Abstract
The COVID-19 pandemic, caused by SARS-CoV-2, has significantly impacted various organ systems, including the eyes. Initially considered a primarily respiratory disease, it is now evident that COVID-19 can induce a range of ocular symptoms. Recognizing these ocular manifestations is crucial for eye care practitioners as they can serve as early indicators of the disease. This review consolidates current evidence on the ocular effects of COVID-19, identifying manifestations such as conjunctivitis, scleritis, uveitis, and retinopathy. The increasing prevalence of these symptoms highlights the importance of thorough eye examinations and detailed patient histories in COVID-19 cases. Potential routes of viral entry into ocular tissues and the underlying mechanisms, including direct infection, immune responses, and vascular involvement, are explored. Additionally, this review addresses ocular side effects associated with COVID-19 vaccines, such as corneal graft rejection, uveitis, and retinal issues. These findings emphasize the need for ongoing surveillance and research to ensure vaccine safety.
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is a highly contagious and pathogenic virus that is known to cause a spectrum of human respiratory tract infections ranging from mild colds to severe respiratory distress syndrome. Coronavirus initially gained widespread attention following the 2003 outbreak of severe acute respiratory syndrome coronavirus (SARS-CoV) [1]. On 30 December 2019, Li Wenliang, an ophthalmologist at Wuhan Central Hospital, alerted fellow medical professionals about a potential outbreak resembling SARS. The World Health Organization (WHO) declared the Wuhan outbreak a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March [2]. Since the onset of the pandemic, the WHO estimates that more than a billion confirmed cases of the coronavirus disease 2019 (COVID-19) and more than 6 million deaths worldwide have occurred [3].
Due to the virus’s potential to cause life-threatening respiratory issues, much of the research on coronaviruses (CoVs) center around the respiratory system. However, COVID-19 demonstrates a multifaceted nature, exhibiting neurotropism and endothelial tropism, alongside the capacity to provoke a systemic inflammatory response [4]. Consequently, a notable portion of COVID-19 patients may experience neurological and vascular symptoms. Manifestations of COVID-19 in the different organ systems should not be overlooked, as they could hint toward an alternative mode of transmission [4].
Ocular manifestations have been reported as potential initial indicators of COVID-19 [5]. Given the rapidly expanding body of literature on COVID-19, it is important to consolidate our current understanding of its ocular effects and discuss the implications for eye care practitioners. This article aims to review the existing evidence regarding the ocular involvement in COVID-19 and the potential underlying pathophysiological mechanisms. We additionally review the existing literature on the ocular complications associated with the COVID-19 vaccines currently in use.
2. Methods
This review utilized PubMed (https://pubmed.ncbi.nlm.nih.gov, accessed on 18 June 2024) and Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com, accessed on 18 June 2024). PubMed, a widely used and trusted biomedical literature database maintained by the National Library of Medicine (NLM), was selected as the primary resource for this research. Its extensive coverage of peer-reviewed journals in medicine and life sciences makes it an ideal tool for retrieving relevant scientific literature. A systematic approach was implemented to develop an effective search strategy. This process included identifying key concepts and terms related to the topic, including relevant medical terminologies, synonyms, and abbreviations. Key terms such as “COVID-19”, “SARS-CoV-2”, “vaccine”, “ocular”, “side effects”, “adverse events”, “eye”, “cornea”, “uvea”, “uveitis”, “retina”, and “retinopathy” were used. Boolean operators (AND, OR, NOT) were used to logically combine these terms, ensuring comprehensive coverage of pertinent literature while minimizing irrelevant results. The review search was updated to June 2024.
3. Ocular Involvement of COVID-19
3.1. Overview of SARS-CoV-2 Viruses
SARS-CoV-2 viruses are a family of positive sense, single-stranded ribonucleic acid (RNA), enveloped viruses. They belong to the Coronaviridae family within the suborder Coronavirineae and order Nidovirales [6]. The family is characterized by a spike (S) glycoprotein facilitating receptor binding and cell entry. Among the seven strains that are commonly reported to cause infection in humans, 229E, NL63, OC43 and HKU1 cause common colds, while Middle East respiratory syndrome (SARS)-CoV, severe acute respiratory syndrome (SARS)-CoV, and SARS-CoV-2 cause more severe respiratory infections [7].
3.2. Viral Transmission
According to the reported literature, COVID-19 transmission likely originated from bats, possibly reaching humans through other intermediate animals, potentially from the local sea food market in Wuhan [8]. Analysis shows a close similarity between the binding proteins Pangolin-CoV, Bat-nCoV and SARS-CoV-2, suggesting viral recombination [9]. Human-to-human transmission mainly occurs through respiratory droplets during coughing and sneezing or via fomites and viral contaminated surfaces [10]. Spike proteins are glycoproteins that protrude from the lipid envelopes of the viral particles and help with binding and entry into the cells. They do so by interacting with the angiotensin converting enzyme-2 (ACE2) receptors present on human cells facilitated by the serine protease TMPRSS2 [11].
3.3. Susceptibility to Ocular Involvement
There is evidence in the literature that the normal human conjunctiva harbors ACE2 receptors, implying the potential for SARS-CoV-2 to bind to the ocular surface [12]. Additionally, ACE1 and ACE2 enzyme expression has also been reported in the cornea and the aqueous humor, respectively [13]. Zhou L et al. reported a higher level of the receptor protein on the cornea and the limbus but reported lower levels in the conjunctiva [14]. Some degree of cross reactivity and binding might also be possible using the α2-3-linked sialic acid lectin binding sites, present in the epithelium of the nasolacrimal duct and lacrimal sac, which are used by adenoviruses and influenza viruses to gain entry into cells [15]. Notably, conjunctival swab samples from the tears of infected individuals have proven positive for SARS-CoV-2 RNA using the reverse-transcriptase polymerase chain reaction (RT-PCR). Other studies have reported the theoretical possibility of viral migration from the nasopharynx via the nasolacrimal duct. The hematogenous spread of the virus to the ocular tissues has also been studied [16]. Cerebral circulation is characterized by a relatively slower blood flow compared to other regions, which can create conditions that are more conducive to the entry and spread of pathogens, including viruses. This slower flow may allow viruses more time to interact with endothelial cells and potentially cross the blood–brain barrier. In the context of the olfactory bulb and cribriform plate, which are in close proximity to the nasal passages, this becomes particularly significant. The literature has explored the possibility of viral spread via the cerebral circulation, taking advantage of the slower blood flow and disseminating through the olfactory bulb and cribriform plate [17].
3.4. Prevalence and Incidence of Ocular Manifestations
Early data reported a lower prevalence of the ocular manifestations of COVID-19. Guan W et al. studied patient data from 552 hospitals with laboratory-confirmed COVID-19 in mainland China between December 2019 and January 2020. Among the 1099 patients included in the study cohort, only 9 (0.8%) were reported to have conjunctival congestion [18]. However, the recent literature has shown a greater prevalence of the ocular involvement of COVID-19. In the systematic review and meta-analysis reported by Nasiri N et al., the reported prevalence of ocular manifestations was estimated to be 11.03% in a study cohort of 8219 patients. They further reported the most common ocular manifestation to be dry eye or foreign body sensation (16%), followed by redness (13.3%), tearing (12.8%), itching (12.6%), eye pain (9.6%) and discharge (8.8%) [19].
3.5. Types of Ocular Manifestations
The studies on the different ocular manifestations of human coronavirus (CoV) infections are limited. However, CoVs have been reported to cause various ocular presentations in animals. Clinical conditions such as conjunctivitis, anterior uveitis, optic neuritis, and retinitis have been documented in feline and murine models [20]. In murine models, the virus is commonly reported to affect the posterior pole of the eye, characterized by choroiditis, retinal detachment, retinal degeneration, and retinal vasculitis [16]. The reported ocular manifestations of SARS-CoV-2 infection in humans are summarized in Table 1.

Table 1.
Ocular complications of an underlying SARS-CoV-2 disease.
3.5.1. Conjunctivitis
Conjunctivitis or excessive tearing has been reported as the initial or the sole symptom in some patients with COVID-19. Scalinci SZ et al. reported data on 5 patients having non-remitting conjunctivitis as the only presenting sign and symptom of COVID-19. These patients did not develop fever, malaise or any respiratory symptoms during the course of their infection and their diagnosis was confirmed using RT-PCR [21]. Navel V et al. reported a case of pseudomembranous and hemorrhagic conjunctivitis in a patient 19 days after onset of SARS-CoV-2 infection. Among children, some cases of COVID-19 have been closely linked with a presentation like that of Kawasaki disease, called multisystem inflammatory syndrome in children (MIS-C). The most common ocular manifestation in these patients was reported to be conjunctivitis [27].
3.5.2. Scleritis and Episcleritis
Feizi S et al. reported two cases of anterior scleritis manifesting after the COVID-19 infection, the first one, in a 67-year-old woman that presented with necrotizing anterior scleritis one week after the onset of COVID-19, and the second one, in a 33-year-old man who presented with sectorial anterior scleritis 2 weeks after the onset of COVID-19. Workup identified no underlying autoimmune disease in any of these patients [22]. Otaif W et al. described the case of a 29-year-old man presenting with episcleritis as the first sign of an underlying COVID-19 infection [23]. Another study reported a case of nodular episcleritis in a patient with underlying COVID-19 infection [28].
3.5.3. Uveitis
During the pandemic, an increase in the number of patients presenting with new-onset uveitis or recurrence of uveitis was noted. In a retrospective observational study conducted at the Beijing Tongren Hospital, 18 patients presented with symptoms of active uveitis within 4 weeks of their positive COVID-19 RT-PCR test [29]. Nine of these patients were reported to have new onset uveitis whereas the other nine had relapsed uveitis. Of the nine patients with new-onset uveitis, seven had a bilateral presentation. Among these patients, four had anterior uveitis, one had sympathetic ophthalmia, three patients had Vogt–Koyanagi–Harada (VKH) syndrome and one had multiple evanescent white dot syndrome (MEWDS) [29]. Patients presenting with various forms of posterior uveitis have also been reported in the literature [30].
3.5.4. Retinopathy
The published literature has documented occurrences of both central retinal vein occlusions (CRVO) and central retinal artery occlusions (CRAO) in patients with underlying COVID-19 infection who do not exhibit the typical systemic vascular risk factors. Walinjkar et al. reported the case of a 17-year-old girl who presented with diminished vision 21 days after an underlying COVID-19 infection [24,25,31,32]. On examination, the patient had swelling of the optic disc, splinter hemorrhages, flame-shaped hemorrhages, and dot and blot hemorrhages. Cystoid macular edema and detachment of the neurosensory layer was noted on an optical coherence tomography examination, and a presumptive diagnosis of CRVO was made [24]. Gaba WH et.al. described a case of a 40-year-old man presenting with bilateral central retinal vein occlusion with severe coronavirus disease pneumonia [25]. Another study reported a case of impending CRVO in a patient with COVID-19. Fundoscopic and imaging examination in this patient revealed a fern-like hypo-autofluorescent appearance that is typical of ischemic CRVO (iCRVO) [32]. Heidarzadeh HR et al. reported a case of CRAO in a 44-year-old with a 1-week history of severe COVID-19 infection [31].
In another cross-sectional study, Invernizzi A et al. noted a direct correlation between retinal vein diameter with the severity of the underlying COVID-19 infection. The mean arterial diameter (MAD) and the mean venous diameter (MVD) were both noted to be significantly higher in COVID-19 patients (p < 0.0001). Tortuous veins were noted in 12.9% of the patient cohort [33]. Marinho et al. studied retinal and optical coherence tomography (OCT) changes in 12 patients with an underlying COVID-19 infection. All patients were reported to have hyper-reflective lesions at the level of the inner plexiform layer and ganglion cell layer. On fundus examination, four of these patients presented microhemorrhages and subtle cotton wool spots [34]. Similarly, Pereira LA et al. studied the ocular findings of 18 patients with confirmed severe COVID-19 and reported that 10 of these patients displayed abnormalities on dilated eye examinations. The common findings that were noted included flame-shaped hemorrhages, cotton wool spots and retinal sectorial pallor [35]. Soni A et al. reported two consecutive cases of acute retinal necrosis as a presenting manifestation in patients with a recent history of COVID-19. PCR from a vitreous sample was positive for Herpes simplex virus (HSV) in both patients. The proposed mechanism was that the immune dysregulation caused by the COVID-19 infection likely caused the reactivation of HSV, which together resulted in the presentation of retinal necrosis [36].
3.5.5. Other Ocular Complications
Verkuli et al. described the case of a 14-year-old girl who presented with new onset abducens nerve palsy and papilledema and had hemorrhages on fundus examination of the optic disc. On lumbar puncture, the opening pressure was found to be 36 cm of H2O [37]. A diagnosis of pseudotumor cerebri syndrome associated with multisystem inflammatory syndrome was made. Other reported ocular complications include optic neuritis, papilledema, and palsy of the third and fourth cranial nerve [38,39,40]. Oscillopsia and central vestibular nystagmus have also been described as complications of an underlying COVID-19 infection [41,42].
4. Clinical Practice and Diagnostic Considerations
For accurate diagnosis of these ocular manifestations, it is of prime importance to take a detailed history regarding the onset and duration of the symptoms along with the accompanying characteristics. All patients should be questioned about any recent history of respiratory symptoms, fever, and travel history to assess the need for further evaluation of COVID-19. Repeated disinfection of equipment, including slit lamps and B-scan probes with 70% ethyl alcohol has shown to reduce coronavirus infectivity. Goldman tonometers can be sterilized with a 10% diluted sodium hypochlorite solution [43]. Ophthalmologists are also advised to prioritize the use of disposable equipment over reusable equipment. TonoSafe are disposable probes that can be used instead of reusable probes for tonometry.
5. Mechanisms of Ocular Infection by SARS-CoV-2
5.1. Entry Routes of the Virus into Ocular Tissues
Initially, the primary mode of viral entry into ocular tissues was thought to be direct inoculation onto the conjunctiva, binding to ACE2 receptors (Figure 1). However, this theory faced limitations due to the absence of activating serine protease in the conjunctiva [12]. Subsequent research has explored alternative pathways for viral transmission into ocular tissues. Literature reports indicate neuronal and hematogenous spread of the infection to the eyes [44]. Studies on the neuronal route suggest viral entry via retrograde transportation from the brain to the optic nerve [45]. Additionally, it has been suggested that following anterior segment inoculation, the virus can spread to other regions via corneal nerves connected to the trigeminal nerve [45,46]. Recent advancements underscore COVID-19 as a vascular disease, spreading hematogenously by infecting capillary endothelial cells. These cells express ACE2 and CD147 receptors, facilitating direct viral binding [47]. Another theory proposes virus transportation to the retina via leukocytes capable of crossing the blood–retinal barrier.
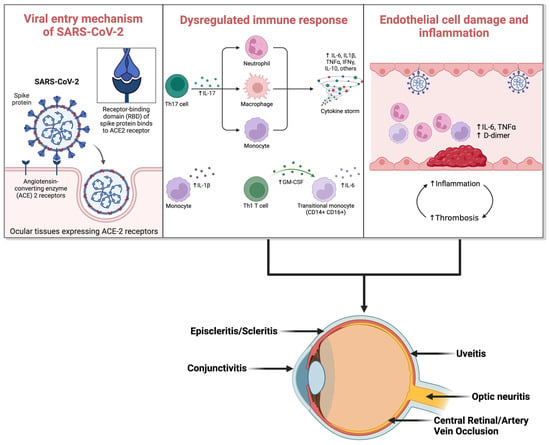
Figure 1.
Viral entry mechanism of SARS-CoV-2 and subsequent immune response dysregulation leading to COVID-19 ocular manifestations. (ACE: Angiotensin-Converting Enzyme; IL-17: Interleukin-17; Th17: T-helper 17 cells; Th1: T-helper 1 cells; IL-6: Interleukin-6; IL-1β: Interleukin-1β; TNF-α: Tumor Necrosis Factor-α; IFN-γ: Interferon-γ; IL-10: Interleukin-10). (Created with Biorender.com).
5.2. Underlying Pathophysiology and Immune Response in Ocular Tissues
The fundamental pathophysiological mechanisms involve viral infection triggering a proinflammatory and prothrombotic state [48]. This leads to the release of cytokines from proinflammatory cells, culminating in a cytokine storm and subsequent inflammatory conditions within the eye, such as conjunctivitis, uveitis, choroiditis, and retinitis. Notably, the cytokine storm induced by SARS-CoV-2 is marked by elevated levels of type 2 cytokines and diminished levels of type 1 cytokines. IL-33, a member of the IL-1 family secreted by epithelial cells, may further amplify this type 2 cytokine response [49]. Some sources liken the underlying pathophysiology to macrophage activation syndrome (MA.S.), particularly concerning the threshold model of SARS-CoV-2 infection [50]. Furthermore, certain studies suggest the involvement of the proptosis pathway and activation of the inflammasome in the inflammatory response [51].
The proposed experimental coronavirus retinopathy (ECOR) model suggests that retinal damage resulting from the viral infection unfolds in two distinct phases. Initially, immune cells infiltrate the retina, triggering the release of inflammatory mediators and instigating retinal inflammation. This phase is succeeded by viral clearance. Subsequently, the second phase ensues, characterized by the production of autoantibodies targeting the retina and pigment epithelium, which leads to photoreceptor and neuroretinal damage [52]. Moreover, in the context of ECOR, the observed retinal damage correlates with elevated levels of TNF-alpha and disrupted TNF signaling pathways [53].
When SARS-CoV-2 utilizes the ACE2 receptor to infiltrate local endothelial cells, it initiates endothelial damage and microangiopathic injury, thereby triggering localized inflammation. The byproducts of microvascular injury activate neutrophils, which, in turn, can harm the endothelial cell glycocalyx [54,55]. This endothelial disruption culminates in platelet activation and an overall state of hypercoagulability. COVID-19 associated coagulopathy (CAC) manifests as an endotheliopathy characterized by severe disturbances in D-dimer levels [56,57]. Alongside this, there is an elevation in fibrinogen levels, while the levels of prothrombin and platelets exhibit only minor alterations [58].
5.3. Association between Ocular Involvement and Disease Severity
In a retrospective study using the electronic medical records of 342 patients at a large tertiary academic medical center in India, the authors reported that 42.9% of patients with severe COVID-19 disease displayed ophthalmic manifestations as compared to 26.7% of patients with non-severe disease. This difference was found to be statistically significant (p value = 0.003). Loffredo et al. reported an overall rate of conjunctivitis in patients with a confirmed COVID-19 infection to be 3% and 0.7% in severe and non-severe illness. They further added that the presence of ocular manifestations may be a warning sign for poor outcomes. Similarly, Navel V et.al. in their study also reported that in individuals diagnosed with COVID-19, the presence of conjunctivitis typically correlates with a heightened severity of illness and a poorer prognosis.
6. Ocular Side Effects of COVID-19 Vaccines
6.1. Overview of COVID-19 Vaccine Types
Since the declaration of COVID-19 as a pandemic by the WHO on 11 March 2020, there have been 336 vaccine candidates developed, with 32 vaccines currently authorized for global use [59] (Table 2). Owing to the urgency of the pandemic and the rapid escalation of cases and fatalities, several of these vaccines proceeded directly to human clinical trials without prior pre-clinical testing on animals. Among them, 11 vaccine candidates received emergency use authorization (EUA) [60].

Table 2.
Vaccines that received Emergency Use Authorization (EUA).
These vaccines can be categorized into four main groups: mRNA vaccines (such as BNT162b2 by Pfizer-BioNTech and mRNA-1273 by Moderna), vector vaccines (including Ad26.COV2 by Janssen Johnson & Johnson and ChAdOx1 nCoV-19/AZD1222 by Oxford-AstraZeneca), protein subunit vaccines (like NVX-CoV2373 by Novavax), and whole virus vaccines (such as PiCoVacc by Sinovac and BBIBP-CorV by Sinopharm) [60].
Among the mRNA vaccines, BioNTech and Pfizer developed two candidates, BNT162b1 and BNT162b2. Both are nucleoside modified, LNP encapsulated mRNA vaccine candidates [72]. mRNA-1273 is an LNP-encapsulated mRNA with an N1-methyl-pseudourine substituting uridine. It was developed by Moderna, and the mRNA encodes the SARS-CoV-2 full length S-2P protein [73]. ARCoV is another LNP encapsulated nucleoside modified mRNA vaccine. This vaccine candidate gave mice complete protection from a mouse-adapted SARS-CoV-2 strain in a preclinical study [61]. The Ad26.COV2 uses modified adenoviral DNA that encodes for a key part of the SARS-CoV-2 virus particle that our immune system can elicit a response against. This vaccine biochemistry is based on stable DNA molecules, so it does not require ultracold storage and is easy to store and distribute [74].
Since most of these vaccines received EUA, the data on the safety profile and potential side effects of these vaccines was limited. To manage this, the Centers for Disease Control and Prevention (CDC) expanded its Vaccine Adverse Event Reporting System (VAERS). VAERS is a national early warning system and functions as a passive surveillance platform for potential vaccine adverse events. The data collected by this platform is available through the Wide-Ranging Online Data for Epidemiologic Research platform (WANDER), which is also developed and operated by CDC [75].
In discussing the ocular side effects of COVID-19 vaccines, it is essential to maintain a balanced perspective. While reporting adverse events is critical for comprehensive understanding, it is equally important to emphasize their rarity relative to the widespread benefits of vaccination. Current data indicates that ocular complications post-vaccination, such as uveitis or retinal issues, are exceedingly rare, occurring in a small fraction of vaccinated individuals. These events must be contextualized within the broader context of millions of vaccine doses administered worldwide, where the overwhelming majority of recipients experience no significant ocular side effects. Emphasizing this rarity underscores the overall safety profile of COVID-19 vaccines while ensuring transparency in reporting potential adverse outcomes.
6.2. Reported Ocular Side Effects
The potential ocular side effects following COVID-19 vaccine administration in human are summarized in Table 3.

Table 3.
Ocular manifestations after vaccination against COVID-19 infection.
6.2.1. Cornea
According to the VAERS database, 73% of the total reported cases of vaccine-associated corneal graft rejection were following vaccination with BNT162b2 vaccine and 26% were following the mRNA-1273 vaccine. The database analysis also reported that 61.87% of the rejections happened following penetrating keratoplasty (PKP), followed by 18.18% with Descemet stripping endothelial keratoplasty and 12.73% with Descemet membrane endothelial keratoplasty [101]. Corneal graft rejection after penetrating keratoplasty (PKP)—Wasser LM et al. reported the cases of two patients aged 56 and 73, with a history of penetrating keratoplasty presenting with acute corneal graft rejection 2 weeks after receiving the first dose of the BNT162b2 mRNA vaccine. Both patients were treated with hourly 0.1% dexamethasone and 60 mg per day of oral prednisone. The management resulted in prompt resolution of the graft rejection [77]. Rallis KI et al. described a case of a 68-year-old man who underwent penetrating keratoplasty presenting with acute corneal graft rejection three days after receiving the first dose of the BNT162b2 mRNA vaccine. The patient presented with discomfort in the eye, conjunctival hyperemia, and epithelial rejection line. Treatment with dexamethasone 0.1% eye drops hourly and oral acyclovir 400 mg five times daily for 1 week resulted in complete resolution of the symptoms [80]. Another study from the USA highlighted a similar presentation of a 51-year-old man with a history of PKP presenting with corneal graft edema and keratoprecipitates as signs of acute graft rejection 3 days after receiving the first dose of the mRNA-1273 vaccine. The patient was treated with topical steroid eye drops and this resulted in complete resolution of the graft rejection [102].
Corneal graft rejection after Descemet’s membrane endothelial keratoplasty (DMEK)—Phylactou M et al., in their study, reported the case of an 83-year-old woman who had undergone bilateral DMEK for Fuch’s endothelial corneal dystrophy and presented with acute endothelial graft rejection in both eyes 3 weeks after receiving the second dose of the BNT162b2 vaccine. She was treated successfully with topical steroids [82]. Crnej A et al. studied the case of a 71-year-old man who had undergone DMEK for endothelial decompensation post phacoemulsification and presented to the clinic with a sudden painless decrease in vision. On examination, he showed conjunctival injection and diffuse corneal edema, and a diagnosis of acute corneal edema was made. The patient was managed with topical steroids and oral valacyclovir resulting in symptom resolution in a week [81].
6.2.2. Uvea
A retrospective observational study of the CDC-VAERS database reported 1094 cases of vaccine-associated uveitis from over 40 countries after SARS-CoV-2 vaccination. Among them, 77.97% of the cases were reported after the BNT162b2 vaccine, followed by 20.11% of the cases with the mRNA-1273 vaccine. A significantly higher number of cases were reported after the first dose of the vaccine and within 1 week of the administration of that dose [103].
Renisi et al. reported a case of a 23-year-old man who presented with a red eye associated with pain and photophobia two weeks after getting the second dose of the BNT162b2 vaccine. The patient had perichoretic and conjunctival hyperemia on examination. A diagnosis of acute anterior uveitis was made, and the patient was treated with topical steroids and cycloplegic drops [83]. Mudie et al. described a case of a 43-year-old woman who came to the clinic with the presentation of pan uveitis three days after receiving the second dose of the Pfizer-Biontech mRNA vaccine. The examination revealed vitreous inflammation and significant thickening of the choroid. The patient was treated with oral and topical steroids that resulted in resolution of the symptoms [87]. Goyal M et al. described a case of bilateral choroiditis following COVID-19 vaccination. The patient showed severe choroidal thickening and a large serous detachment of the macula on examination. There was a rapid improvement of the symptoms after initiation of oral steroids [28,104].
6.2.3. Retina
Bøhler et al. described the case of a 27-year-old woman who presented to the clinic with visual disturbances a few days after receiving the first dose of the AstraZeneca vaccine. On examination, a paracentral scotoma was noted on perimetry in the upper temporal quadrant of the left eye. On fundoscopic examination, a tear-shaped macular lesion was noted, which was better visualized on optical coherence tomography. A diagnosis of acute macular neuro-retinopathy (A.M.N) was established [94]. Another study published a case of a 41-year-old woman who presented to the clinic with foggy vision 2 days after receiving the AstraZeneca vaccine. On fundoscopic examination, disc edema was noted in the right eye and a dome shaped serous detachment was noted in the upper quadrant of the left eye. A diagnosis of idiopathic optic disc edema was made for the right eye and that of central serous chorioretinopathy was made for the left eye [105].
Singh RB et al. analyzed cases of retinal vessel occlusion post COVID-19 vaccination from the VAERS database and reported that a majority (74.17%) of the cases of retinal vessel occlusion were reported after vaccination with the BNT162b2 vaccine, and 41.12% of the cases of retinal venous occlusion (RVO) and 48.27% of the cases of retinal arterial occlusion presented within the first week after vaccination [106]. Subramony R et al. reported the case of an otherwise healthy 22-year-old woman who presented to the emergency department with progressive painless loss of vision in her right eye a few days after receiving the Moderna SARS-CoV-2 vaccine. On examination, a point of care ultrasound showed bilateral retinal detachment, for which she subsequently underwent bilateral vitrectomies for management [26]. Based on the cases described, it is suggested that vaccine administration may have direct or indirect correlations with local ocular effects such as acute macular neuro-retinopathy, idiopathic optic disc edema, central serous chorioretinopathy, and retinal vessel occlusions, highlighting the need for further investigation into these associations.
6.2.4. Other Reported Ocular Side Effects
Garcia-Estarada C et al. reported a case of a 19-year-old woman who developed optic neuritis 1 week after receiving a first dose of the Ad26.COV2.S vaccine [95]. Another study described a case of acute abducens nerve palsy in a 23-year-old man 1 week after receiving the Covishield vaccine [107]. Colella G et al. studied the case of a healthy 37-year-old who developed facial palsy within a few days after receiving the BNT162b2 mRNA vaccine [108]. Additional reports in the published literature have documented ocular manifestations following COVID-19 vaccination, including conditions such as thyroid eye disease, Miller Fisher syndrome, glaucoma, superior ophthalmic vein syndrome, and herpetic eye disease [109,110,111,112,113].
7. Mechanisms of Ocular Side Effects
Cunningham et al. attributed the immune responses observed in vaccine-associated uveitis (VAU) to a delayed type of hypersensitivity response to the molecular similarities between the vaccine peptides and the uveal self-peptides [114]. Rabinovitch et al. described a type 1 hypersensitivity reaction with the upregulation of proinflammatory molecules after interaction with the vaccine components [115]. The presence of autoimmune processes due to the upregulation of RNA sensing molecules like TLR3 and MDA5 in response to the mRNA molecules used in vaccine delivery was also noted [116]. There is evidence of similarities between the uveitic reaction observed post adenoviral infection, which was attributed to an antigen–antibody complex mediated type three hypersensitivity reaction and VAU in COVID-19 [117].
The complication of corneal graft rejection post vaccination is postulated to be due to cross reactivity between the human leukocyte antigens of the corneal endothelial cells and the viral antigen-specific T cells [83]. Immunologically, it has been observed that the proinflammatory state due to the body’s immune response to the SARS-CoV-2 vaccines leads to upregulation of the CD4 Th1 cells, and these cells have been shown to be one of the primary mediators of corneal graft rejection [82]. Furthermore, the interaction between the vaccine components and platelet factor 4 causing subsequent platelet activation has been postulated to be the underlying mechanism of the prothrombotic side effects of the SARS-CoV-2 vaccines like CRAO and CRVO [118,119].
8. Conclusions
In summary, ocular involvement in COVID-19 presents a complex challenge that warrants ongoing attention. From conjunctivitis to retinopathy and potential vaccine-related complications, thorough eye examinations and patient histories are crucial. Future research should focus on clarifying how SARS-CoV-2 affects different ocular tissues, understanding the long-term implications, and assessing emerging therapies. Vigilant surveillance and targeted studies will enhance our understanding and management of ocular manifestations in COVID-19, contributing to improved patient outcomes.
Author Contributions
Conceptualization, P.L.S. and R.B.S.; methodology, U.P.S.P., P.L.S., A.R., R.B.S. and M.Z.; software, U.P.S.P. and R.B.S.; validation, U.P.S.P., P.L.S., R.B.S., M.M., A.S., G.S., A.M., S.L., F.D., A.R., A.L., C.G. and M.Z.; investigation, U.P.S.P., P.L.S., R.B.S., A.R., M.M. and A.S.; resources, C.G. and M.Z.; data curation, U.P.S.P., P.L.S., R.B.S., M.M., A.S., G.S., A.M., S.L., F.D., A.R., A.L., C.G. and M.Z.; writing—original draft preparation, U.P.S.P., P.L.S., R.B.S., A.S. and G.S.; writing—review and editing, U.P.S.P., P.L.S., R.B.S., M.M., A.R., A.S., G.S., A.M., S.L., F.D., A.L., C.G. and M.Z.; visualization, U.P.S.P., P.L.S., R.B.S., M.M., A.S., G.S., A.M., A.R., S.L., F.D., A.L., C.G. and M.Z.; supervision, P.L.S., C.G. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Loon, S.C.; Lun, K. SARS: A Timely Reminder. Br. J. Ophthalmol. 2013, 97, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Novel Coronavirus (2019-NCoV): Situation Report, 11. World Health Organization. 2020. Available online: https://iris.who.int/handle/10665/330776 (accessed on 12 May 2024).
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Martin, D.; Bremond-Gignac, D. Ocular Manifestation as First Sign of Coronavirus Disease 2019 (COVID-19): Interest of Telemedicine during the Pandemic Context. J. Fr. Ophtalmol. 2020, 43, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Ichhpujani, P.; Parmar, U.P.S.; Duggal, S.; Kumar, S. COVID-19 Vaccine-Associated Ocular Adverse Effects: An Overview. Vaccines 2022, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation and Characterization of 2019-NCoV-like Coronavirus from Malayan Pangolins. bioRxiv 2020. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, C.-B.; Jhanji, V.; Xu, C.; Yuan, X.-L.; Liang, J.-J.; Huang, Y.; Cen, L.-P.; Ng, T.K. Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Human Primary Conjunctival and Pterygium Cell Lines and in Mouse Cornea. Eye 2020, 34, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Holappa, M.; Vapaatalo, H.; Vaajanen, A. Many Faces of Renin-Angiotensin System—Focus on Eye. Open Ophthalmol. J. 2017, 11, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 Are Expressed on the Human Ocular Surface, Suggesting Susceptibility to SARS-CoV-2 Infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Rota, P.A.; Tumpey, T.M. Ocular Tropism of Respiratory Viruses. Microbiol. Mol. Biol. Rev. 2013, 77, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Sharifi, H.; Bazrafshan, A.; Noori, A.; Karamouzian, M.; Sharifi, A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-Analysis. J. Ophthalmic Vis. Res. 2021, 16, 103. [Google Scholar] [CrossRef]
- Doherty, M.J. Ocular Manifestations of Feline Infectious Peritonitis. J. Am. Vet. Med. Assoc. 1971, 159, 417–424. [Google Scholar] [PubMed]
- Scalinci, S.Z.; Trovato Battagliola, E. Conjunctivitis Can Be the Only Presenting Sign and Symptom of COVID-19. IDCases 2020, 20, e00774. [Google Scholar] [CrossRef] [PubMed]
- Danthuluri, V.; Grant, M.B. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmol. Ther. 2020, 9, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Meshksar, A.; Naderi, A.; Esfandiari, H. Anterior Scleritis Manifesting After Coronavirus Disease 2019: A Report of Two Cases. Cornea 2021, 40, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Otaif, W.; Al Somali, A.I.; Al Habash, A. Episcleritis as a Possible Presenting Sign of the Novel Coronavirus Disease: A Case Report. Am. J. Ophthalmol. Case Rep. 2020, 20, 100917. [Google Scholar] [CrossRef] [PubMed]
- Méndez Mangana, C.; Barraquer Kargacin, A.; Barraquer, R.I. Episcleritis as an Ocular Manifestation in a Patient with COVID-19. Acta Ophthalmol. 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhao, M.; Mo, J.; Cao, X.; Chen, W.; Wang, H. New Onset or Recurrence of Uveitis Following COVID-19 Infection. BMC Ophthalmol. 2024, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Murthy, S.I.; Annum, S. Bilateral Multifocal Choroiditis Following COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Walinjkar, J.; Makhija, S.; Sharma, H.; Morekar, S.; Natarajan, S. Central Retinal Vein Occlusion with COVID-19 Infection as the Presumptive Etiology. Indian J. Ophthalmol. 2020, 68, 2572. [Google Scholar] [CrossRef] [PubMed]
- Gaba, W.H.; Ahmed, D.; Al Nuaimi, R.K.; Dhanhani, A.A.; Eatamadi, H. Bilateral Central Retinal Vein Occlusion in a 40-Year-Old Man with Severe Coronavirus Disease 2019 (COVID-19) Pneumonia. Am. J. Case Rep. 2020, 21, e927691-1. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Messenio, D.; Cereda, M.; Olivieri, P.; Brambilla, A.M.; Staurenghi, G. Impending Central Retinal Vein Occlusion in a Patient with Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, H.R.; Abrishami, M.; Motamed Shariati, M.; Ghavami Shahri, S.H.; Ansari Astaneh, M.R. Atypical Central Retinal Artery Occlusion Following COVID-19 Infection: A Case Report. Case Rep. Ophthalmol. 2023, 14, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal Findings in Patients with COVID-19: Results from the SERPICO-19 Study. EClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef] [PubMed]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal Findings in Patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A.; Soares, L.C.M.; Nascimento, P.A.; Cirillo, L.R.N.; Sakuma, H.T.; da Veiga, G.L.; Fonseca, F.L.A.; Lima, V.L.; Abucham-Neto, J.Z. Retinal Findings in Hospitalised Patients with Severe COVID-19. Br. J. Ophthalmol. 2022, 106, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Narayanan, R.; Tyagi, M.; Belenje, A.; Basu, S. Acute Retinal Necrosis as a Presenting Ophthalmic Manifestation in COVID-19 Recovered Patients. Ocul. Immunol. Inflamm. 2021, 29, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Verkuil, L.D.; Liu, G.T.; Brahma, V.L.; Avery, R.A. Pseudotumor Cerebri Syndrome Associated with MIS-C: A Case Report. Lancet 2020, 396, 532. [Google Scholar] [CrossRef] [PubMed]
- Belghmaidi, S.; Nassih, H.; Boutgayout, S.; El Fakiri, K.; El Qadiry, R.; Hajji, I.; Bourrahouat, A.; Moutaouakil, A. Third Cranial Nerve Palsy Presenting with Unilateral Diplopia and Strabismus in a 24-Year-Old Woman with COVID-19. Am. J. Case Rep. 2020, 21, e925897-1. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.D.M.C.D.; Santos, D.H.; Olivetti, B.C.; Takahashi, J.T. Bilateral Trochlear Nerve Palsy Due to Cerebral Vasculitis Related to COVID-19 Infection. Arq. Neuropsiquiatr. 2020, 78, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.M.; Díaz-Maroto, I.; Segura, T. Multiple Sclerosis Following SARS-CoV-2 Infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef] [PubMed]
- Malayala, S.V.; Raza, A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus 2020, 12, e8918. [Google Scholar] [CrossRef]
- Llorente Ayuso, L.; Torres Rubio, P.; Beijinho do Rosário, R.F.; Giganto Arroyo, M.L.; Sierra-Hidalgo, F. Bickerstaff Encephalitis after COVID-19. J. Neurol. 2021, 268, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Patel, J.; Swiston, C.; Patel, B.C. Ophthalmic Manifestations of Coronavirus (COVID-19); StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Raony, Í.; de Figueiredo, C.S.; Pandolfo, P.; Giestal-de-Araujo, E.; Oliveira-Silva Bomfim, P.; Savino, W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front. Immunol. 2020, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the Human Corneal Innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; García-Feijoó, J.; Sáenz-Francés, F.; Arriola-Villalobos, P.; Martinez-de-la-Casa, J.M.; Benítez-del-Castillo, J.M.; Herrera de la Muela, M. Conjunctivitis in COVID-19 Patients: Frequency and Clinical Presentation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, C.S.; Raony, Í.; Giestal-de-Araujo, E. SARS-CoV-2 Targeting the Retina: Host–Virus Interaction and Possible Mechanisms of Viral Tropism. Ocul. Immunol. Inflamm. 2020, 28, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, L.E.; Binkhorst, M.; Bourgonje, A.R.; Offringa, A.K.; Mulder, D.J.; Bos, E.M.; Kolundzic, N.; Abdulle, A.E.; van der Voort, P.H.; Olde Rikkert, M.G.; et al. COVID-19: Immunopathology, Pathophysiological Mechanisms, and Treatment Options. J. Pathol. 2021, 254, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ge, Y.; Sun, J. IL-33 in COVID-19: Friend or Foe? Cell. Mol. Immunol. 2021, 18, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Cron, R.Q. The Genetics of Macrophage Activation Syndrome. Genes Immun. 2020, 21, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hooks, J.J.; Percopo, C.; Wang, Y.; Detrick, B. Retina and Retinal Pigment Epithelial Cell Autoantibodies Are Produced during Murine Coronavirus Retinopathy. J. Immunol. 1993, 151, 3381–3389. [Google Scholar] [CrossRef]
- Hooper, L.C.; Chin, M.S.; Detrick, B.; Hooks, J.J. Retinal Degeneration in Experimental Coronavirus Retinopathy (ECOR) Is Associated with Increased TNF-α, Soluble TNFR2 and Altered TNF-α Signaling. J. Neuroimmunol. 2005, 166, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Moriki, T.; Igari, A.; Matsubara, Y.; Ohnishi, T.; Hosokawa, K.; Murata, M. Studies of a Microchip Flow-Chamber System to Characterize Whole Blood Thrombogenicity in Healthy Individuals. Thromb. Res. 2013, 132, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Levy, J.H.; Iba, T. The Influence of Hyperglycemia on Neutrophil Extracellular Trap Formation and Endothelial Glycocalyx Damage in a Mouse Model of Type 2 Diabetes. Microcirculation 2020, 27, e12617. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High Risk of Thrombosis in Patients with Severe SARS-CoV-2 Infection: A Multicenter Prospective Cohort Study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Swinnen, T.; Kampmann, B.; Parker, E.P.K. An Interactive Website Tracking COVID-19 Vaccine Development. Lancet Glob. Health 2021, 9, e590–e592. [Google Scholar] [CrossRef] [PubMed]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA 2021, 325, 1575. [Google Scholar] [CrossRef] [PubMed]
- The Vaccine Adverse Event Reporting System (VAERS) about. Available online: https://wonder.cdc.gov/controller/datarequest/D8 (accessed on 13 May 2024).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and Reactogenic Efficacy of Covaxin and Covishield: A Comparative Review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef]
- Soheili, M.; Khateri, S.; Moradpour, F.; Mohammadzedeh, P.; Zareie, M.; Mortazavi, S.M.M.; Manifar, S.; Kohan, H.G.; Moradi, Y. The Efficacy and Effectiveness of COVID-19 Vaccines around the World: A Mini-Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 Vaccine Efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.-Y.; Lu, Q.-B.; Cui, F. Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Protection against SARS-CoV-2 Related Disease. Vaccines 2022, 10, 920. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Zhang, X.; Li, J.; Zhu, F. CoronaVac: A Review of Efficacy, Safety, and Immunogenicity of the Inactivated Vaccine against SARS-CoV-2. Hum. Vaccines Immunother. 2022, 18, 2096970. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.; Kalita, C.; Neog, N.; Goswami, C.; Sarma, M.K.; Hazarika, I. A Comparative Analysis on the Safety and Efficacy of Covaxin versus Other Vaccines against COVID-19: A Review. Z. Naturforschung C 2022, 77, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Fujio, K.; Sung, J.; Nakatani, S.; Yamamoto, K.; Iwagami, M.; Fujimoto, K.; Shokirova, H.; Okumura, Y.; Akasaki, Y.; Nagino, K.; et al. Characteristics and Clinical Ocular Manifestations in Patients with Acute Corneal Graft Rejection after Receiving the COVID-19 Vaccine: A Systematic Review. J. Clin. Med. 2022, 11, 4500. [Google Scholar] [CrossRef] [PubMed]
- Wasser, L.M.; Roditi, E.; Zadok, D.; Berkowitz, L.; Weill, Y. Keratoplasty Rejection After the BNT162b2 Messenger RNA Vaccine. Cornea 2021, 40, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, T.R.J.; Jørgensen, C.; Kyhn, M.C.; Cabrerizo, J. Acute Bilateral Descemet Membrane Endothelial Keratoplasty Graft Rejection After the BNT162b2 MRNA COVID-19 Vaccine. Int. Med. Case Rep. J. 2022, 15, 201–204. [Google Scholar] [CrossRef]
- Crnej, A.; Khoueir, Z.; Cherfan, G.; Saad, A. Acute Corneal Endothelial Graft Rejection Following COVID-19 Vaccination. J. Fr. Ophtalmol. 2021, 44, e445–e447. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.I.; Ting, D.S.J.; Said, D.G.; Dua, H.S. Corneal Graft Rejection Following COVID-19 Vaccine. Eye 2022, 36, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Molero-Senosiain, M.; Houben, I.; Savant, S.; Savant, V. Five Cases of Corneal Graft Rejection after Recent COVID-19 Vaccinations and a Review of the Literature. Cornea 2022, 41, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Phylactou, M.; Li, J.-P.O.; Larkin, D.F.P. Characteristics of Endothelial Corneal Transplant Rejection Following Immunisation with SARS-CoV-2 Messenger RNA Vaccine. Br. J. Ophthalmol. 2021, 105, 893–896. [Google Scholar] [CrossRef]
- Renisi, G.; Lombardi, A.; Stanzione, M.; Invernizzi, A.; Bandera, A.; Gori, A. Anterior Uveitis Onset after Bnt162b2 Vaccination: Is This Just a Coincidence? Int. J. Infect. Dis. 2021, 110, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, A.-W.; Razok, A.; Al-Allaf, Y.; Aker, L. Post-COVID-19 Vaccine Medium-Vessel Vasculitis and Acute Anterior Uveitis, Causation vs. Temporal Relation; Case Report and Literature Review. Ann. Med. Surg. 2022, 75. [Google Scholar] [CrossRef]
- Ortiz-Egea, J.M.; Sánchez, C.G.; López-Jiménez, A.; Navarro, O.D. Herpetic Anterior Uveitis Following Pfizer–BioNTech Coronavirus Disease 2019 Vaccine: Two Case Reports. J. Med. Case Rep. 2022, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Seo, M.-H.; Choi, K.-E.; Lee, S.; Choi, B.; Yun, C.; Kim, S.-W.; Kim, Y.Y. Vision-Threatening Ocular Adverse Events after Vaccination against Coronavirus Disease 2019. J. Clin. Med. 2022, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Mudie, L.I.; Zick, J.D.; Dacey, M.S.; Palestine, A.G. Panuveitis Following Vaccination for COVID-19. Ocul. Immunol. Inflamm. 2021, 29, 741–742. [Google Scholar] [CrossRef] [PubMed]
- van Dam, C.S.; Lede, I.; Schaar, J.; Al-Dulaimy, M.; Rösken, R.; Smits, M. Herpes Zoster after COVID Vaccination. Int. J. Infect. Dis. 2021, 111, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, P.; Chicharro, P.; Cabrera, L.-M.; Seguí, M.; Morales-Caballero, Á.; Llamas-Velasco, M.; Sánchez-Pérez, J. Varicella-Zoster Virus Reactivation after SARS-CoV-2 BNT162b2 MRNA Vaccination: Report of 5 Cases. JAAD Case Rep. 2021, 12, 58–59. [Google Scholar] [CrossRef]
- Aksu, S.B.; Öztürk, G.Z. A Rare Case of Shingles after COVID-19 Vaccine: Is It a Possible Adverse Effect? Clin. Exp. Vaccine Res. 2021, 10, 198. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Mambretti, M.; Huemer, J.; Torregrossa, G.; Ullrich, M.; Findl, O.; Casalino, G. Acute Macular Neuroretinopathy Following Coronavirus Disease 2019 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Book, B.A.J.; Schmidt, B.; Foerster, A.M.H. Bilateral Acute Macular Neuroretinopathy After Vaccination Against SARS-CoV-2. JAMA Ophthalmol. 2021, 139, e212471. [Google Scholar] [CrossRef]
- Bøhler, A.D.; Strøm, M.E.; Sandvig, K.U.; Moe, M.C.; Jørstad, Ø.K. Acute Macular Neuroretinopathy Following COVID-19 Vaccination. Eye 2022, 36, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Subramony, R.; Lin, L.C.; Knight, D.K.; Aminlari, A.; Belovarski, I. Bilateral Retinal Detachments in a Healthy 22-Year-Old Woman After Moderna SARS-CoV-2 Vaccination. J. Emerg. Med. 2021, 61, e146–e150. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, C.; Gómez-Figueroa, E.; Alban, L.; Arias-Cárdenas, A. Optic Neuritis after COVID-19 Vaccine Application. Clin. Exp. Neuroimmunol. 2022, 13, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nagasato, D.; Nakakura, S.; Nagasawa, T.; Wakuda, H.; Kurusu, A.; Mitamura, Y.; Tabuchi, H. Branch Retinal Vein Occlusion Post Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination. Taiwan J. Ophthalmol. 2022, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Cheng, S.S.H.; Sharma, A.; Moloney, T.P. Retinal Vein Occlusion Following COVID-19 Vaccination. Clin. Exp. Ophthalmol. 2022, 50, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, N.; Yadav, D.; Kota, A.; Singh, H. Central Retinal Vein Occlusion Post-COVID-19 Vaccination. Indian J. Ophthalmol. 2022, 70, 308. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.S.C.; Finamor, L.P.S.; Andrade, G.C.; Lima, L.H.; Zett, C.; Muccioli, C.; Sarraff, E.P.; Marinho, P.M.; Peruchi, J.; Oliveira, R.D.d.L.; et al. Vascular Retinal Findings after COVID-19 Vaccination in 11 Cases: A Coincidence or Consequence? Arq. Bras. Oftalmol. 2022, 85, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nagasato, D.; Nakakura, S.; Tanabe, H.; Nagasawa, T.; Wakuda, H.; Imada, Y.; Mitamura, Y.; Tabuchi, H. Exacerbation of Branch Retinal Vein Occlusion Post SARS-CoV2 Vaccination. Medicine 2021, 100, e28236. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Li, J.; Parmar, U.P.S.; Jeng, B.H.; Jhanji, V. Vaccine-Associated Corneal Graft Rejection Following SARS-CoV-2 Vaccination: A CDC-VAERS Database Analysis. Br. J. Ophthalmol. 2024, 108, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ritterband, D.C.; Mehta, I. Acute Corneal Transplant Rejection After Severe Acute Respiratory Syndrome Coronavirus 2 MRNA-1273 Vaccination. Cornea 2022, 41, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Kahale, F.; Agarwal, A.; Tsui, E. Vaccine-Associated Uveitis after COVID-19 Vaccination. Ophthalmology 2023, 130, 179–186. [Google Scholar] [CrossRef]
- Cafiero, C.; Re, A.; Micera, A.; Palmirotta, R.; Monaco, D.; Romano, F.; Fabrizio, C.; Di Francia, R.; Cacciamani, A.; Surico, P.L.; et al. Pharmacogenomics and Pharmacogenetics: In Silico Prediction of Drug Effects in Treatments for Novel Coronavirus SARS-CoV-2 Disease. Pharmacogenom. Pers. Med. 2020, 13, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Wu, H.; Cheng, K.; Chang, Y. Disc Edema in One Eye and Central Serous Chorioretinopathy in the Other Eye Shortly after AstraZeneca COVID-19 Vaccination. Kaohsiung J. Med. Sci. 2022, 38, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Gupta, R.; Garcia, A.J.V.; Cho, W.; Singh, K.P.; Agarwal, A. Retinal Vascular Occlusion after Severe Acute Respiratory Syndrome Coronavirus Vaccination. Ophthalmol. Sci. 2024, 4, 100354. [Google Scholar] [CrossRef] [PubMed]
- Pawar, N.; Ravindran, M.; Padmavathy, S.; Chakrabarty, S. Acute Abducens Nerve Palsy after COVID-19 Vaccination in a Young Adult. Indian J. Ophthalmol. 2021, 69, 3764. [Google Scholar] [CrossRef]
- Colella, G.; Orlandi, M.; Cirillo, N. Bell’s Palsy Following COVID-19 Vaccination. J. Neurol. 2021, 268, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, T.J. Thyroid Eye Disease Following COVID-19 Vaccine in a Patient With a History Graves’ Disease: A Case Report. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e221–e223. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Ichhpujani, P.; Jeng, B.H.; Jhanji, V. Herpetic Eye Disease After SARS-CoV-2 Vaccination: A CDC-VAERS Database Analysis. Cornea 2023, 42, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Abičić, A.; Adamec, I.; Habek, M. Miller Fisher Syndrome Following Pfizer COVID-19 Vaccine. Neurol. Sci. 2022, 43, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Cho, W.; Ichhpujani, P. Glaucoma Cases Following SARS-CoV-2 Vaccination: A VAERS Database Analysis. Vaccines 2022, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Menacher, M.; Christ, M.; Behrens, L.; Rank, A.; Naumann, M. Bilateral Superior Ophthalmic Vein Thrombosis, Ischaemic Stroke, and Immune Thrombocytopenia after ChAdOx1 NCoV-19 Vaccination. Lancet 2021, 397, e11. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.T.; Moorthy, R.S.; Fraunfelder, F.W.; Zierhut, M. Vaccine-Associated Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, T.; Ben-Arie-Weintrob, Y.; Hareuveni-Blum, T.; Shaer, B.; Vishnevskia-Dai, V.; Shulman, S.; Newman, H.; Biadsy, M.; Masarwa, D.; Fischer, N.; et al. Uveitis after the BNT162b2 mRNA Vaccination against SARS-CoV-2 Infection. Retina 2021, 41, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.A.; Solyman, O.; Abushanab, M.M.; Abo Obaia, A.S.; Elhusseiny, A.M. Ocular Complications Following Vaccination for COVID-19: A One-Year Retrospective. Vaccines 2022, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Sawant, O.B.; Singh, S.; Wright, R.E.; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in Human Post-Mortem Ocular Tissues. Ocul. Surf. 2021, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Sheth, J.; Narayanan, R.; Goyal, J.; Goyal, V. Retinal Vein Occlusion in COVID-19: A Novel Entity. Indian J. Ophthalmol. 2020, 68, 2291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).