Peritumoral Adipose Tissue Features Derived from [18F]fluoro-2-deoxy-2-d-glucose Positron Emission Tomography/Computed Tomography as Predictors for Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Response Assessment
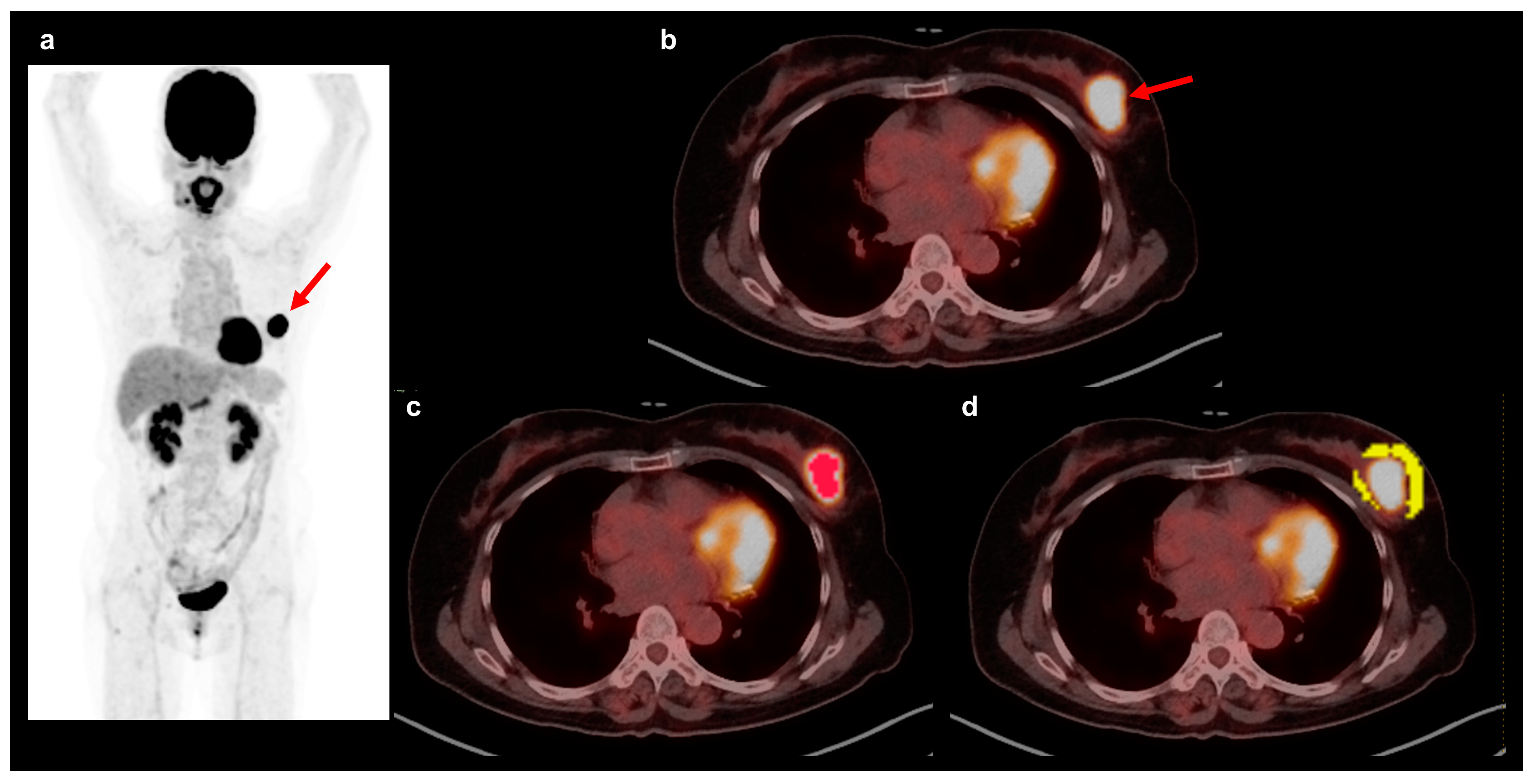
2.3. FDG PET/CT and Textural Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Pathological Outcomes
3.2. PET/CT Textural Features and Molecular Subtypes
3.3. PET/CT Textural Features and Pathological Response
3.4. Survival Analysis for PFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, X.E.; Tian, J.H.; Yang, X.J.; Wang, Y.F.; Yang, K.H. Survival benefit of neoadjuvant chemotherapy for resectable breast cancer: A meta-analysis. Medicine 2018, 97, e10634. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- van der Hage, J.A.; van de Velde, C.J.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef]
- Prowell, T.M.; Pazdur, R. Pathological complete response and accelerated drug approval in early breast cancer. N. Engl. J. Med. 2012, 366, 2438–2441. [Google Scholar] [CrossRef]
- Kong, X.; Moran, M.S.; Zhang, N.; Haffty, B.; Yang, Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur. J. Cancer. 2011, 47, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Oliveira, C.; Oliveira, F.; Vaz, S.C.; Marques, H.P.; Cardoso, F. Prediction of pathological response after neoadjuvant chemotherapy using baseline FDG PET heterogeneity features in breast cancer. Br. J. Radiol. 2023, 96, 20220655. [Google Scholar] [CrossRef]
- Pesapane, F.; Agazzi, G.M.; Rotili, A.; Ferrari, F.; Cardillo, A.; Penco, S.; Dominelli, V.; D’Ecclesiis, O.; Vignati, S.; Raimondi, S.; et al. Prediction of the pathological response to neoadjuvant chemotherapy in breast cancer patients with MRI-radiomics: A systematic review and meta-analysis. Curr. Probl. Cancer 2022, 46, 100883. [Google Scholar] [CrossRef]
- Kong, E.; Choi, J. The new perspective of PET/CT for axillary nodal staging in early breast cancer patients according to ACOSOG Z0011 trial PET/CT axillary staging according to Z0011. Nucl. Med. Commun. 2021, 42, 1369–1374. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Lee, H.J.; Heo, N.H.; Lee, S.M. [(18)F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020, 10, 72. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, Y.; Chung, J.; Kim, B.S. Predicting neo-adjuvant chemotherapy response and progression-free survival of locally advanced breast cancer using textural features of intratumoral heterogeneity on F-18 FDG PET/CT and diffusion-weighted MR imaging. Breast J. 2019, 25, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.P.; Choi, J.Y.; Choi, J.H.; Cho, Y.S.; Hur, S.M.; Kim, Z.; Lim, C.W.; Seo, S.; Moon, J.E.; Woo, S.K.; et al. Prognostic value of axillary lymph node texture parameters measured by pretreatment (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in locally advanced breast cancer with neoadjuvant chemotherapy. Diagnostics 2022, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Shen, G.; Deng, Y.; Diao, W.; Jia, Z. The accuracy of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: A meta-analysis and systematic review. Eur. Radiol. 2017, 27, 4786–4796. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Kreis, N.N.; Hoock, S.C.; Solbach, C.; Louwen, F.; Yuan, J. Adipose tissue-derived mesenchymal stromal/stem cells, obesity and the tumor microenvironment of breast cancer. Cancers 2022, 14, 3908. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.N.; Roth, S.; Friemel, A.; Safdar, B.K.; Hoock, S.C.; Wildner, J.M.; Allert, R.; Louwen, F.; Solbach, C.; et al. Cancer-educated mammary adipose tissue-derived stromal/stem cells in obesity and breast cancer: Spatial regulation and function. J. Exp. Clin. Cancer Res. 2023, 42, 35. [Google Scholar] [CrossRef]
- Rybinska, I.; Mangano, N.; Tagliabue, E.; Triulzi, T. Cancer-associated adipocytes in breast cancer: Causes and consequences. Int. J. Mol. Sci. 2021, 22, 3775. [Google Scholar] [CrossRef]
- Kalezic, A.; Udicki, M.; Srdic Galic, B.; Aleksic, M.; Korac, A.; Jankovic, A.; Korac, B. Tissue-specific warburg effect in breast cancer and cancer-associated adipose tissue-relationship between AMPK and glycolysis. Cancers 2021, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Hong, S.H.; Lee, S.M.; Jo, I.Y. Clinical significance of peritumoral adipose tissue PET/CT imaging features for predicting axillary lymph node metastasis in patients with breast cancer. J. Pers. Med. 2021, 11, 1029. [Google Scholar] [CrossRef]
- Ahn, H.; Won Lee, J.; Jang, S.H.; Ju Lee, H.; Lee, J.H.; Oh, M.H.; Mi Lee, S. Prognostic significance of imaging features of peritumoral adipose tissue in FDG PET/CT of patients with colorectal cancer. Eur. J. Radiol. 2021, 145, 110047. [Google Scholar] [CrossRef]
- Ahn, H.; Song, G.J.; Jang, S.H.; Son, M.W.; Lee, H.J.; Lee, M.S.; Lee, J.H.; Oh, M.H.; Jeong, G.C.; Yun, J.H.; et al. Predicting the recurrence of gastric cancer using the textural features of perigastric adipose tissue on [(18)F]FDG PET/CT. Int. J. Mol. Sci. 2022, 23, 11985. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, C.; Bourhis, D.; Orlhac, F.; Robin, P.; Corre, J.B.; Delcroix, O.; Gobel, Y.; Schick, U.; Salaün, P.Y.; Abgral, R. Inter-observer and segmentation method variability of textural analysis in pre-therapeutic FDG PET/CT in head and neck cancer. PLoS ONE 2019, 14, e0214299. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.H.; Lee, H.S.; Cho, E.S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Park, C.; Yeu, Y.; Clemenceau, J.R.; et al. Radiomics features of (18)F-fluorodeoxyglucose positron-emission tomography as a novel prognostic signature in colorectal cancer. Cancers 2021, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef]
- Verras, G.I.; Tchabashvili, L.; Chlorogiannis, D.D.; Mulita, F.; Argentou, M.I. Updated clinical evidence on the role of adipokines and breast cancer: A review. Cancers 2023, 15, 1572. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Quiroga, D.; Jalilvand, A.; Torres Matias, G.S.; Wright, V.P.; Liu, J.; Yu, L.; Bradley, D.; Hsueh, W.A.; Carson, W.E., 3rd. Characterization of inflammatory changes in the breast cancer associated adipose tissue and comparison to the unaffected contralateral breast. Surg. Oncol. 2021, 39, 101659. [Google Scholar] [CrossRef]
- Zoico, E.; Rizzatti, V.; Darra, E.; Budui, S.L.; Franceschetti, G.; Vinante, F.; Pedrazzani, C.; Guglielmi, A.; De Manzoni, G.; Mazzali, G.; et al. Morphological and functional changes in the peritumoral adipose tissue of colorectal cancer patients. Obesity 2017, 25 (Suppl. 2), S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, S.; Li, J.J.; Yuan, J.P.; Sun, S.R.; Wu, Q. Adipose tissue macrophages: Implications for obesity-associated cancer. Mil. Med. Res. 2023, 10, 1. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.H.; Ahn, H.; Lee, S.M.; Jang, S.J. Predicting survival in patients with pancreatic cancer by integrating bone marrow FDG uptake and radiomic features of primary tumor in PET/CT. Cancers 2021, 13, 3563. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, X.; Xiao, F.; Zhang, H.; Li, J.; Liao, M. Magnetic resonance imaging texture analysis in differentiating benign and malignant breast lesions of breast imaging reporting and data system 4: A Preliminary study. J. Comput. Assist. Tomogr. 2020, 44, 83–89. [Google Scholar] [CrossRef]
- Wu, C.; Dong, S.; Huang, R.; Chen, X. Cancer-associated adipocytes and breast cancer: Intertwining in the tumor microenvironment and challenges for cancer therapy. Cancers 2023, 15, 726. [Google Scholar] [CrossRef] [PubMed]
- Annovazzi, A.; Ferraresi, V.; Covello, R.; Ascione, A.; Vari, S.; Petrongari, M.G.; Baldi, J.; Biagini, R.; Sciuto, R. Prognostic value of pre-treatment [18F]FDG PET/CT texture analysis in undifferentiated soft-tissue sarcoma. J. Clin. Med. 2022, 12, 279. [Google Scholar] [CrossRef]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The value of semiquantitative parameters derived from (18)F-FDG PET/CT for predicting response to neoadjuvant chemotherapy in a cohort of patients with different molecular subtypes of breast cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, R.; Qian, K.; Lu, J.; Zhang, Y. Clinical and radiomic factors for predicting invasiveness in pulmonary ground-glass opacity. Exp. Ther. Med. 2022, 24, 685. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Han, J. Analysis of tumor microenvironment heterogeneity among breast cancer subtypes to identify subtype-specific signatures. Genes 2022, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
| Characteristics | Number of Patients (Percentage) n= 147 |
|---|---|
| Median age, years (range) | 47 (23–79) |
| Obesity | |
| Underweight/normal | 56 (38.1%) |
| Overweight/obesity | 91 (61.9%) |
| Menopausal status | |
| Premenopausal | 80 (54.4%) |
| Postmenopausal | 67 (45.6%) |
| Histopathology | |
| Invasive ductal carcinoma | 141 (95.9%) |
| Others | 6 (4.1%) |
| Histologic grade | |
| Grade 1 | 15 (10.2%) |
| Grade 2 | 79 (53.7%) |
| Grade 3 | 49 (33.3%) |
| Not specified | 4 (2.7%) |
| Molecular subtypes | |
| Luminal A | 28 (19.0%) |
| Luminal B-like HER2-negative | 19 (12.9%) |
| Luminal B-like HER2-positive | 60 (40.8%) |
| HER2-enriched | 21 (14.3%) |
| Triple-negative | 19 (12.9%) |
| Clinical T stage | |
| T1–T2 | 90 (61.2%) |
| T3–T4 | 57 (38.8%) |
| Clinical N stage | |
| N0 | 11 (7.5%) |
| N1 | 63 (42.9%) |
| N2–N3 | 73 (49.7%) |
| Clinical TNM stage | |
| Stage II | 40 (27.2%) |
| Stage III | 107 (72.8%) |
| Neoadjuvant chemotherapy regimen | |
| Doxorubicin and docetaxel | 52 (35.4%) |
| Doxorubicin, cyclophosphamide, and docetaxel | 40 (27.2%) |
| Docetaxel, carboplatin, trastuzumab, and pertuzumab | 25 (17.0%) |
| Doxorubicin and cyclophosphamide | 22 (15.0%) |
| Doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab | 8 (5.4%) |
| Parameters | Luminal A | Luminal B-like HER2 Negative | Luminal B-like HER2 Positive | HER2-Enriched | Triple Negative | p-Value |
|---|---|---|---|---|---|---|
| Primary tumor | ||||||
| Maximum SUV | 7.79 (5.58–11.66) | 8.17 (6.26–9.56) | 10.04 (5.19–14.75) | 14.02 (10.82–18.36) | 13.01 (9.51–18.13) | <0.001 |
| MTV | 6.76 (3.61–10.95) | 10.47 (5.25–21.84) | 8.45 (3.87–18.85) | 9.06 (5.39–14.63) | 8.79 (4.61–13.72) | 0.633 |
| TLG | 27.64 (19.13–75.96) | 68.48 (20.07–93.17) | 39.46 (20.67–82.19) | 75.82 (52.18–144.57) | 79.93 (31.42–134.41) | 0.039 |
| Peritumoral AT | ||||||
| First-order features | ||||||
| Maximum SUV | 2.44 (2.22–2.80) | 2.43 (2.26–2.58) | 2.53 (2.10–2.86) | 2.47 (2.23–2.83) | 2.52 (2.36–2.96) | 0.440 |
| Mean SUV | 0.74 (0.66–0.89) | 0.78 (0.64–0.86) | 0.80 (0.69–0.90) | 0.78 (0.69–0.87) | 0.79 (0.70–0.97) | 0.770 |
| Standard deviation SUV | 0.35 (0.34–0.39) | 0.38 (0.34–0.43) | 0.37 (0.30–0.43) | 0.35 (0.30–0.45) | 0.37 (0.34–0.48) | 0.366 |
| 25th percentile SUV | 0.46 (0.43–0.58) | 0.46 (0.38–0.58) | 0.52 (0.43–0.62) | 0.54 (0.44–0.63) | 0.49 (0.44–0.70) | 0.473 |
| 50th percentile SUV | 0.66 (0.57–0.79) | 0.62 (0.58–0.75) | 0.70 (0.60–0.80) | 0.71 (0.60–0.78) | 0.81 (0.60–0.89) | 0.368 |
| 75th percentile SUV | 0.93 (0.82–1.09) | 0.99 (0.78–1.09) | 0.97 (0.83–1.12) | 0.92 (0.82–1.07) | 0.95 (0.83–1.23) | 0.927 |
| SUV histogram kurtosis | 4.32 (3.84–5.74) | 4.10 (3.61–4.65) | 4.93 (4.06–5.94) | 5.27 (4.40–6.26) | 5.59 (3.92–6.84) | 0.071 |
| SUV histogram skewness | 1.14 (0.92–1.30) | 1.05 (0.91–1.18) | 1.21 (0.96–1.47) | 1.27 (0.94–1.47) | 1.37 (0.98–1.67) | 0.294 |
| SUV histogram energy | 0.29 (0.25–0.31) | 0.27 (0.25–0.30) | 0.29 (0.26–0.34) | 0.29 (0.25–0.35) | 0.28 (0.22–0.32) | 0.629 |
| SUV histogram entropy | 2.08 (2.03–2.22) | 2.17 (2.05–2.31) | 2.06 (1.88–2.25) | 2.18 (1.84–2.33) | 2.18 (2.09–2.60) | 0.078 |
| GLCM features | ||||||
| Contrast | 1.15 (0.94–1.65) | 1.25 (0.88–1.79) | 1.17 (0.85–1.56) | 1.22 (0.97–1.43) | 1.33 (0.96–2.00) | 0.808 |
| Correlation | 0.50 (0.42–0.57) | 0.51 (0.45–0.56) | 0.48 (0.35–0.57) | 0.47 (0.37–0.58) | 0.57 (0.43–0.63) | 0.519 |
| Dissimilarity | 0.70 (0.63–0.89) | 0.73 (0.57–0.94) | 0.71 (0.57–0.81) | 0.72 (0.60–0.82) | 0.74 (0.57–0.98) | 0.837 |
| Energy | 0.14 (0.11–0.16) | 0.13 (0.09–0.16) | 0.15 (0.12–0.18) | 0.13 (0.11–0.17) | 0.13 (0.09–0.19) | 0.421 |
| Entropy | 3.55 (3.16–3.81) | 3.59 (3.40–4.10) | 3.34 (3.03–3.82) | 3.61 (3.15–3.89) | 3.94 (3.41–4.13) | 0.057 |
| Homogeneity | 0.72 (0.67–0.75) | 0.70 (0.65–0.73) | 0.72 (0.69–0.76) | 0.71 (0.67–0.75) | 0.70 (0.63–0.76) | 0.554 |
| NGLDM features | ||||||
| Busyness | 2.09 (1.39–3.89) | 3.29 (2.72–4.81) | 2.80 (1.68–4.12) | 3.36 (2.44–4.29) | 2.17 (1.48–3.00) | 0.061 |
| Coarseness | 0.019 (0.011–0.039) | 0.015 (0.009–0.019) | 0.016 (0.009–0.029) | 0.011 (0.008–0.014) | 0.012 (0.009–0.015) | 0.032 |
| Contrast | 0.028 (0.020–0.035) | 0.027 (0.023–0.046) | 0.027 (0.020–0.034) | 0.023 (0.017–0.029) | 0.029 (0.022–0.039) | 0.215 |
| Parameter | Responders (n = 36) | Non-Responders (n = 111) | p-Value |
|---|---|---|---|
| Primary tumor | |||
| Maximum SUV | 11.89 (7.57–15.68) | 9.49 (6.25–14.81) | 0.151 |
| MTV | 6.56 (3.29–15.51) | 8.81 (4.29–15.21) | 0.269 |
| TLG | 51.86 (21.58–123.98) | 59.57 (20.83–91.38) | 0.650 |
| Peritumoral AT | |||
| First-order features | |||
| Maximum SUV | 2.40 (2.21–2.76) | 2.52 (2.24–2.82) | 0.270 |
| Mean SUV | 0.72 (0.64–0.82) | 0.80 (0.70–0.90) | 0.011 |
| Standard deviation SUV | 0.35 (0.31–0.40) | 0.37 (0.33–0.45) | 0.137 |
| 25th percentile SUV | 0.44 (0.40–0.56) | 0.52 (0.45–0.64) | 0.017 |
| 50th percentile SUV | 0.60 (0.54–0.71) | 0.71 (0.61–0.85) | <0.001 |
| 75th percentile SUV | 0.89 (0.78–1.00) | 0.99 (0.84–1.14) | 0.009 |
| SUV histogram kurtosis | 5.05 (4.16–6.95) | 4.68 (3.81–5.83) | 0.121 |
| SUV histogram skewness | 1.31 (1.05–1.56) | 1.17 (0.91–1.42) | 0.072 |
| SUV histogram energy | 0.30 (0.27–0.34) | 0.28 (0.25–0.32) | 0.051 |
| SUV histogram entropy | 2.02 (1.88–2.18) | 2.14 (2.01–2.35) | 0.020 |
| GLCM features | |||
| Contrast | 1.07 (0.83–1.36) | 1.28 (0.96–1.71) | 0.052 |
| Correlation | 0.49 (0.43–0.57) | 0.50 (0.38–0.58) | 0.986 |
| Dissimilarity | 0.66 (0.54–0.77) | 0.74 (0.61–0.88) | 0.028 |
| Energy | 0.14 (0.12–0.19) | 0.13 (0.10–0.17) | 0.054 |
| Entropy | 3.12 (2.94–3.65) | 3.63 (3.32–3.94) | <0.001 |
| Homogeneity | 0.75 (0.70–0.78) | 0.70 (0.66–0.74) | 0.003 |
| NGLDM features | |||
| Busyness | 3.10 (1.77–4.20) | 2.80 (1.72–4.07) | 0.604 |
| Coarseness | 0.015 (0.011–0.029) | 0.014 (0.009–0.022) | 0.439 |
| Contrast | 0.026 (0.020–0.032) | 0.027 (0.020–0.036) | 0.592 |
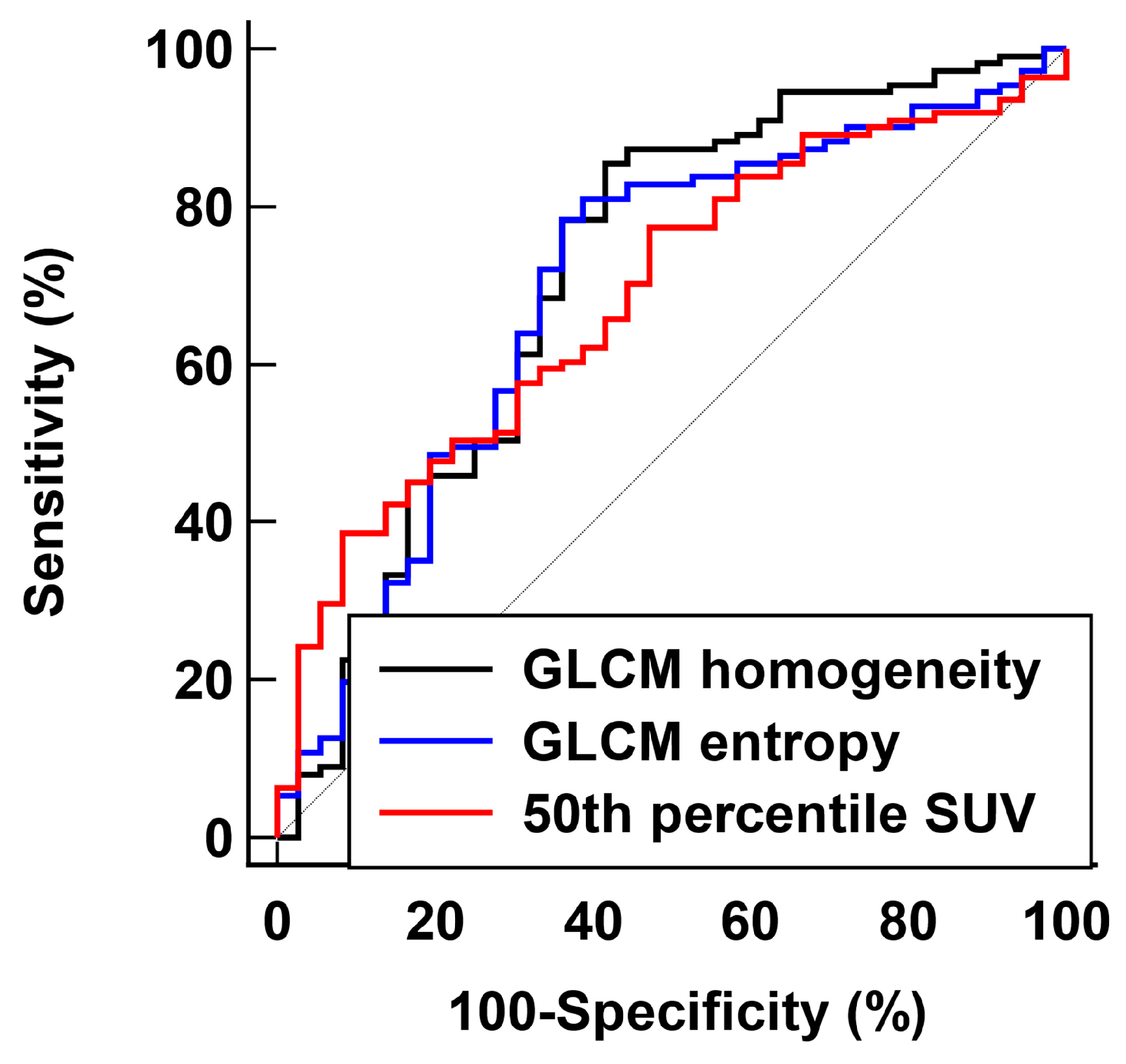
| Parameter | AUC (95% Confidence Interval) | Cut-Off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Primary tumor | ||||
| Maximum SUV | 0.580 (0.476–0.677) | 10.77 | 58.3 | 59.5 |
| MTV | 0.561 (0.454–0.669) | 3.48 | 30.6 | 84.7 |
| TLG | 0.534 (0.424–0.638) | 85.90 | 38.9 | 73.0 |
| Peritumoral AT | ||||
| First-order features | ||||
| Maximum SUV | 0.561 (0.454–0.655) | 2.45 | 61.1 | 56.8 |
| Mean SUV | 0.642 (0.531–0.731) | 0.85 | 86.1 | 39.6 |
| Standard deviation SUV | 0.583 (0.469–0.677) | 0.37 | 66.7 | 52.3 |
| 25th percentile SUV | 0.633 (0.528–0.728) | 0.45 | 52.8 | 74.8 |
| 50th percentile SUV | 0.686 (0.583–0.768) | 0.78 | 91.7 | 38.7 |
| 75th percentile SUV | 0.645 (0.535–0.731) | 1.04 | 86.1 | 41.4 |
| SUV histogram kurtosis | 0.586 (0.475–0.687) | 7.04 | 25.0 | 93.7 |
| SUV histogram skewness | 0.600 (0.942–0.702) | 1.35 | 50.0 | 69.4 |
| SUV histogram energy | 0.608 (0.501–0.706) | 0.26 | 80.6 | 39.6 |
| SUV histogram entropy | 0.653 (0.552–0.750) | 2.20 | 86.1 | 44.1 |
| GLCM features | ||||
| Contrast | 0.608 (0.486–0.707) | 1.25 | 72.2 | 52.3 |
| Correlation | 0.505 (0.400–0.608) | 0.64 | 97.2 | 12.6 |
| Dissimilarity | 0.622 (0.510–0.719) | 0.73 | 72.2 | 52.3 |
| Energy | 0.607 (0.500–0.699) | 0.11 | 86.1 | 36.0 |
| Entropy | 0.697 (0.585–0.791) | 3.24 | 63.9 | 78.4 |
| Homogeneity | 0.717 (0.600–0.811) | 0.75 | 58.3 | 85.6 |
| NGLDM features | ||||
| Busyness | 0.529 (0.419–0.630) | 2.70 | 66.7 | 47.7 |
| Coarseness | 0.543 (0.434–0.643) | 0.011 | 77.8 | 36.0 |
| Contrast | 0.530 (0.418–0.627) | 0.023 | 41.7 | 69.4 |
| Parameter | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p-Value | Odds Ratio (95% Confidence Interval) | p-Value | Odds Ratio (95% Confidence Interval) | |
| Primary tumor | ||||
| Maximum SUV | 0.355 | |||
| MTV | 0.380 | |||
| TLG | 0.385 | |||
| Peritumoral AT | ||||
| First-order features | ||||
| Maximum SUV | 0.258 | |||
| Mean SUV | 0.016 | 23.70 (1.83–307.75) | 0.012 | 26.92 (2.06–351.31) |
| Standard deviation SUV | 0.174 | |||
| 25th percentile SUV | 0.025 | 29.49 (1.54–564.71) | 0.139 | |
| 50th percentile SUV | 0.004 | 55.27 (3.70–826.21) | 0.002 | 76.37 (4.80–1215.22) |
| 75th percentile SUV | 0.015 | 12.47 (1.62–95.81) | 0.015 | 12.46 (1.64–94.70) |
| SUV histogram kurtosis | 0.074 | |||
| SUV histogram skewness | 0.059 | |||
| SUV histogram energy | 0.074 | |||
| SUV histogram entropy | 0.029 | 3.98 (1.15–13.77) | 0.034 | 4.11 (1.12–15.14) |
| GLCM features | ||||
| Contrast | 0.426 | |||
| Correlation | 0.840 | |||
| Dissimilarity | 0.174 | |||
| Energy | 0.037 | 0.02 (0.01–0.70) | 0.083 | |
| Entropy | 0.001 | 3.49 (1.65–7.37) | 0.001 | 3.67 (1.65–8.16) |
| Homogeneity | 0.001 | 0.32 (0.16–0.63) | 0.002 | 0.33 (0.16–0.66) |
| NGLDM features | ||||
| Busyness | 0.402 | |||
| Coarseness | 0.843 | |||
| Contrast | 0.668 | |||
| Parameter | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p-Value | Hazard Ratio (95% Confidence Interval) | p-Value | Hazard Ratio (95% Confidence Interval) | |
| Primary tumor | ||||
| Maximum SUV | 0.332 | |||
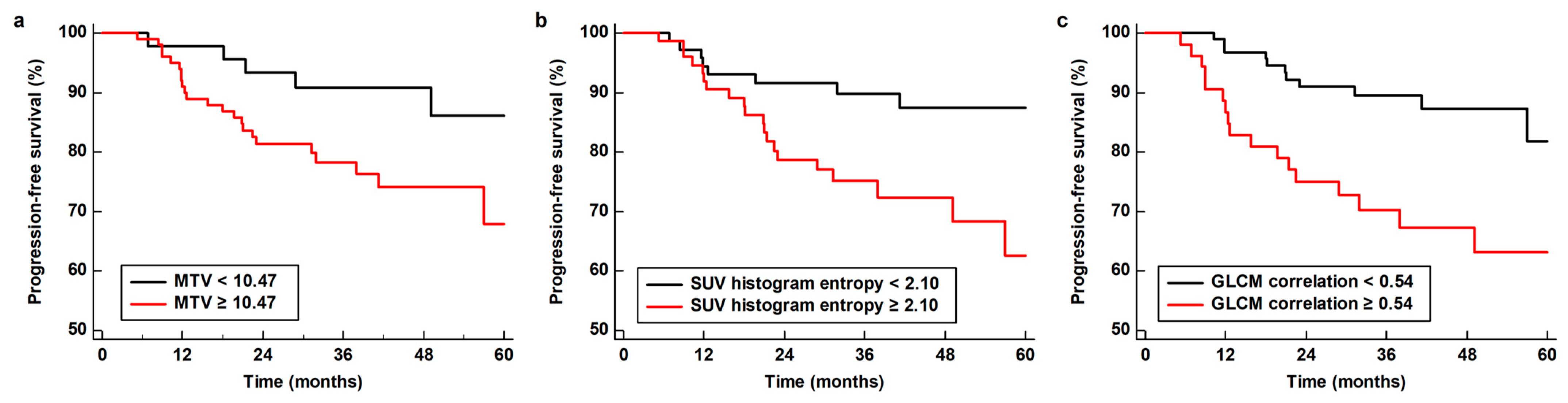
| MTV | 0.002 | 1.01 (1.01–1.02) | 0.039 | 1.01 (1.00–1.02) |
| TLG | 0.008 | 1.00 (1.00–1.01) | 0.178 | |
| Peritumoral AT | ||||
| First-order features | ||||
| Maximum SUV | 0.360 | |||
| Mean SUV | 0.238 | |||
| Standard deviation SUV | 0.600 | |||
| 25th percentile SUV | 0.310 | |||
| 50th percentile SUV | 0.022 | 8.60 (1.37–53.88) | 0.083 | |
| 75th percentile SUV | 0.224 | |||
| SUV histogram kurtosis | 0.555 | |||
| SUV histogram skewness | 0.535 | |||
| SUV histogram energy | 0.221 | |||
| SUV histogram entropy | 0.012 | 4.23 (1.38–12.93) | 0.042 | 2.71 (1.10–10.54) |
| GLCM features | ||||
| Contrast | 0.487 | |||
| Correlation | 0.019 | 38.25 (1.83–796.39) | 0.040 | 29.32 (1.17–734.56) |
| Dissimilarity | 0.542 | |||
| Energy | 0.418 | |||
| Entropy | 0.045 | 1.87 (1.01–3.46) | 0.437 | |
| Homogeneity | 0.546 | |||
| NGLDM features | ||||
| Busyness | 0.998 | |||
| Coarseness | 0.028 | 0.63 (0.42–0.95) | 0.082 | |
| Contrast | 0.378 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Won, Y.K.; Ahn, H.; Lee, J.E.; Han, S.W.; Kim, S.Y.; Jo, I.Y.; Lee, S.M. Peritumoral Adipose Tissue Features Derived from [18F]fluoro-2-deoxy-2-d-glucose Positron Emission Tomography/Computed Tomography as Predictors for Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. J. Pers. Med. 2024, 14, 952. https://doi.org/10.3390/jpm14090952
Lee JW, Won YK, Ahn H, Lee JE, Han SW, Kim SY, Jo IY, Lee SM. Peritumoral Adipose Tissue Features Derived from [18F]fluoro-2-deoxy-2-d-glucose Positron Emission Tomography/Computed Tomography as Predictors for Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. Journal of Personalized Medicine. 2024; 14(9):952. https://doi.org/10.3390/jpm14090952
Chicago/Turabian StyleLee, Jeong Won, Yong Kyun Won, Hyein Ahn, Jong Eun Lee, Sun Wook Han, Sung Yong Kim, In Young Jo, and Sang Mi Lee. 2024. "Peritumoral Adipose Tissue Features Derived from [18F]fluoro-2-deoxy-2-d-glucose Positron Emission Tomography/Computed Tomography as Predictors for Response to Neoadjuvant Chemotherapy in Breast Cancer Patients" Journal of Personalized Medicine 14, no. 9: 952. https://doi.org/10.3390/jpm14090952
APA StyleLee, J. W., Won, Y. K., Ahn, H., Lee, J. E., Han, S. W., Kim, S. Y., Jo, I. Y., & Lee, S. M. (2024). Peritumoral Adipose Tissue Features Derived from [18F]fluoro-2-deoxy-2-d-glucose Positron Emission Tomography/Computed Tomography as Predictors for Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. Journal of Personalized Medicine, 14(9), 952. https://doi.org/10.3390/jpm14090952






