from Kamiya Biomedical Company, Seattle, WA, USA. Dilution 1:10,000. Incubation time: 30 min [35].
2.3. Statistical Analysis
3. Results
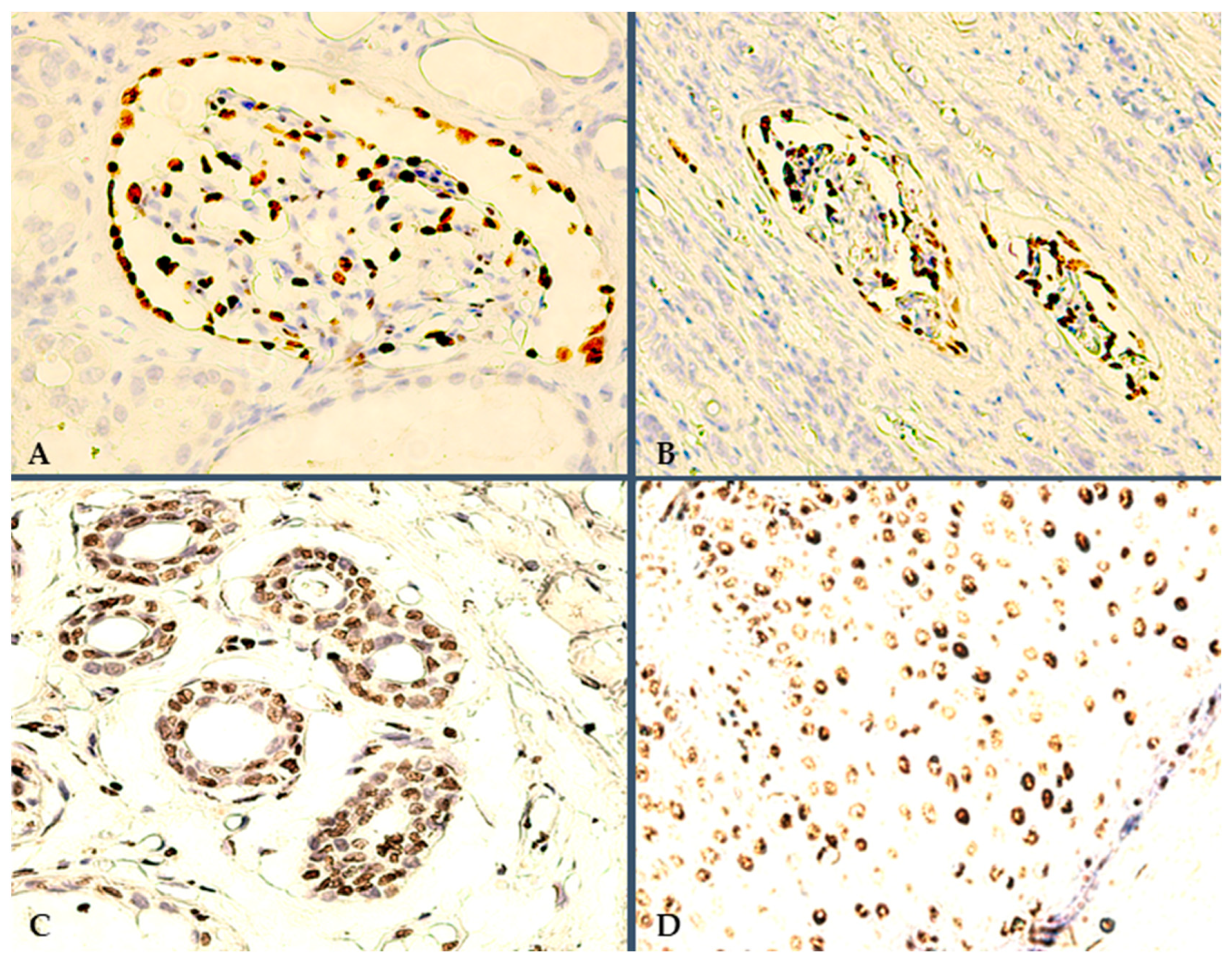
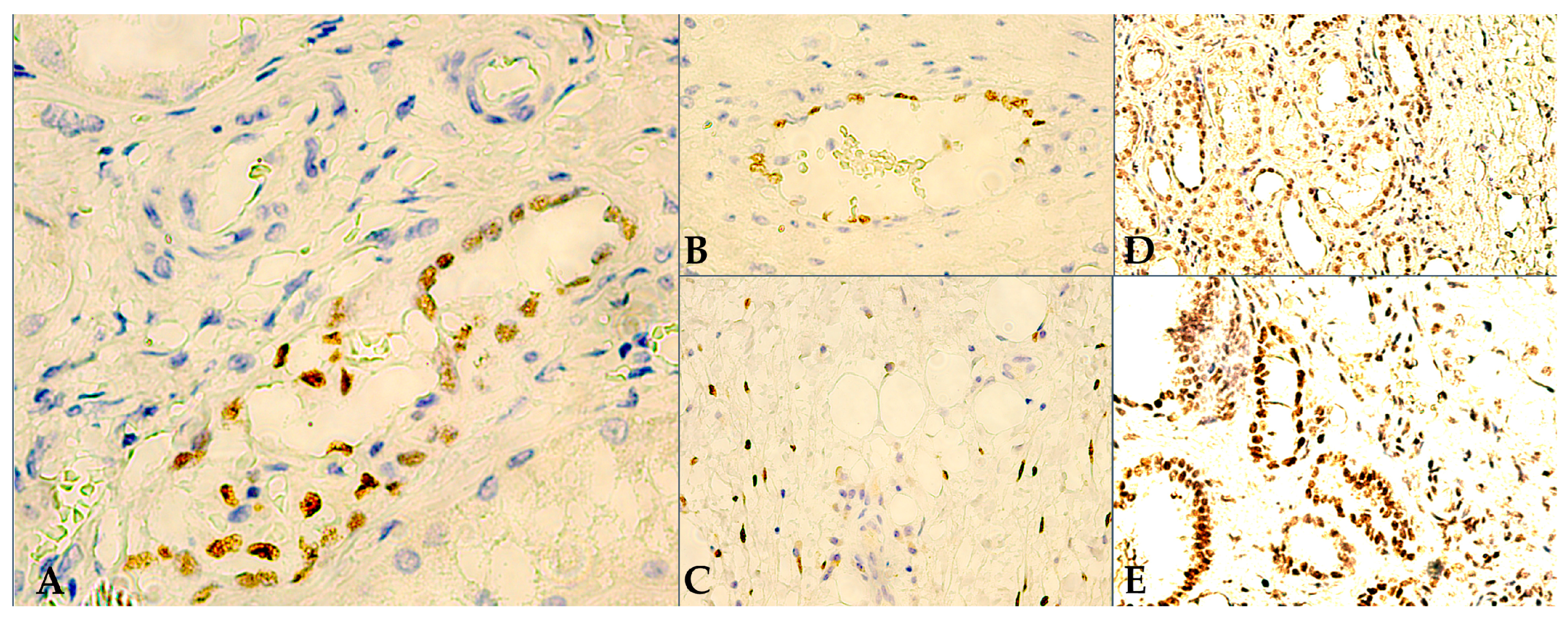
3.1. Microscopy and Immunoexpression Patterns
3.2. Data Processing and Interpretation of IHC Results
3.2.1. Background Analysis
3.2.2. Clinical and IHC Findings
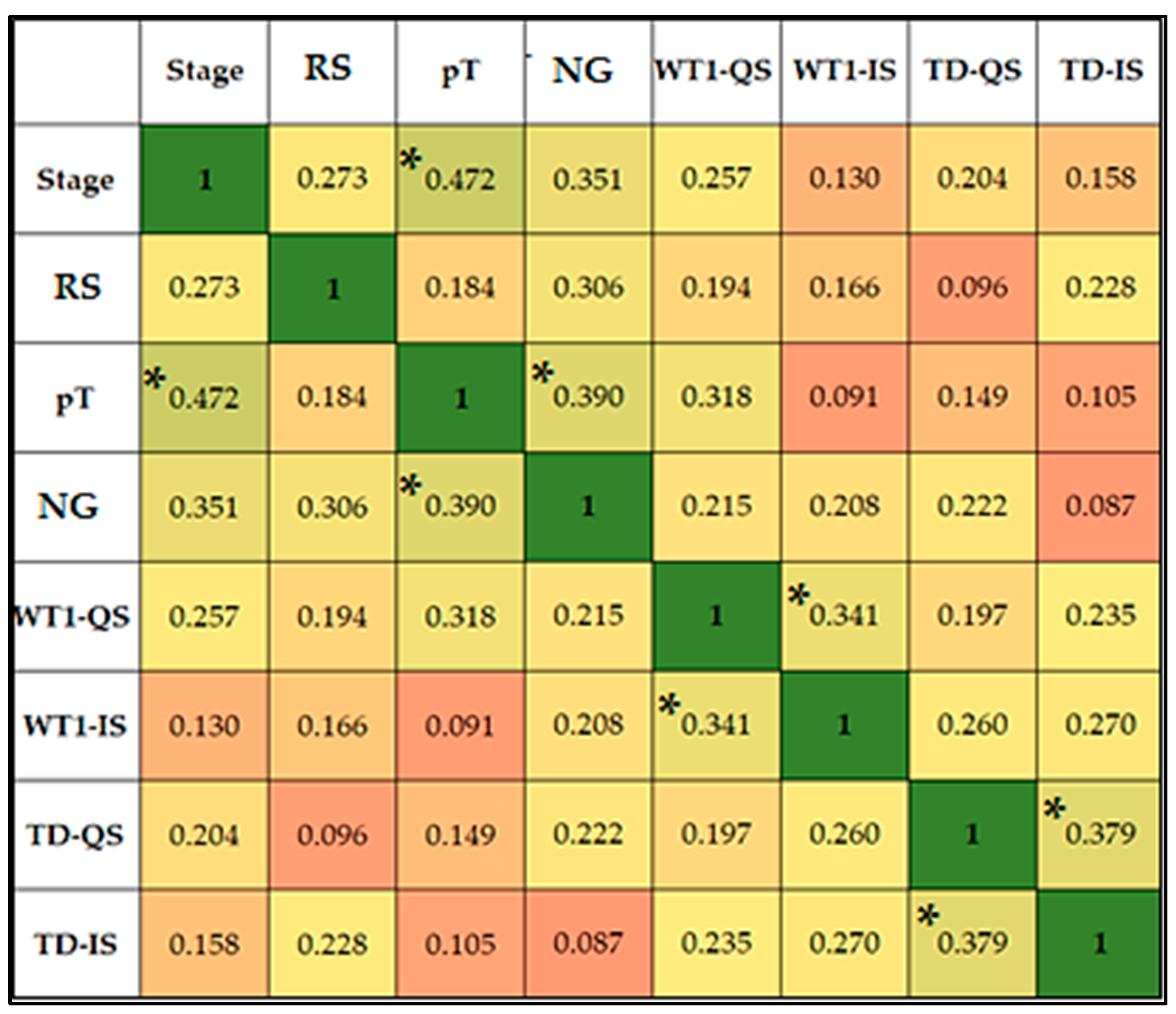
3.2.3. Statistical Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Decastro, G.J.; McKiernan, J.M. Epidemiology, Clinical Staging, and Presentation of Renal Cell Carcinoma. Urol. Clin. N. Am. 2008, 35, 581–592. [Google Scholar] [CrossRef]
- Kane, C.J.; Mallin, K.; Ritchey, J.; Cooperberg, M.R.; Carroll, P.R. Renal Cell Cancer Stage Migration: Analysis of the National Cancer Data Base. Cancer 2008, 113, 78–83. [Google Scholar] [CrossRef]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-Related Approach to Kidney Cancer Management: Moving Forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Reuter, V.E.; Argani, P.; Zhou, M.; Delahunt, B.; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best Practices Recommendations in the Application of Immunohistochemistry in the Kidney Tumors: Report from the International Society of Urologic Pathology Consensus Conference. Am. J. Surg. Pathol. 2014, 38, e35–e49. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Epstein, J.I.; Ulbright, T.M.; Humphrey, P.A.; Egevad, L.; Montironi, R.; Grignon, D.; Trpkov, K.; Lopez-Beltran, A.; Zhou, M.; et al. Best Practices Recommendations in the Application of Immunohistochemistry in Urologic Pathology: Report from the International Society of Urological Pathology Consensus Conference. Am. J. Surg. Pathol. 2014, 38, 1017–1022. [Google Scholar] [CrossRef]
- Novacescu, D.; Feciche, B.O.; Cumpanas, A.A.; Bardan, R.; Rusmir, A.V.; Bitar, Y.A.; Barbos, V.I.; Cut, T.G.; Raica, M.; Latcu, S.C. Contemporary Clinical Definitions, Differential Diagnosis, and Novel Predictive Tools for Renal Cell Carcinoma. Biomedicines 2022, 10, 2926. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Baderca, F.; Zăhoi, D.E.; Lighezan, R.; Izvernariu, D.; Raica, M. Clinical Significance of Her2/Neu Overexpression in Urothelial Carcinomas. Rom. J. Morphol. Embryol. 2010, 51, 277–282. [Google Scholar]
- Novacescu, D.; Latcu, S.; Bardan, R.; Daminescu, L.; Cumpanas, A. Contemporary Biomarkers for Renal Transplantation: A Narrative Overview. J. Pers. Med. 2023, 13, 1216. [Google Scholar] [CrossRef]
- Alexa, A.; Baderca, F.; Lighezan, R.; Izvernariu, D. Myofibroblasts Reaction in Urothelial Carcinomas. Rom. J. Morphol. Embryol. 2009, 50, 639–643. [Google Scholar]
- Cumpănas, A.A.; Cimpean, A.M.; Ferician, O.; Ceausu, R.A.; Sarb, S.; Barbos, V.; Dema, A.; Raica, M. The Involvement of PDGF-B/PDGFRβ Axis in the Resistance to Antiangiogenic and Antivascular Therapy in Renal Cancer. Anticancer Res. 2016, 36, 2291–2295. [Google Scholar] [PubMed]
- Radu-Cosnita, A.D.; Nesiu, A.; Berzava, P.L.; Cerbu, S.; Cosma, A.; Comsa, S.; Sarb, S.; Ferician, A.M.; Ferician, O.C.; Cimpean, A.M. Anti-Chloride Intracellular Channel Protein 1 (CLIC1) Antibodies Induce Tumour Necrosis and Angiogenesis Inhibition on In Vivo Experimental Models of Human Renal Cancer. Anticancer Res. 2022, 42, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Ferician, A.M.; Ferician, O.C.; Cumpanas, A.D.; Berzava, P.L.; Nesiu, A.; Barmayoun, A.; Cimpean, A.M. Heterogeneity of Platelet Derived Growth Factor Pathway Gene Expression Profile Defines Three Distinct Subgroups of Renal Cell Carcinomas. Cancer Genom. Proteom. 2022, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ferician, A.M.; Ferician, O.C.; Nesiu, A.; Cosma, A.A.; Caplar, B.D.; Melnic, E.; Cimpean, A.M. The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC). Cancers 2022, 14, 5981. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Joo, J.W.; Lee, S.J.; Cho, Y.A.; Park, C.K.; Cho, N.H. Comprehensive Immunoprofiles of Renal Cell Carcinoma Subtypes. Cancers 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Roma, A.; Magi-Galluzzi, C. The Usefulness of Immunohistochemical Markers in the Differential Diagnosis of Renal Neoplasms. Clin. Lab. Med. 2005, 25, 247–257. [Google Scholar] [CrossRef]
- Skinnider, B.F.; Amin, M.B. An Immunohistochemical Approach to the Differential Diagnosis of Renal Tumors. Semin. Diagn. Pathol. 2005, 22, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Cheng, L.; Rioux-Leclercq, N.; Merino, M.J.; Netto, G.; Reuter, V.E.; Shen, S.S.; Grignon, D.J.; Montironi, R.; Egevad, L.; et al. Renal Tumors: Diagnostic and Prognostic Biomarkers. Am. J. Surg. Pathol. 2013, 37, 1518–1531. [Google Scholar] [CrossRef]
- Heidegger, I.; Pircher, A.; Pichler, R. Targeting the Tumor Microenvironment in Renal Cell Cancer Biology and Therapy. Front. Oncol. 2019, 9, 490. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. A Phase 3, Randomized, Open-Label Study of Nivolumab Combined with Ipilimumab Versus Sunitinib Monotherapy in Subjects with Previously Untreated, Advanced or Metastatic Renal Cell Carcinoma; Clinical trial registration NCT02231749; Clinicaltrials.gov: Bethesda, MD, USA, 2022.
- Fan, D.; Liu, Q.; Wu, F.; Liu, N.; Qu, H.; Yuan, Y.; Li, Y.; Gao, H.; Ge, J.; Xu, Y.; et al. Prognostic Significance of PI3K/AKT/MTOR Signaling Pathway Members in Clear Cell Renal Cell Carcinoma. PeerJ 2020, 8, e9261. [Google Scholar] [CrossRef]
- Sabatino, M.; Kim-Schulze, S.; Panelli, M.C.; Stroncek, D.; Wang, E.; Taback, B.; Kim, D.W.; Deraffele, G.; Pos, Z.; Marincola, F.M.; et al. Serum Vascular Endothelial Growth Factor and Fibronectin Predict Clinical Response to High-Dose Interleukin-2 Therapy. J. Clin. Oncol. 2009, 27, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, G.; Gentil-Perret, A.; Genin, C.; Tostain, J. Serum Carbonic Anhydrase 9 Level Is Associated with Postoperative Recurrence of Conventional Renal Cell Cancer. J. Urol. 2008, 180, 510–513, discussion 513–514. [Google Scholar] [CrossRef]
- Sim, S.H.; Messenger, M.P.; Gregory, W.M.; Wind, T.C.; Vasudev, N.S.; Cartledge, J.; Thompson, D.; Selby, P.J.; Banks, R.E. Prognostic Utility of Pre-Operative Circulating Osteopontin, Carbonic Anhydrase IX and CRP in Renal Cell Carcinoma. Br. J. Cancer 2012, 107, 1131–1137. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Pal, S.K.; McDermott, D.F.; Morrissey, S.; Ferguson, K.C.; Holland, J.; Kaelin, W.G.; Dutcher, J.P. A Phase I Study of Cabozantinib (XL184) in Patients with Renal Cell Cancer. Ann. Oncol. 2014, 25, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, A.; Sepe, P.; Zattarin, E.; Mennitto, A.; Stellato, M.; Claps, M.; Guadalupi, V.; Verzoni, E.; de Braud, F.; Procopio, G. Predictive Biomarkers of Response to Immunotherapy in Metastatic Renal Cell Cancer. Front. Oncol. 2020, 10, 1644. [Google Scholar] [CrossRef]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic Factors and Prognostic Models for Renal Cell Carcinoma: A Literature Review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Vaziri, S.A.J.; Jaeger, E.; Elson, P.; Wood, L.; Bhalla, I.P.; Small, E.J.; Weinberg, V.; Sein, N.; Simko, J.; et al. Von Hippel-Lindau Gene Status and Response to Vascular Endothelial Growth Factor Targeted Therapy for Metastatic Clear Cell Renal Cell Carcinoma. J. Urol. 2008, 180, 860–865, discussion 865–866. [Google Scholar] [CrossRef]
- Cho, D.; Signoretti, S.; Dabora, S.; Regan, M.; Seeley, A.; Mariotti, M.; Youmans, A.; Polivy, A.; Mandato, L.; McDermott, D.; et al. Potential Histologic and Molecular Predictors of Response to Temsirolimus in Patients with Advanced Renal Cell Carcinoma. Clin. Genitourin. Cancer 2007, 5, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lidgren, A.; Hedberg, Y.; Grankvist, K.; Rasmuson, T.; Bergh, A.; Ljungberg, B. Hypoxia-Inducible Factor 1α Expression in Renal Cell Carcinoma Analyzed by Tissue Microarray. Eur. Urol. 2006, 50, 1272–1277. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; Riggs, S.B.; Leppert, J.T.; Berkman, M.K.; Kleid, M.D.; Yu, H.; Kabbinavar, F.F.; Pantuck, A.J.; Belldegrun, A.S. Hypoxia-Inducible Factor 1 Alpha in Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 7388–7393. [Google Scholar] [CrossRef]
- Rini, B.I.; Michaelson, M.D.; Rosenberg, J.E.; Bukowski, R.M.; Sosman, J.A.; Stadler, W.M.; Hutson, T.E.; Margolin, K.; Harmon, C.S.; DePrimo, S.E.; et al. Antitumor Activity and Biomarker Analysis of Sunitinib in Patients with Bevacizumab-Refractory Metastatic Renal Cell Carcinoma. JCO 2008, 26, 3743–3748. [Google Scholar] [CrossRef]
- Novacescu, D.; Cut, T.G.; Cumpanas, A.A.; Latcu, S.C.; Bardan, R.; Ferician, O.; Secasan, C.-C.; Rusmir, A.; Raica, M. Evaluating Established Roles, Future Perspectives and Methodological Heterogeneity for Wilms’ Tumor 1 (WT1) Antigen Detection in Adult Renal Cell Carcinoma, Using a Novel N-Terminus Targeted Antibody (Clone WT49). Biomedicines 2022, 10, 912. [Google Scholar] [CrossRef]
- Novacescu, D.; Cut, T.G.; Cumpanas, A.A.; Bratosin, F.; Ceausu, R.A.; Raica, M. Novel Expression of Thymine Dimers in Renal Cell Carcinoma, Demonstrated through Immunohistochemistry. Biomedicines 2022, 10, 2673. [Google Scholar] [CrossRef]
- Call, K.M.; Glaser, T.; Ito, C.Y.; Buckler, A.J.; Pelletier, J.; Haber, D.A.; Rose, E.A.; Kral, A.; Yeger, H.; Lewis, W.H. Isolation and Characterization of a Zinc Finger Polypeptide Gene at the Human Chromosome 11 Wilms’ Tumor Locus. Cell 1990, 60, 509–520. [Google Scholar] [CrossRef]
- Gessler, M.; Poustka, A.; Cavenee, W.; Neve, R.L.; Orkin, S.H.; Bruns, G.A. Homozygous Deletion in Wilms Tumours of a Zinc-Finger Gene Identified by Chromosome Jumping. Nature 1990, 343, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.; Pritchard-Jones, K. WT1 Mutation in Childhood Cancer. Methods Mol. Biol. 2016, 1467, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salvatorelli, L.; Parenti, R.; Leone, G.; Musumeci, G.; Vasquez, E.; Magro, G. Wilms Tumor 1 (WT1) Protein: Diagnostic Utility in Pediatric Tumors. Acta Histochem. 2015, 117, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hastie, N.D. Wilms’ Tumour 1 (WT1) in Development, Homeostasis and Disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef]
- Discenza, M.T.; Pelletier, J. Insights into the Physiological Role of WT1 from Studies of Genetically Modified Mice. Physiol. Genom. 2004, 16, 287–300. [Google Scholar] [CrossRef]
- Hohenstein, P.; Hastie, N.D. The Many Facets of the Wilms’ Tumour Gene, WT1. Hum. Mol. Genet. 2006, 15, R196–R201. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.G.E. Transcriptional Regulation by WT1 in Development. Curr. Opin. Genet. Dev. 2005, 15, 542–547. [Google Scholar] [CrossRef]
- Chau, Y.-Y.; Hastie, N.D. The Role of Wt1 in Regulating Mesenchyme in Cancer, Development, and Tissue Homeostasis. Trends Genet. 2012, 28, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Parenti, R.; Puzzo, L.; Vecchio, G.M.; Gravina, L.; Salvatorelli, L.; Musumeci, G.; Vasquez, E.; Magro, G. Immunolocalization of Wilms’ Tumor Protein (WT1) in Developing Human Peripheral Sympathetic and Gastroenteric Nervous System. Acta Histochem. 2014, 116, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Huff, V. Wilms’ Tumours: About Tumour Suppressor Genes, an Oncogene and a Chameleon Gene. Nat. Rev. Cancer 2011, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Vicent, S.; Chen, R.; Sayles, L.C.; Lin, C.; Walker, R.G.; Gillespie, A.K.; Subramanian, A.; Hinkle, G.; Yang, X.; Saif, S.; et al. Wilms Tumor 1 (WT1) Regulates KRAS-Driven Oncogenesis and Senescence in Mouse and Human Models. J. Clin. Investig. 2010, 120, 3940–3952. [Google Scholar] [CrossRef]
- Sugiyama, H. WT1 (Wilms’ Tumor Gene 1): Biology and Cancer Immunotherapy. Jpn. J. Clin. Oncol. 2010, 40, 377–387. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Suarez Saiz, F.; Saurez Saiz, F.; Minden, M.D. A Tumor Suppressor and Oncogene: The WT1 Story. Leukemia 2007, 21, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S. The Molecular Perspective: Ultraviolet Light and Pyrimidine Dimers. Oncologist 2001, 6, 298–299. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D. DNA Repair and Mutagenesis, 2nd ed.; ASM Press: Washington, DC, USA, 2006; pp. 109–110. [Google Scholar]
- Ramasamy, K.; Shanmugam, M.; Balupillai, A.; Govindhasamy, K.; Gunaseelan, S.; Muthusamy, G.; Robert, B.M.; Nagarajan, R.P. Ultraviolet Radiation-Induced Carcinogenesis: Mechanisms and Experimental Models. J. Radiat. Cancer Res. 2017, 8, 4. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA Damage-Induced Cell Death: From Specific DNA Lesions to the DNA Damage Response and Apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Mallet, J.D.; Dorr, M.M.; Drigeard Desgarnier, M.-C.; Bastien, N.; Gendron, S.P.; Rochette, P.J. Faster DNA Repair of Ultraviolet-Induced Cyclobutane Pyrimidine Dimers and Lower Sensitivity to Apoptosis in Human Corneal Epithelial Cells than in Epidermal Keratinocytes. PLoS ONE 2016, 11, e0162212. [Google Scholar] [CrossRef]
- Brenner, M.; Degitz, K.; Besch, R.; Berking, C. Differential Expression of Melanoma-Associated Growth Factors in Keratinocytes and Fibroblasts by Ultraviolet A and Ultraviolet B Radiation. Br. J. Dermatol. 2005, 153, 733–739. [Google Scholar] [CrossRef]
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet Radiation and Cutaneous Malignant Melanoma. Oncogene 2003, 22, 3099–3112. [Google Scholar] [CrossRef]
- Kanjilal, S.; Pierceall, W.E.; Cummings, K.K.; Kripke, M.L.; Ananthaswamy, H.N. High Frequency of P53 Mutations in Ultraviolet Radiation-Induced Murine Skin Tumors: Evidence for Strand Bias and Tumor Heterogeneity. Cancer Res. 1993, 53, 2961–2964. [Google Scholar]
- van Kranen, H.J.; de Gruijl, F.R.; de Vries, A.; Sontag, Y.; Wester, P.W.; Senden, H.C.; Rozemuller, E.; van Kreijl, C.F. Frequent P53 Alterations but Low Incidence of Ras Mutations in UV-B-Induced Skin Tumors of Hairless Mice. Carcinogenesis 1995, 16, 1141–1147. [Google Scholar] [CrossRef]
- You, Y.H.; Szabó, P.E.; Pfeifer, G.P. Cyclobutane Pyrimidine Dimers Form Preferentially at the Major P53 Mutational Hotspot in UVB-Induced Mouse Skin Tumors. Carcinogenesis 2000, 21, 2113–2117. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. Does GammaH2AX Foci Formation Depend on the Presence of DNA Double Strand Breaks? Cancer Lett. 2005, 229, 171–179. [Google Scholar] [CrossRef]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Delinasios, G.J.; Premi, S.; Young, A.R.; Cooke, M.S. Perspectives on Cyclobutane Pyrimidine Dimers—Rise of the Dark Dimers†. Photochem. Photobiol. 2022, 98, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gibson-D’Ambrosio, R.E.; Leong, Y.; D’Ambrosio, S.M. DNA Repair Following Ultraviolet and N-Ethyl-N-Nitrosourea Treatment of Cells Cultured from Human Fetal Brain, Intestine, Kidney, Liver, and Skin. Cancer Res. 1983, 43, 5846–5850. [Google Scholar]
- Beukers, R.; Eker, A.P.M.; Lohman, P.H.M. 50 Years Thymine Dimer. DNA Repair 2008, 7, 530–543. [Google Scholar] [CrossRef]
- Karami, S.; Boffetta, P.; Stewart, P.; Rothman, N.; Hunting, K.L.; Dosemeci, M.; Berndt, S.I.; Brennan, P.; Chow, W.-H.; Moore, L.E. Occupational Sunlight Exposure and Risk of Renal Cell Carcinoma. Cancer 2010, 116, 2001–2010. [Google Scholar] [CrossRef]
- Protić-Sabljić, M.; Tuteja, N.; Munson, P.J.; Hauser, J.; Kraemer, K.H.; Dixon, K. UV Light-Induced Cyclobutane Pyrimidine Dimers Are Mutagenic in Mammalian Cells. Mol. Cell. Biol. 1986, 6, 3349–3356. [Google Scholar] [PubMed]
- Clingen, P.H.; Arlett, C.F.; Cole, J.; Waugh, A.P.; Lowe, J.E.; Harcourt, S.A.; Hermanova, N.; Roza, L.; Mori, T.; Nikaido, O. Correlation of UVC and UVB Cytotoxicity with the Induction of Specific Photoproducts in T-Lymphocytes and Fibroblasts from Normal Human Donors. Photochem. Photobiol. 1995, 61, 163–170. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Theis, R.P.; Dolwick Grieb, S.M.; Burr, D.; Siddiqui, T.; Asal, N.R. Smoking, Environmental Tobacco Smoke, and Risk of Renal Cell Cancer: A Population-Based Case-Control Study. BMC Cancer 2008, 8, 387. [Google Scholar] [CrossRef]
- Tsivian, M.; Moreira, D.M.; Caso, J.R.; Mouraviev, V.; Polascik, T.J. Cigarette Smoking Is Associated with Advanced Renal Cell Carcinoma. JCO 2011, 29, 2027–2031. [Google Scholar] [CrossRef]
- Ho, W.J.; Simon, M.S.; Yildiz, V.O.; Shikany, J.M.; Kato, I.; Beebe-Dimmer, J.L.; Cetnar, J.P.; Bock, C.H. Antioxidant Micronutrients and the Risk of Renal Cell Carcinoma in the Women’s Health Initiative Cohort. Cancer 2015, 121, 580–588. [Google Scholar] [CrossRef]
- Do ‘Dark’ DNA Dimers Drive Disease and Create Targets for Immunotherapy for People with Renal Cell Carcinoma? ASCO Daily News. Available online: https://dailynews.ascopubs.org/do/10.1200/ADN.23.201300/full (accessed on 7 May 2023).
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E Inhibits the UVAI Induction of “Light” and “Dark” Cyclobutane Pyrimidine Dimers, and Oxidatively Generated DNA Damage, in Keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Wang, Y.; Lebwohl, M. Inhibition of Ultraviolet Light-Induced Oxidative Events in the Skin and Internal Organs of Hairless Mice by Isoflavone Genistein. Cancer Lett. 2002, 185, 21–29. [Google Scholar] [CrossRef]
- Chan, C.-M.; Huang, C.-H.; Li, H.-J.; Hsiao, C.-Y.; Su, C.-C.; Lee, P.-L.; Hung, C.-F. Protective Effects of Resveratrol against UVA-Induced Damage in ARPE19 Cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef]
- Sherwani, M.A.; Ahmad, I.; Lewis, M.J.; Abdelgawad, A.; Rashid, H.; Yang, K.; Chen, C.-Y.; Raman, C.; Elmets, C.A.; Yusuf, N. Type I Interferons Enhance the Repair of Ultraviolet Radiation-Induced DNA Damage and Regulate Cutaneous Immune Suppression. Int. J. Mol. Sci. 2022, 23, 1822. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Block, K. Renal Carcinogenesis, Tumor Heterogeneity, and Reactive Oxygen Species: Tactics Evolved. Antioxid. Redox Signal. 2016, 25, 685–701. [Google Scholar] [CrossRef]
- Courtney, K.D.; Bezwada, D.; Mashimo, T.; Pichumani, K.; Vemireddy, V.; Funk, A.M.; Wimberly, J.; McNeil, S.S.; Kapur, P.; Lotan, Y.; et al. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell Metab. 2018, 28, 793–800. [Google Scholar] [CrossRef]
- Hervouet, E.; Simonnet, H.; Godinot, C. Mitochondria and Reactive Oxygen Species in Renal Cancer. Biochimie 2007, 89, 1080–1088. [Google Scholar] [CrossRef]
- Wagner, K.-D.; Wagner, N.; Wellmann, S.; Schley, G.; Bondke, A.; Theres, H.; Scholz, H. Oxygen-Regulated Expression of the Wilms’ Tumor Suppressor Wt1 Involves Hypoxia-Inducible Factor-1 (HIF-1). FASEB J. 2003, 17, 1364–1366. [Google Scholar] [CrossRef]
- Wagner, K.-D.; Wagner, N.; Bondke, A.; Nafz, B.; Flemming, B.; Theres, H.; Scholz, H. The Wilms’ Tumor Suppressor Wt1 Is Expressed in the Coronary Vasculature after Myocardial Infarction. FASEB J. 2002, 16, 1117–1119. [Google Scholar] [CrossRef]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De Novo Cardiomyocytes from within the Activated Adult Heart after Injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef]
- Mier, J.W. The Tumor Microenvironment in Renal Cell Cancer. Curr. Opin. Oncol. 2019, 31, 194–199. [Google Scholar] [CrossRef]
- Cano, E.; Carmona, R.; Ruiz-Villalba, A.; Rojas, A.; Chau, Y.-Y.; Wagner, K.D.; Wagner, N.; Hastie, N.D.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. Extracardiac Septum Transversum/Proepicardial Endothelial Cells Pattern Embryonic Coronary Arterio–Venous Connections. Proc. Natl. Acad. Sci. USA 2016, 113, 656–661. [Google Scholar] [CrossRef]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 Is Required for Early Kidney Development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 Is Required for Cardiovascular Progenitor Cell Formation through Transcriptional Control of Snail and E-Cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Ota, S. Vaccination of Urological Cancer Patients with WT1 Peptide-Pulsed Dendritic Cells in Combination with Molecular Targeted Therapy or Conventional Chemotherapy Induces Immunological and Clinical Responses. Ther. Apher. Dial. 2018, 22, 266–277. [Google Scholar] [CrossRef]
- Kashima, S.; Maeda, T.; Masuda, K.; Nagano, S.; Inoue, T.; Takeda, M.; Kono, Y.; Kobayashi, T.; Saito, S.; Higuchi, T.; et al. Cytotoxic T Lymphocytes Regenerated from IPS Cells Have Therapeutic Efficacy in a Patient-Derived Xenograft Solid Tumor Model. iScience 2020, 23, 100998. [Google Scholar] [CrossRef]
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; Bakir, M.A.; et al. Insertion-and-Deletion-Derived Tumour-Specific Neoantigens and the Immunogenic Phenotype: A Pan-Cancer Analysis. Lancet Oncol. 2017, 18, 1009–1021. [Google Scholar] [CrossRef]
- Hansen, U.K.; Ramskov, S.; Bjerregaard, A.-M.; Borch, A.; Andersen, R.; Draghi, A.; Donia, M.; Bentzen, A.K.; Marquard, A.M.; Szallasi, Z.; et al. Tumor-Infiltrating T Cells From Clear Cell Renal Cell Carcinoma Patients Recognize Neoepitopes Derived From Point and Frameshift Mutations. Front. Immunol. 2020, 11, 373. [Google Scholar] [CrossRef]
- Chae, Y.K.; Viveiros, P.; Lopes, G.; Sukhadia, B.; Sheikh, M.M.; Saravia, D.; Florou, V.; Sokol, E.S.; Frampton, G.M.; Chalmers, Z.R.; et al. Clinical and Immunological Implications of Frameshift Mutations in Lung Cancer. J. Thorac. Oncol. 2019, 14, 1807–1817. [Google Scholar] [CrossRef]
- Sena, L.A.; Fountain, J.; Isaacsson Velho, P.; Lim, S.J.; Wang, H.; Nizialek, E.; Rathi, N.; Nussenzveig, R.; Maughan, B.L.; Velez, M.G.; et al. Tumor Frameshift Mutation Proportion Predicts Response to Immunotherapy in Mismatch Repair-Deficient Prostate Cancer. Oncologist 2021, 26, e270–e278. [Google Scholar] [CrossRef]
- Kanemura, H.; Hayashi, H.; Tomida, S.; Tanizaki, J.; Suzuki, S.; Kawanaka, Y.; Tsuya, A.; Fukuda, Y.; Kaneda, H.; Kudo, K.; et al. The Tumor Immune Microenvironment and Frameshift Neoantigen Load Determine Response to PD-L1 Blockade in Extensive-Stage SCLC. JTO Clin. Res. Rep. 2022, 3, 10037. [Google Scholar] [CrossRef]
- Iiyama, T.; Udaka, K.; Takeda, S.; Takeuchi, T.; Adachi, Y.C.; Ohtsuki, Y.; Tsuboi, A.; Nakatsuka, S.; Elisseeva, O.A.; Oji, Y.; et al. WT1 (Wilms’ Tumor 1) Peptide Immunotherapy for Renal Cell Carcinoma. Microbiol. Immunol. 2007, 51, 519–530. [Google Scholar] [CrossRef]
- Gao, L.; Bellantuono, I.; Elsässer, A.; Marley, S.B.; Gordon, M.Y.; Goldman, J.M.; Stauss, H.J. Selective Elimination of Leukemic CD34(+) Progenitor Cells by Cytotoxic T Lymphocytes Specific for WT1. Blood 2000, 95, 2198–2203. [Google Scholar] [CrossRef]
- Mailänder, V.; Scheibenbogen, C.; Thiel, E.; Letsch, A.; Blau, I.W.; Keilholz, U. Complete Remission in a Patient with Recurrent Acute Myeloid Leukemia Induced by Vaccination with WT1 Peptide in the Absence of Hematological or Renal Toxicity. Leukemia 2004, 18, 165–166. [Google Scholar] [CrossRef]
- Ohminami, H.; Yasukawa, M.; Fujita, S. HLA Class I-Restricted Lysis of Leukemia Cells by a CD8(+) Cytotoxic T-Lymphocyte Clone Specific for WT1 Peptide. Blood 2000, 95, 286–293. [Google Scholar] [CrossRef]
- Oka, Y.; Elisseeva, O.A.; Tsuboi, A.; Ogawa, H.; Tamaki, H.; Li, H.; Oji, Y.; Kim, E.H.; Soma, T.; Asada, M.; et al. Human Cytotoxic T-Lymphocyte Responses Specific for Peptides of the Wild-Type Wilms’ Tumor Gene (WT1) Product. Immunogenetics 2000, 51, 99–107. [Google Scholar] [CrossRef]
- Oka, Y.; Udaka, K.; Tsuboi, A.; Elisseeva, O.A.; Ogawa, H.; Aozasa, K.; Kishimoto, T.; Sugiyama, H. Cancer Immunotherapy Targeting Wilms’ Tumor Gene WT1 Product. J. Immunol. 2000, 164, 1873–1880. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Letsch, A.; Thiel, E.; Schmittel, A.; Mailaender, V.; Baerwolf, S.; Nagorsen, D.; Keilholz, U. CD8 T-Cell Responses to Wilms Tumor Gene Product WT1 and Proteinase 3 in Patients with Acute Myeloid Leukemia. Blood 2002, 100, 2132–2137. [Google Scholar] [CrossRef]
- Tsuboi, A.; Oka, Y.; Ogawa, H.; Elisseeva, O.A.; Li, H.; Kawasaki, K.; Aozasa, K.; Kishimoto, T.; Udaka, K.; Sugiyama, H. Cytotoxic T-Lymphocyte Responses Elicited to Wilms’ Tumor Gene WT1 Product by DNA Vaccination. J. Clin. Immunol. 2000, 20, 195–202. [Google Scholar] [CrossRef]
- Gillmore, R.; Xue, S.-A.; Holler, A.; Kaeda, J.; Hadjiminas, D.; Healy, V.; Dina, R.; Parry, S.C.; Bellantuono, I.; Ghani, Y.; et al. Detection of Wilms’ Tumor Antigen–Specific CTL in Tumor-Draining Lymph Nodes of Patients with Early Breast Cancer. Clin. Cancer Res. 2006, 12, 34–42. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Matsui, M.S.; Mukhtar, H. Kinetics of UV Light-Induced Cyclobutane Pyrimidine Dimers in Human Skin in Vivo: An Immunohistochemical Analysis of Both Epidermis and Dermis. Photochem. Photobiol. 2000, 72, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Bertona, M.; Altabas, K.; Altabas, V.; Emanuele, E. Reduced Ultraviolet-Induced DNA Damage and Apoptosis in Human Skin with Topical Application of a Photolyase-Containing DNA Repair Enzyme Cream: Clues to Skin Cancer Prevention. Mol. Med. Rep. 2012, 5, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Adar, S.; Selby, C.P.; Lieb, J.D.; Sancar, A. Genome-Wide Analysis of Human Global and Transcription-Coupled Excision Repair of UV Damage at Single-Nucleotide Resolution. Genes Dev. 2015, 29, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S.; Koji, T.; Taguchi, T.; Harada, T.; Nakane, P.K. In Situ Localization of Type III and Type IV Collagen-Expressing Cells in Human Diabetic Nephropathy. J. Pathol. 1994, 174, 131–138. [Google Scholar] [CrossRef]
- Bisceglia, M.; Vairo, M.; Galliani, C.; Lastilla, G.; Parafioriti, A.; De Maglio, G. Immunohistochemical Investigation of WT1 Expression in 117 Embryonal Tumors. Pathologica 2011, 103, 182–183. [Google Scholar]
- Parenti, R.; Perris, R.; Vecchio, G.M.; Salvatorelli, L.; Torrisi, A.; Gravina, L.; Magro, G. Immunohistochemical Expression of Wilms’ Tumor Protein (WT1) in Developing Human Epithelial and Mesenchymal Tissues. Acta Histochem. 2013, 115, 70–75. [Google Scholar] [CrossRef]
- Parenti, R.; Cardile, V.; Graziano, A.C.E.; Parenti, C.; Venuti, A.; Bertuccio, M.P.; Furno, D.L.; Magro, G. Wilms’ Tumor Gene 1 (WT1) Silencing Inhibits Proliferation of Malignant Peripheral Nerve Sheath Tumor SNF96.2 Cell Line. PLoS ONE 2014, 9, e114333. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Salvatorelli, L.; Vecchio, G.M.; Musumeci, G.; Rita, A.; Parenti, R. Cytoplasmic Expression of Wilms Tumor Transcription Factor-1 (WT1): A Useful Immunomarker for Young-Type Fibromatoses and Infantile Fibrosarcoma. Acta Histochem. 2014, 116, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Longo, F.; Salvatorelli, L.; Vecchio, G.M.; Parenti, R. Wilms’ Tumor Protein (WT1) in Mammary Myofibroblastoma: An Immunohistochemical Study. Acta Histochem. 2014, 116, 905–910. [Google Scholar] [CrossRef]
- Magro, G.; Salvatorelli, L.; Puzzo, L.; Musumeci, G.; Bisceglia, M.; Parenti, R. Oncofetal Expression of Wilms’ Tumor 1 (WT1) Protein in Human Fetal, Adult and Neoplastic Skeletal Muscle Tissues. Acta Histochem. 2015, 117, 492–504. [Google Scholar] [CrossRef]
- Magro, G.; Longo, F.R.; Angelico, G.; Spadola, S.; Amore, F.F.; Salvatorelli, L. Immunohistochemistry as Potential Diagnostic Pitfall in the Most Common Solid Tumors of Children and Adolescents. Acta Histochem. 2015, 117, 397–414. [Google Scholar] [CrossRef]
- Niksic, M.; Slight, J.; Sanford, J.R.; Caceres, J.F.; Hastie, N.D. The Wilms’ Tumour Protein (WT1) Shuttles between Nucleus and Cytoplasm and Is Present in Functional Polysomes. Hum. Mol. Genet. 2004, 13, 463–471. [Google Scholar] [CrossRef]
- Reddy, J.C.; Morris, J.C.; Wang, J.; English, M.A.; Haber, D.A.; Shi, Y.; Licht, J.D. WT1-Mediated Transcriptional Activation Is Inhibited by Dominant Negative Mutant Proteins. J. Biol. Chem. 1995, 270, 10878–10884. [Google Scholar] [CrossRef]
- Moffett, P.; Bruening, W.; Nakagama, H.; Bardeesy, N.A.B.E.E.L.; Housman, D.; Housman, D.E.; Pelletier, J. Antagonism of WT1 Activity by Protein Self-Association. Proc. Natl. Acad. Sci. USA 1995, 92, 11105–11109. [Google Scholar] [CrossRef]
- Kennedy, D.; Ramsdale, T.; Mattick, J.; Little, M. An RNA Recognition Motif in Wilms’ Tumour Protein (WT1) Revealed by Structural Modelling. Nat. Genet. 1996, 12, 329–331. [Google Scholar] [CrossRef]

| Variables | HKTE(+) (n = 12) | HKTE(−) (n = 18) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 63.2 ± 7.5 | 67.2 ± 8.8 | 0.207 |
| Age range | 51–71 | 55–84 | - |
| Sex | 0.215 | ||
| Men | 6 (50.0%) | 13 (72.2%) | |
| Women | 6 (50.0%) | 5 (27.8%) | |
| Stage at diagnosis | 0.368 | ||
| 1 | 5 (41.7%) | 12 (66.7%) | |
| 2 | 3 (25.0%) | 2 (11.1%) | |
| 3 | 3 (25.0%) | 4 (22.2%) | |
| 4 | 1 (8.3%) | 0 (0.0%) |
| Variables | HKTE(+) (n = 12) | HKTE(−) (n = 18) | p-Value |
|---|---|---|---|
| Cancer type | 0.149 | ||
| ccRCC | 7 (58.3%) | 12 (66.7%) | |
| pRCC | 1 (8.3%) | 5 (27.8%) | |
| chRCC | 2 (16.7%) | 1 (5.6%) | |
| svRCC | 2 (16.7%) | 0 (0.0%) | |
| Local extension (pT) | 0.632 | ||
| 1A | 3 (25.0%) | 7 (38.9%) | |
| 1B | 2 (16.7%) | 3 (16.7%) | |
| 2A | 2 (16.7%) | 3 (16.7%) | |
| 2B | 1 (8.3%) | 3 (16.7%) | |
| 3A | 4 (33.3%) | 2 (11.1%) | |
| Positive lymph nodes (cN) | 0.576 | ||
| Yes | 3 (25.0%) | 3 (16.7%) | |
| No | 9 (75.0%) | 15 (83.3%) | |
| Distant metastasis (cM) | 0.212 | ||
| Yes | 1 (8.3%) | 0 (0.0%) | |
| No | 11 (91.7%) | 18 (100%) | |
| Nuclear grade | 0.509 | ||
| G1 | 3 (25.0%) | 9 (50.0%) | |
| G2 | 5 (41.7%) | 7 (38.9%) | |
| G3 | 2 (16.7%) | 1 (5.6%) | |
| G4 | 2 (16.7%) | 1 (5.6%) |
| Variables | HKTE(+) (n = 12) | HKTE(−) (n = 18) | p-Value |
|---|---|---|---|
| Intratumoral WT1 expression | 0.661 | ||
| Yes | 1 (8.3%) | 1 (5.6%) | |
| No | 11 (91.7%) | 17 (94.4%) | |
| Density of intratumoral WT1 positive cellularity (WT1-QS) | 0.908 | ||
| High (>25%) | 0 (0%) | 0 (0%) | |
| Rare/Moderate (1–25%) | 1 (8.3%) | 1 (5.6%) | |
| No expression | 11 (91.7%) | 17 (94.4%) | |
| Intensity of intratumoral WT1 nuclear reactions (WT1-IS) | 0.453 | ||
| Strong | 1 (8.3%) | 1 (5.6%) | |
| Weak/Moderate | 0 (0.0%) | 0 (0%) | |
| No expression | 11 (91.7%) | 17 (94.4%) | |
| WT1 expression in tumor-adjacent HRT—endothelial cells | 0.072 | ||
| Yes | 2 (16.7%) | 0 (0.0%) | |
| No | 10 (83.3%) | 18 (100%) | |
| WT1 expression in tumor-adjacent HRT—fibroblasts | 0.765 | ||
| Yes | 1 (8.3%) | 1 (5.6%) | |
| No | 11 (91.7%) | 17 (94.4%) | |
| WT1 expression in tumor-adjacent HRT—healthy stroma | 0.124 | ||
| Yes | 3 (25.0%) | 1 (5.6%) | |
| No | 9 (75.0%) | 17 (94.4%) |
| Variables | HKTE(+) (n = 12) | HKTE(−) (n = 18) | p-Value |
|---|---|---|---|
| Intratumoral TD expression | 0.112 | ||
| Yes | 11 (91.7%) | 12 (66.7%) | |
| No | 1 (8.3%) | 6 (33.3%) | |
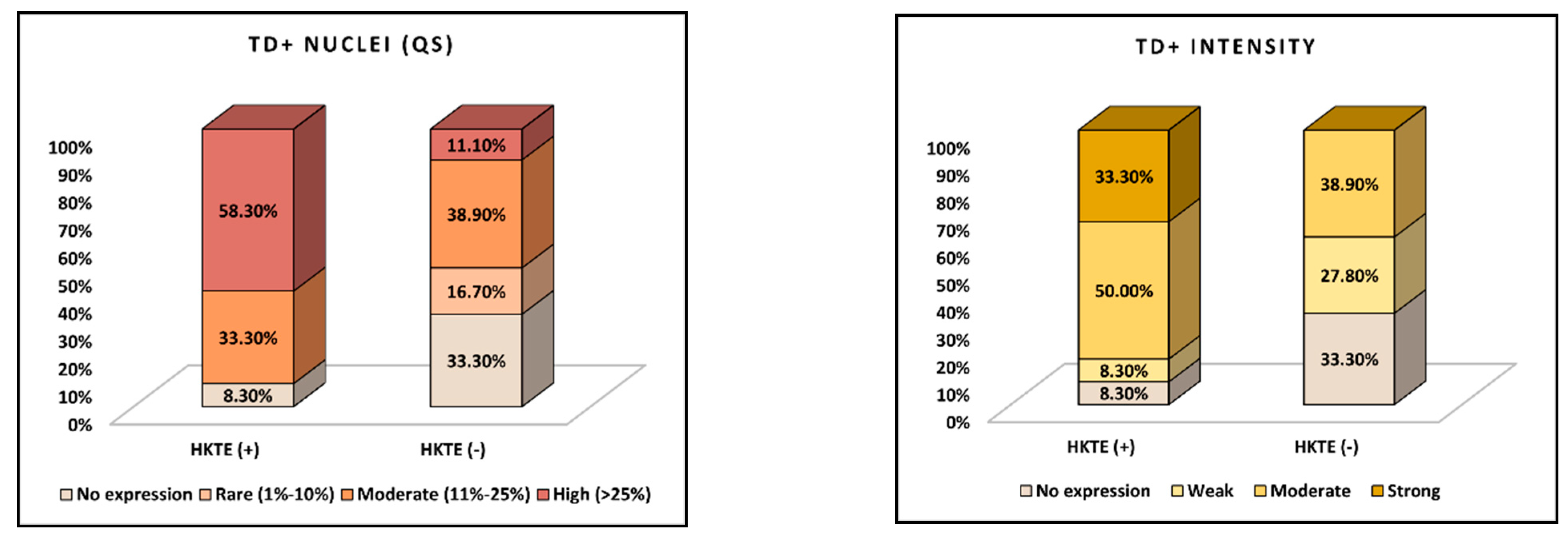
| Density of intratumoral TD positive cellularity (TD-QS) | 0.025 | ||
| High (>25%) | 7 (58.3%) | 2 (11.1%) | |
| Moderate (11–25%) | 4 (33.3%) | 7 (38.9%) | |
| Rare (1–10%) | 0 (0.0%) | 3 (16.7%) | |
| No expression | 1 (8.3%) | 6 (33.3%) | |
| Intensity of intratumoral TD nuclear reactions (TD-IS) | 0.023 | ||
| Strong | 4 (33.3%) | 0 (0.0%) | |
| Moderate | 6 (50.0%) | 7 (38.9%) | |
| Weak | 1 (8.3%) | 5 (27.8%) | |
| No expression | 1 (8.3%) | 6 (33.3%) |
| Variables | Odds Ratio (OR) | 95% CI | p-Value |
|---|---|---|---|
| Age (per 1-year increase) | 0.95 | 0.86–1.04 | 0.248 |
| Sex (female vs. male) | 1.85 | 0.52–6.53 | 0.351 |
| Stage (reference: Stage 1) | |||
| Stage 2 | 0.95 | 0.38–8.07 | 0.650 |
| Stage 3 | 1.44 | 0.52–4.28 | 0.736 |
| Stage 4 | 4.02 | 0.90–20.15 | 0.413 |
| RCC subtype (reference: ccRCC) | |||
| pRCC | 0.68 | 0.27–2.24 | 0.742 |
| chRCC | 0.45 | 0.52–1.75 | 0.290 |
| svRCC | 3.71 | 0.52–16.6 | 0.306 |
| Nuclear grade (reference: G1) | |||
| G2 | 0.90 | 0.33–2.68 | 0.880 |
| G3 | 1.17 | 0.67–5.90 | 0.512 |
| G4 | 2.56 | 0.81–6.06 | 0.268 |
| WT1 immunoexpression | |||
| Intratumoral WT1 positivity (Yes vs. No) | 1.60 | 0.25–8.30 | 0.630 |
| WT1 endothelial cell positivity (Yes vs. No) | 2.09 | 0.77–10.91 | 0.302 |
| WT1 fibroblast positivity (Yes vs. No) | 1.13 | 0.82–3.07 | 0.759 |
| WT1 healthy stroma positivity (Yes vs. No) | 3.44 | 0.92–18.50 | 0.180 |
| Quantitative TD intratumoral expression (reference: no expression) | |||
| Rare (1–10%) | 1.39 | 0.36–2.81 | 0.664 |
| Moderate (11–25%) | 3.17 | 0.92–7.03 | 0.061 |
| High (>25%) | 3.62 | 1.04–6.90 | 0.040 |
| Intensity of TD intratumoral expression (reference: no expression) | |||
| Weak | 2.18 | 0.86–6.29 | 0.220 |
| Moderate | 1.94 | 0.62–6.55 | 0.391 |
| Strong | 4.33 | 0.92–11.61 | 0.114 |
| Intratumoral TD positivity (Yes vs. No) | 4.18 | 0.96–8.42 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latcu, S.C.; Bardan, R.; Cumpanas, A.A.; Barbos, V.; Baderca, F.; Gaje, P.N.; Ceausu, R.A.; Comsa, S.; Dumitru, C.-S.; Dumache, R.; et al. Immunotherapy Applications for Thymine Dimers and WT1 Antigen in Renal Cancers: A Comparative Statistical Analysis. J. Pers. Med. 2024, 14, 557. https://doi.org/10.3390/jpm14060557
Latcu SC, Bardan R, Cumpanas AA, Barbos V, Baderca F, Gaje PN, Ceausu RA, Comsa S, Dumitru C-S, Dumache R, et al. Immunotherapy Applications for Thymine Dimers and WT1 Antigen in Renal Cancers: A Comparative Statistical Analysis. Journal of Personalized Medicine. 2024; 14(6):557. https://doi.org/10.3390/jpm14060557
Chicago/Turabian StyleLatcu, Silviu Constantin, Razvan Bardan, Alin Adrian Cumpanas, Vlad Barbos, Flavia Baderca, Pusa Nela Gaje, Raluca Amalia Ceausu, Serban Comsa, Cristina-Stefania Dumitru, Raluca Dumache, and et al. 2024. "Immunotherapy Applications for Thymine Dimers and WT1 Antigen in Renal Cancers: A Comparative Statistical Analysis" Journal of Personalized Medicine 14, no. 6: 557. https://doi.org/10.3390/jpm14060557








