Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals/Reagents
2.2. Animals and Experimental Design
2.3. Blood Sampling—Hematocrit Measurement
2.4. Measurement of EPO Levels in Blood Serum
2.5. Histochemical Staining of Hematoxylin–Eosin (H-E)
2.6. Immunohistochemical Assessments
2.7. Statistical Analysis
3. Results
3.1. Bodyweight, Hematocrit, and Erythropoietin Measurements
3.2. Histological Examination
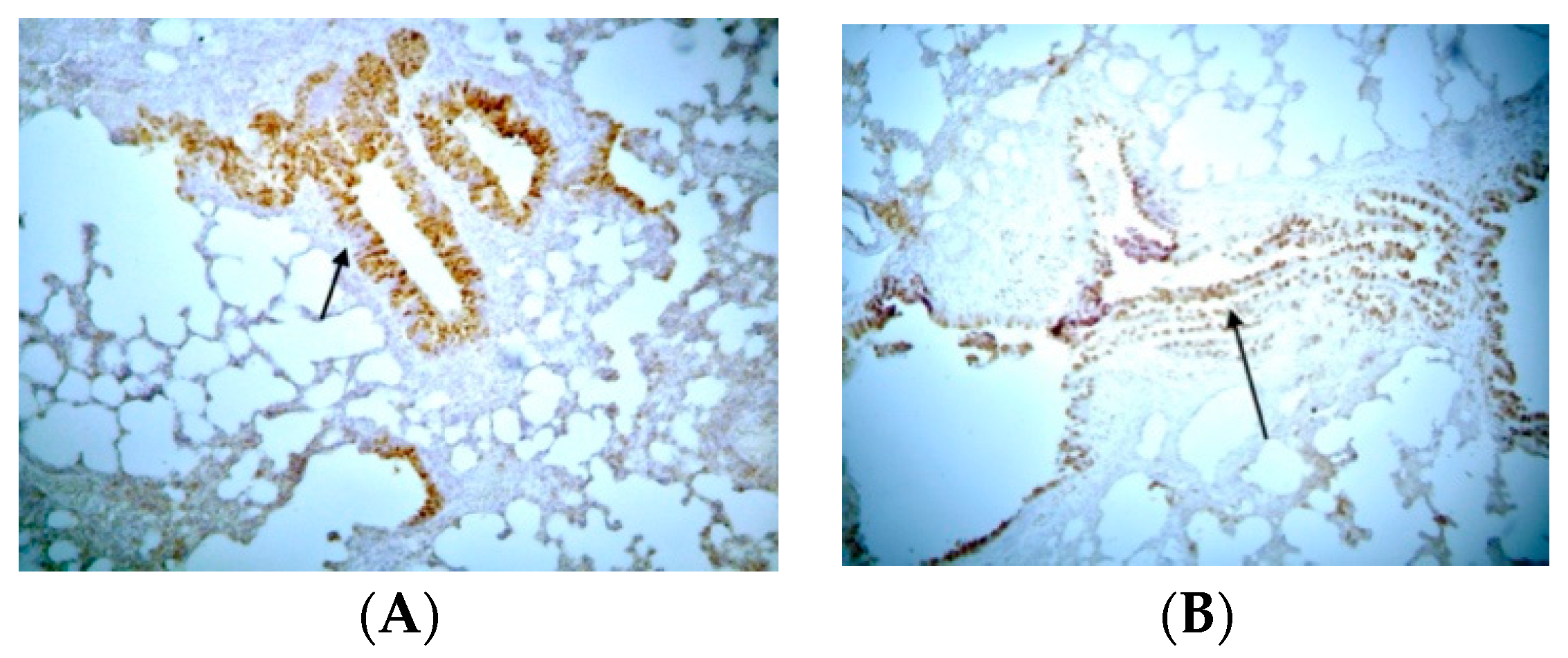
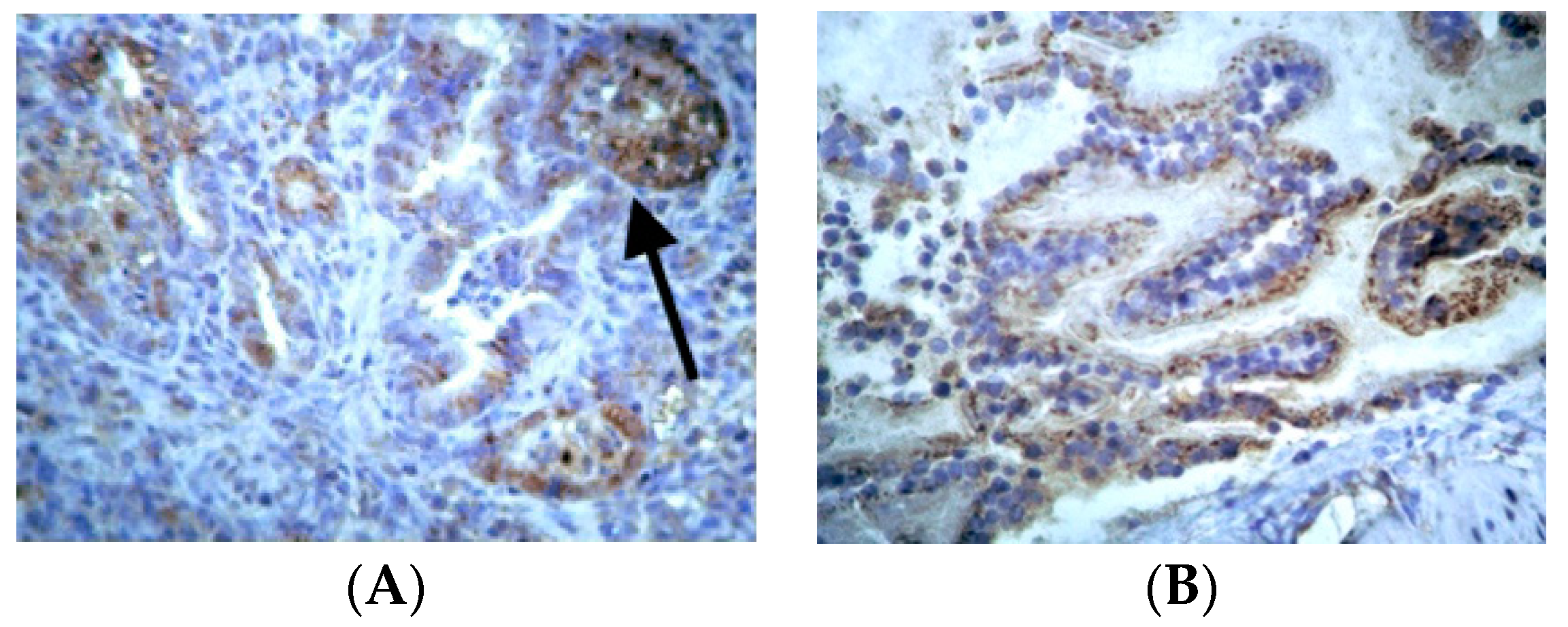
3.3. Immunohistochemical Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic pulmonary fibrosis: State of the art for 2023. Eur. Respir. J. 2023, 61, 2200957. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thusen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Hawgood, S.; Hook-Barnard, I.G.; O’Brien, T.C.; Yamamoto, K.R. Precision medicine: Beyond the inflection point. Sci. Transl. Med. 2015, 7, 300ps17. [Google Scholar] [CrossRef]
- Shang, Y.; Jiang, Y.X.; Xu, S.P.; Wu, Y.; Wu, Z.Y.; Yuan, S.Y.; Yao, S.L. Reduction of pulmonary inflammatory response by erythropoietin in a rat model of endotoxaemia. Chin. Med. J. 2009, 122, 834–838. [Google Scholar]
- Spandou, E.; Papadopoulou, Z.; Soubasi, V.; Karkavelas, G.; Simeonidou, C.; Pazaiti, A.; Guiba-Tziampiri, O. Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats. Brain Res. 2005, 1045, 22–30. [Google Scholar] [CrossRef]
- Spandou, E.; Tsouchnikas, I.; Karkavelas, G.; Dounousi, E.; Simeonidou, C.; Guiba-Tziampiri, O.; Tsakiris, D. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol. Dial Transplant. 2006, 21, 330–336. [Google Scholar] [CrossRef]
- Nishida, A.; Nishida, M.; Iehara, T. Delayed treatment with erythropoietin attenuates renal fibrosis in mouse model of unilateral ureteral obstruction. Int. J. Urol. 2024, 31, 685–692. [Google Scholar] [CrossRef]
- Elbaset, M.A.; Mohamed, B.M.S.A.; Moustafa, P.E.; Mansour, D.F.; Afifi, S.M.; Esatbeyoglu, T.; Abdelrahman, S.S.M.; Fayed, H.M. Erythropoietin Suppresses the Hepatic Fibrosis Caused by Thioacetamide: Role of the PI3K/Akt and TLR4 Signaling Pathways. Oxid. Med. Cell. Longev. 2023, 2023, 5514248. [Google Scholar] [CrossRef]
- Haine, L.; Yegen, C.H.; Marchant, D.; Richalet, J.P.; Boncoeur, E.; Voituron, N. Cytoprotective effects of erythropoietin: What about the lung? Biomed. Pharmacother. 2021, 139, 111547. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z. New avenues of exploration for erythropoietin. JAMA 2005, 293, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, G.; Liu, H.; Xu, B.; Li, D.; Jing, H. Erythropoietin attenuates ischemia-reperfusion induced lung injury by inhibiting tumor necrosis factor-alpha and matrix metalloproteinase-9 expression. Eur. J. Pharmacol. 2009, 602, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Xue, X.D. Anti-inflammatory effects of erythropoietin on hyperoxia-induced bronchopulmonary dysplasia in newborn rats. Zhonghua Er Ke Za Zhi 2009, 47, 446–451. [Google Scholar] [PubMed]
- Zheng, D.H.; Han, Z.Q.; Wang, X.X.; Ma, D.; Zhang, J. Erythropoietin attenuates high glucose-induced oxidative stress and inhibition of osteogenic differentiation in periodontal ligament stem cell (PDLSCs). Chem. Biol. Interact. 2019, 305, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Banaei, S.; Ahmadiasl, N.; Alihemmati, A. Combination Anti-Apoptotic Effect of Erythropoietin and Melatonin on Ischemia Reperfusion-Induced Renal Injury in Rats. Acta Med. Iran. 2016, 54, 624–630. [Google Scholar]
- Tascilar, O.; Cakmak, G.K.; Tekin, I.O.; Emre, A.U.; Ucan, B.H.; Bahadir, B.; Acikgoz, S.; Irkorucu, O.; Karakaya, K.; Balbaloglu, H.; et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J. Gastroenterol. 2007, 13, 6172–6182. [Google Scholar] [CrossRef]
- Portillo, K.; Martinez-Rivera, C.; Ruiz-Manzano, J. Anaemia in chronic obstructive pulmonary disease. Does it really matter? Int. J. Clin. Pract. 2013, 67, 558–565. [Google Scholar] [CrossRef]
- Bajo, M.A.; Selgas, R.; Castro, M.J.; Jimenez, C.; Fernandez-Reyes, M.J.; Del Peso, G.; De Alvaro, F.; Sanchez-Sicilia, L. Erythropoietin treatment decreases cardiovascular morbidity and mortality in CAPD patients. Perit. Dial. Int. 1997, 17, 129–135. [Google Scholar] [CrossRef]
- Tsakiris, D. Morbidity and mortality reduction associated with the use of erythropoietin. Nephron 2000, 85 (Suppl. S1), 2–8. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Al-Saedi, H.F.S.; Mohammed, A.Q.; Amir, A.A.; Radi, U.K.; Sattar, R.; Ahmad, I.; Ramadan, M.F.; Alshahrani, M.Y.; Balasim, H.M.; et al. Mechanisms of Bleomycin-induced Lung Fibrosis: A Review of Therapeutic Targets and Approaches. Cell Biochem. Biophys. 2024. [Google Scholar] [CrossRef] [PubMed]
- Barbayianni, I.; Kanellopoulou, P.; Fanidis, D.; Nastos, D.; Ntouskou, E.D.; Galaris, A.; Harokopos, V.; Hatzis, P.; Tsitoura, E.; Homer, R.; et al. SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis. Nat. Commun. 2023, 14, 5882. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.A.; Belalcazar, V.; de Bolos, C.; Gutierrez, R.; Llop, E.; Segura, J. Recombinant erythropoietin and analogues: A challenge for doping control. Ther. Drug Monit. 2004, 26, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.M.; Khan, K.N.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Singh, V.; Ulasov, I.; Gupta, S.; Singh, A.; Roy, V.K.; Kharwar, R.K. Idiopathic Pulmonary Fibrosis: Where do We Stand and How Far to Go? Discov. Med. 2024, 36, 22–47. [Google Scholar] [CrossRef]
- Ozer, E.A.; Kumral, A.; Ozer, E.; Yilmaz, O.; Duman, N.; Ozkal, S.; Koroglu, T.; Ozkan, H. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr. Res. 2005, 58, 38–41. [Google Scholar] [CrossRef][Green Version]
- Katz, O.; Stuible, M.; Golishevski, N.; Lifshitz, L.; Tremblay, M.L.; Gassmann, M.; Mittelman, M.; Neumann, D. Erythropoietin treatment leads to reduced blood glucose levels and body mass: Insights from murine models. J. Endocrinol. 2010, 205, 87–95. [Google Scholar] [CrossRef]
- Yoshimi, M.; Maeyama, T.; Yamada, M.; Hamada, N.; Fukumoto, J.; Kawaguchi, T.; Kuwano, K.; Nakanishi, Y. Recombinant human erythropoietin reduces epithelial cell apoptosis and attenuates bleomycin-induced pneumonitis in mice. Respirology 2008, 13, 639–645. [Google Scholar] [CrossRef]
- Segel, M.J.; Or, R.; Tzurel, A.; Lucey, E.C.; Goldstein, R.H.; Izbicki, G.; Breuer, R. All-trans-retinoic acid (ATRA) is of no benefit in bleomycin-induced lung injury. Pulm. Pharmacol. Ther. 2001, 14, 403–407. [Google Scholar] [CrossRef]
- Lippl, F.J.; Neubauer, S.; Schipfer, S.; Lichter, N.; Tufman, A.; Otto, B.; Fischer, R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity 2010, 18, 675–681. [Google Scholar] [CrossRef]
- Yasui, H.; Gabazza, E.C.; Tamaki, S.; Kobayashi, T.; Hataji, O.; Yuda, H.; Shimizu, S.; Suzuki, K.; Adachi, Y.; Taguchi, O. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am. J. Respir. Crit. Care Med. 2001, 163, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.J.; Demers, E.J.; Juul, S.E. Safety of high-dose recombinant erythropoietin in a neonatal rat model. Neonatology 2007, 91, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Martinez-Bello, V.E.; Gomez-Cabrera, M.C.; Vina, J. It is not hypoxia itself, but how you use it. Eur. J. Appl. Physiol. 2010, 109, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Arabul, M.; Gullulu, M.; Yilmaz, Y.; Eren, M.A.; Baran, B.; Gul, C.B.; Kocamaz, G.; Dilek, K. Influence of erythropoietin therapy on serum prohepcidin levels in dialysis patients. Med. Sci. Monit. 2009, 15, CR583–CR587. [Google Scholar] [PubMed]
- Tsantes, A.; Bonovas, S.; Tassiopoulos, S.; Filioussi, K.; Vlachou, A.; Meletis, J.; Papadhimitriou, S.; Vaiopoulos, G. A comparative study of the role of erythropoietin in the pathogenesis of deficient erythropoiesis in idiopathic pulmonary fibrosis as opposed to chronic obstructive pulmonary disease. Med. Sci. Monit. 2005, 11, CR177–CR181. [Google Scholar]
- Satoh, K.; Kagaya, Y.; Nakano, M.; Ito, Y.; Ohta, J.; Tada, H.; Karibe, A.; Minegishi, N.; Suzuki, N.; Yamamoto, M.; et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006, 113, 1442–1450. [Google Scholar] [CrossRef]
- Sebti, S.M.; Mignano, J.E.; Jani, J.P.; Srimatkandada, S.; Lazo, J.S. Bleomycin hydrolase: Molecular cloning, sequencing, and biochemical studies reveal membership in the cysteine proteinase family. Biochemistry 1989, 28, 6544–6548. [Google Scholar] [CrossRef]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef]
- Zhao, Y.; Lan, X.; Wang, Y.; Xu, X.; Lu, S.; Li, X.; Zhang, B.; Shi, G.; Gu, X.; Du, C.; et al. Human Endometrial Regenerative Cells Attenuate Bleomycin-Induced Pulmonary Fibrosis in Mice. Stem Cells Int. 2018, 2018, 3475137. [Google Scholar] [CrossRef]
- Peng, R.; Sridhar, S.; Tyagi, G.; Phillips, J.E.; Garrido, R.; Harris, P.; Burns, L.; Renteria, L.; Woods, J.; Chen, L.; et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: A model for “active” disease. PLoS ONE 2013, 8, e59348. [Google Scholar] [CrossRef]
- MacRedmond, R.; Singhera, G.K.; Dorscheid, D.R. Erythropoietin inhibits respiratory epithelial cell apoptosis in a model of acute lung injury. Eur. Respir. J. 2009, 33, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Sigounas, G.; Salleng, K.J.; Mehlhop, P.D.; Sigounas, D.G. Erythropoietin ameliorates chemotherapy-induced fibrosis of the lungs in a preclinical murine model. Int. J. Cancer 2008, 122, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Toumpanakis, D.; Noussia, O.; Sigala, I.; Litsiou, E.; Loverdos, K.; Zacharatos, P.; Karavana, V.; Michailidou, T.; Magkou, C.; Zhou, Z.; et al. Inspiratory resistive breathing induces MMP-9 and MMP-12 expression in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L683–L692. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, P.; Hubbs, A.; Battelli, L.; Castranova, V. Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis. J. Toxicol. Environ. Health A 2004, 67, 1001–1026. [Google Scholar] [CrossRef]
- Joo, M.; Wright, J.G.; Hu, N.N.; Sadikot, R.T.; Park, G.Y.; Blackwell, T.S.; Christman, J.W. Yin Yang 1 enhances cyclooxygenase-2 gene expression in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1219–L1226. [Google Scholar] [CrossRef]
- Choi, J.K.; Lee, S.G.; Lee, J.Y.; Nam, H.Y.; Lee, W.K.; Lee, K.H.; Kim, H.J.; Lim, Y. Silica induces human cyclooxygenase-2 gene expression through the NF-kappaB signaling pathway. J. Environ. Pathol. Toxicol. Oncol. 2005, 24, 163–174. [Google Scholar] [CrossRef]
- Azim, A.C.; Wang, X.; Park, G.Y.; Sadikot, R.T.; Cao, H.; Mathew, B.; Atchison, M.; van Breemen, R.B.; Joo, M.; Christman, J.W. NF-kappaB-inducing kinase regulates cyclooxygenase 2 gene expression in macrophages by phosphorylation of PU. 1. J. Immunol. 2007, 179, 7868–7875. [Google Scholar] [CrossRef]
- Jenkins, G.; Hart, S.L.; Hodges, R.J.; Meng, Q.H.; Kinnon, C.; Laurent, G.J.; McAnulty, R.J. Cyclooxygenase-2 overexpression, using an integrin-targeted gene delivery system (the LID vector), inhibits fibroblast proliferation in vitro and leads to increased prostaglandin E(2) in the lung. Chest 2002, 121 (Suppl. S3), 102S–104S. [Google Scholar] [CrossRef]
- Keerthisingam, C.B.; Jenkins, R.G.; Harrison, N.K.; Hernandez-Rodriguez, N.A.; Booth, H.; Laurent, G.J.; Hart, S.L.; Foster, M.L.; McAnulty, R.J. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 2001, 158, 1411–1422. [Google Scholar] [CrossRef]
- Park, G.Y.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L797–L805. [Google Scholar] [CrossRef]
- Genovese, T.; Cuzzocrea, S.; Di Paola, R.; Failla, M.; Mazzon, E.; Sortino, M.A.; Frasca, G.; Gili, E.; Crimi, N.; Caputi, A.P.; et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir. Res. 2005, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.D.; Golden, T.; Guo, C.-J.; Tu, S.P.; Scott, P.; Lee, M.-J.; Yang, C.S.; Gow, A.J. Tocopherol supplementation reduces NO production and pulmonary inflammatory response to bleomycin. Nitric Oxide 2013, 34, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Coldewey, S.M.; Patel, N.S.; Rogazzo, M.; Collino, M.; Yaqoob, M.M.; Radermacher, P.; Kapoor, A.; Thiemermann, C. Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the beta-common receptor. Dis. Model Mech. 2013, 6, 1021–1030. [Google Scholar] [PubMed]
- Kumral, A.; Baskın, H.; Duman, N.; Yilmaz, O.; Tatli, M.; Özer, E.; Gökmen, N.; Genc, S.; Özkan, H. Erythropoietin protects against necrotizing enterocolitis of newborn rats by the inhibiting nitric oxide formation. Biol. Neonate 2003, 84, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Liu, X.M.; Zhou, G.P. Changes of matrix metalloproteinase 2,9 and tissue inhibitor of metalloproteinase 1, 2, 3 expression level in aged rat lung. Beijing Da Xue Xue Bao Yi Xue Ban 2008, 40, 101–104. [Google Scholar]
- Wang, L.; Antonini, J.M.; Rojanasakul, Y.; Castranova, V.; Scabilloni, J.F.; Mercer, R.R. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. J. Cell. Physiol. 2003, 194, 215–224. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, L.; Chao, S.; Liu, X.; Li, K.; Wang, Y.; Zhang, Z. Mesenchymal stem cells in combination with erythropoietin repair hyperoxia-induced alveoli dysplasia injury in neonatal mice via inhibition of TGF-beta1 signaling. Oncotarget 2016, 7, 47082–47094. [Google Scholar] [CrossRef]
- Shi, M.; Flores, B.; Li, P.; Gillings, N.; McMillan, K.L.; Ye, J.; Huang, L.J.-S.; Sidhu, S.S.; Zhong, Y.-P.; Grompe, M.T.; et al. Effects of erythropoietin receptor activity on angiogenesis, tubular injury, and fibrosis in acute kidney injury: A “U-shaped” relationship. Am. J. Physiol. Renal. Physiol. 2018, 314, F501–F516. [Google Scholar] [CrossRef]
- Yang, J.C.; Cortopassi, G.A. Induction of the mitochondrial permeability transition causes release of the apoptogenic factor cytochrome c. Free Radic Biol. Med. 1998, 24, 624–631. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Konga, D.B.; Kim, Y.; Hong, S.C.; Roh, Y.M.; Lee, C.M.; Kim, K.Y.; Lee, S.M. Oxidative stress and antioxidant defenses in asthmatic murine model exposed to printer emissions and environmental tobacco smoke. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Han, B.H.; Li, Y.; Keogh, C.L.; Holtzman, D.M.; Yu, S.P. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J. Pharmacol. Exp. Ther. 2006, 317, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Cayla, J.L.; Maire, P.; Duvallet, A.; Wahrmann, J.P. Erythropoietin induces a shift of muscle phenotype from fast glycolytic to slow oxidative. Int. J. Sports Med. 2008, 29, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Thomsen, J.J.; Rentsch, R.L.; Lundby, C. Effects of prolonged recombinant human erythropoietin administration on muscle membrane transport systems and metabolic marker enzymes. Eur. J. Appl. Physiol. 2007, 102, 41–44. [Google Scholar] [CrossRef]




| Body Weight (g) | Hematocrit (%) | Erythropoietin (pg/mL) | ||||
|---|---|---|---|---|---|---|
| Group | Day 1 | Day 14 | Day 1 | Day 14 | Day 1 | Day 14 |
| CNT | 260.4 ± 12.5 * | 325.2 ± 14.4 * | 48.2 ± 1.3 | 47.8 ± 1.3 | 5.9 ± 0.2 | 5.8 ± 0.2 |
| CNT SAL | 273 ± 46.8 * | 321.2 ± 43.2 * | 48 ± 0.7 | 48.6 ± 0.8 | 6.1 ± 0.5 | 5.7 ± 0.5 |
| CNT SAL/EPO | 268 ± 47.2 * | 301 ± 41.2 * | 47.4 ± 0.8 | 48.2 ± 1.3 | 6.3 ± 0.3 | 5.7 ± 0.6 |
| BLM/SAL | 259.3 ± 23.5 * | 227.2 ± 23 * | 48.3 ± 1.1 | 48.6 ± 0.7 | 5.9 ± 0.4 | 5.1 ± 0.9 |
| BLM/EPO | 263.7 ± 46.1 * | 289.2 ± 51.8 * | 48.5 ± 1 | 49.1 ± 1.1 | 6.4 ± 0.4 | 6 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsavlis, D.; Domvri, K.; Porpodis, K.; Papoutsopoulou, S.; Anestakis, D.; Tzoumaka, A.; Meditskou, S.; Symeonidoy, K.; Spandou, E. Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis. J. Pers. Med. 2024, 14, 972. https://doi.org/10.3390/jpm14090972
Tsavlis D, Domvri K, Porpodis K, Papoutsopoulou S, Anestakis D, Tzoumaka A, Meditskou S, Symeonidoy K, Spandou E. Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis. Journal of Personalized Medicine. 2024; 14(9):972. https://doi.org/10.3390/jpm14090972
Chicago/Turabian StyleTsavlis, Drosos, Kalliopi Domvri, Konstantinos Porpodis, Stamatia Papoutsopoulou, Doxakis Anestakis, Anna Tzoumaka, Soultana Meditskou, Konstantina Symeonidoy, and Evangelia Spandou. 2024. "Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis" Journal of Personalized Medicine 14, no. 9: 972. https://doi.org/10.3390/jpm14090972
APA StyleTsavlis, D., Domvri, K., Porpodis, K., Papoutsopoulou, S., Anestakis, D., Tzoumaka, A., Meditskou, S., Symeonidoy, K., & Spandou, E. (2024). Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis. Journal of Personalized Medicine, 14(9), 972. https://doi.org/10.3390/jpm14090972









