Outcomes of Free Vascularized Fibular Grafts in Treating Massive Forearm Skeletal Defects
Abstract
1. Introduction
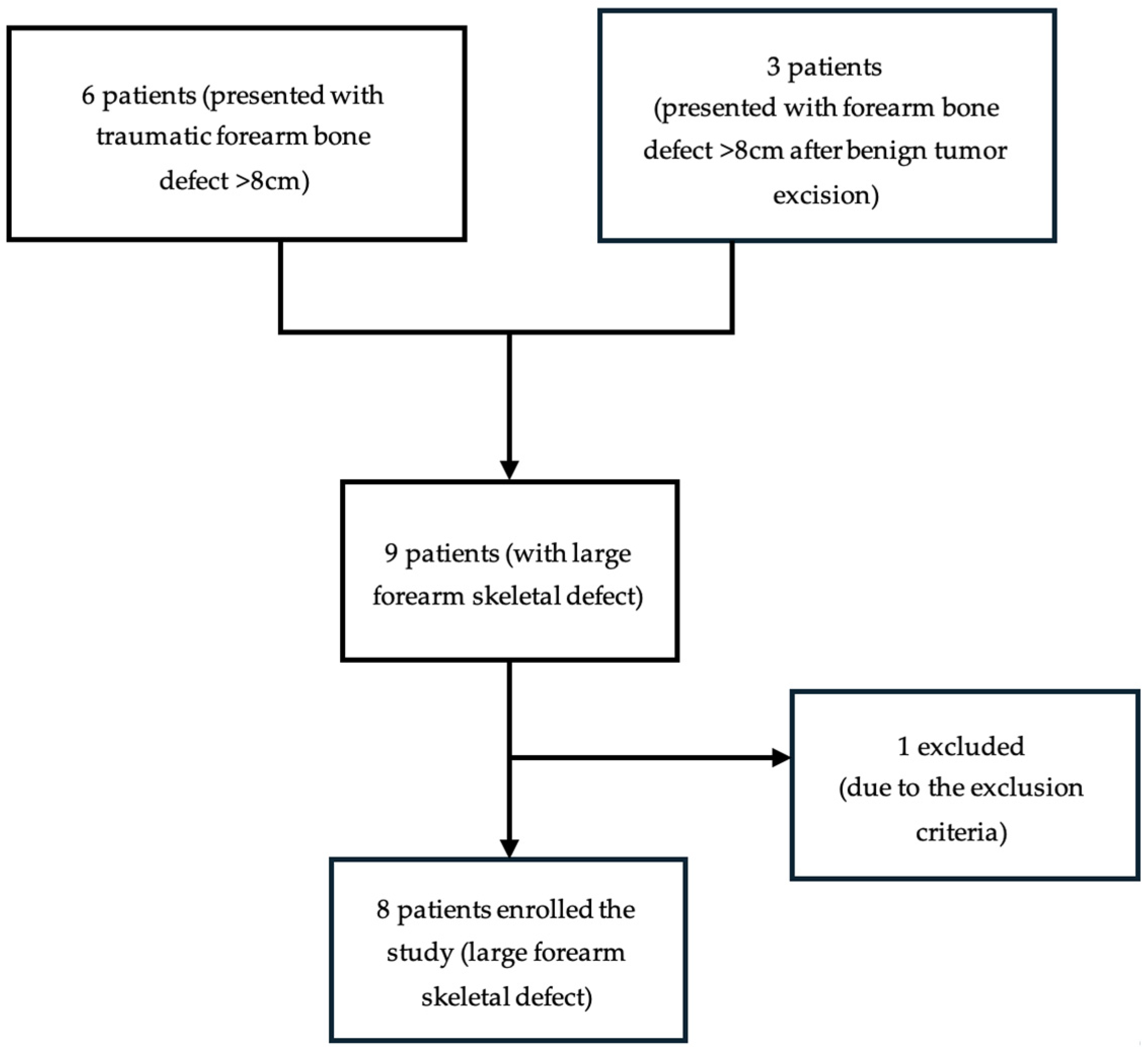
2. Patients and Methods
2.1. Study Population
2.2. Data Collection
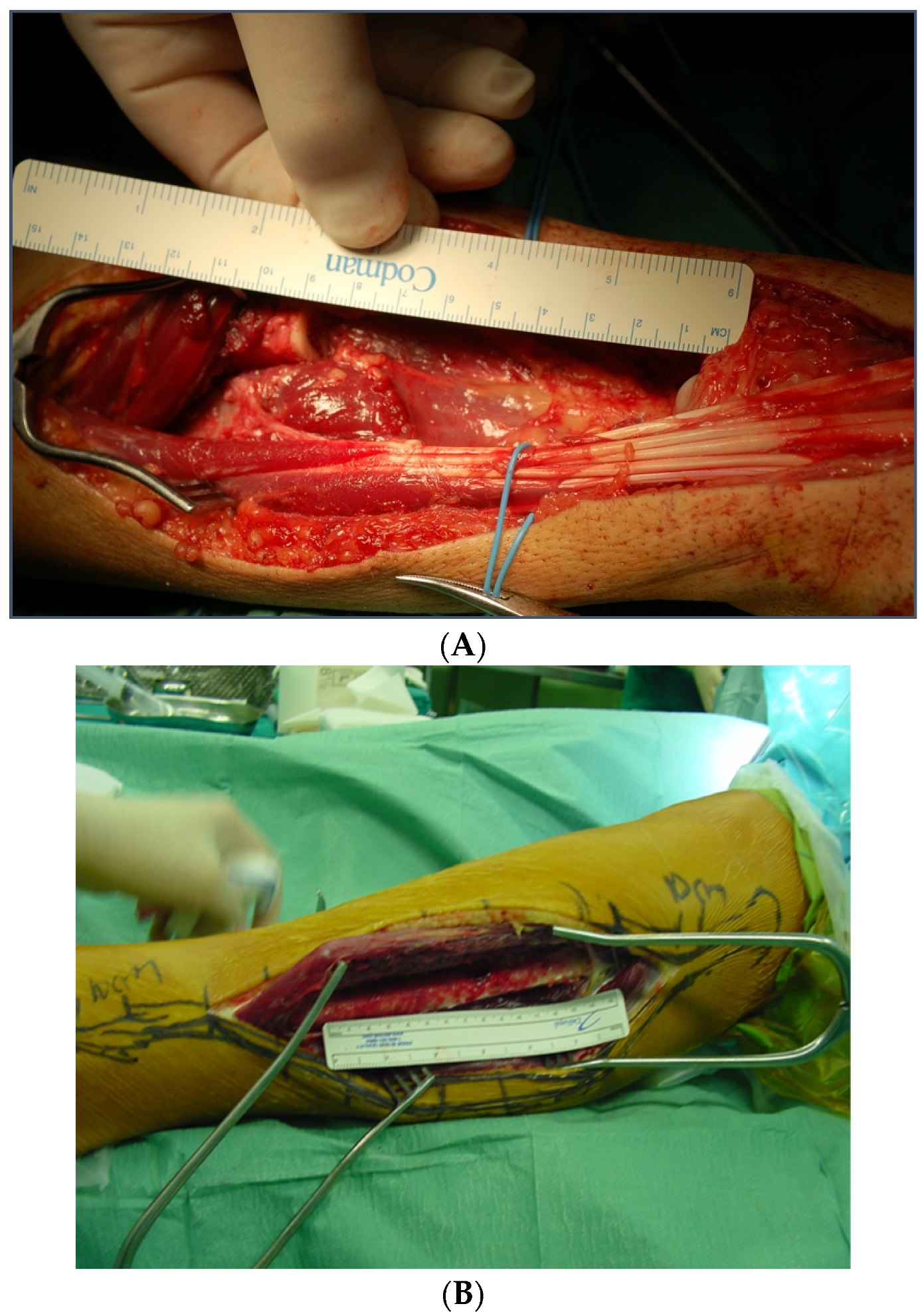
2.3. Surgical Technique
2.4. Post-Operative Treatment
2.5. Study Outcomes
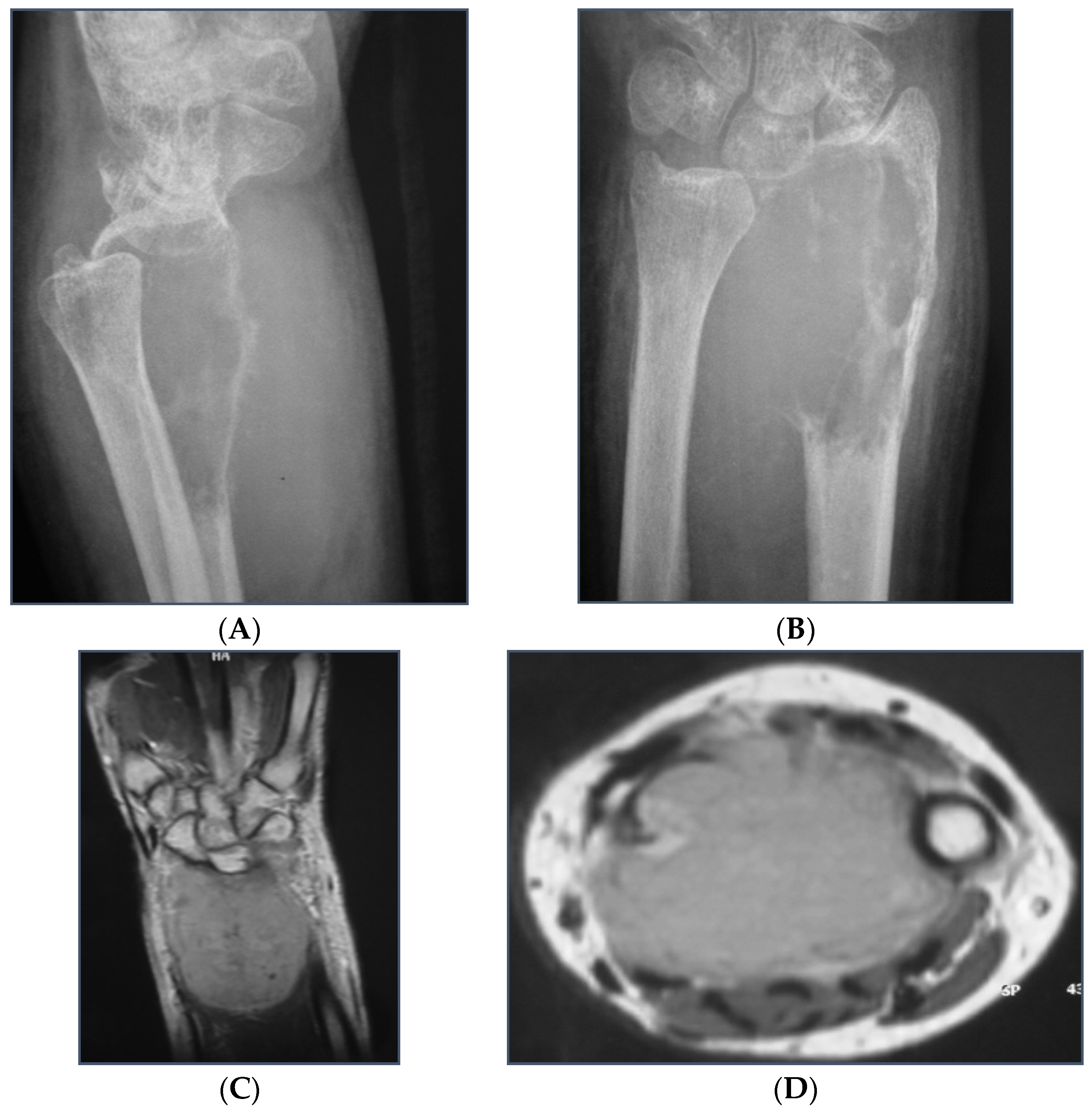
3. Results
3.1. Demographics
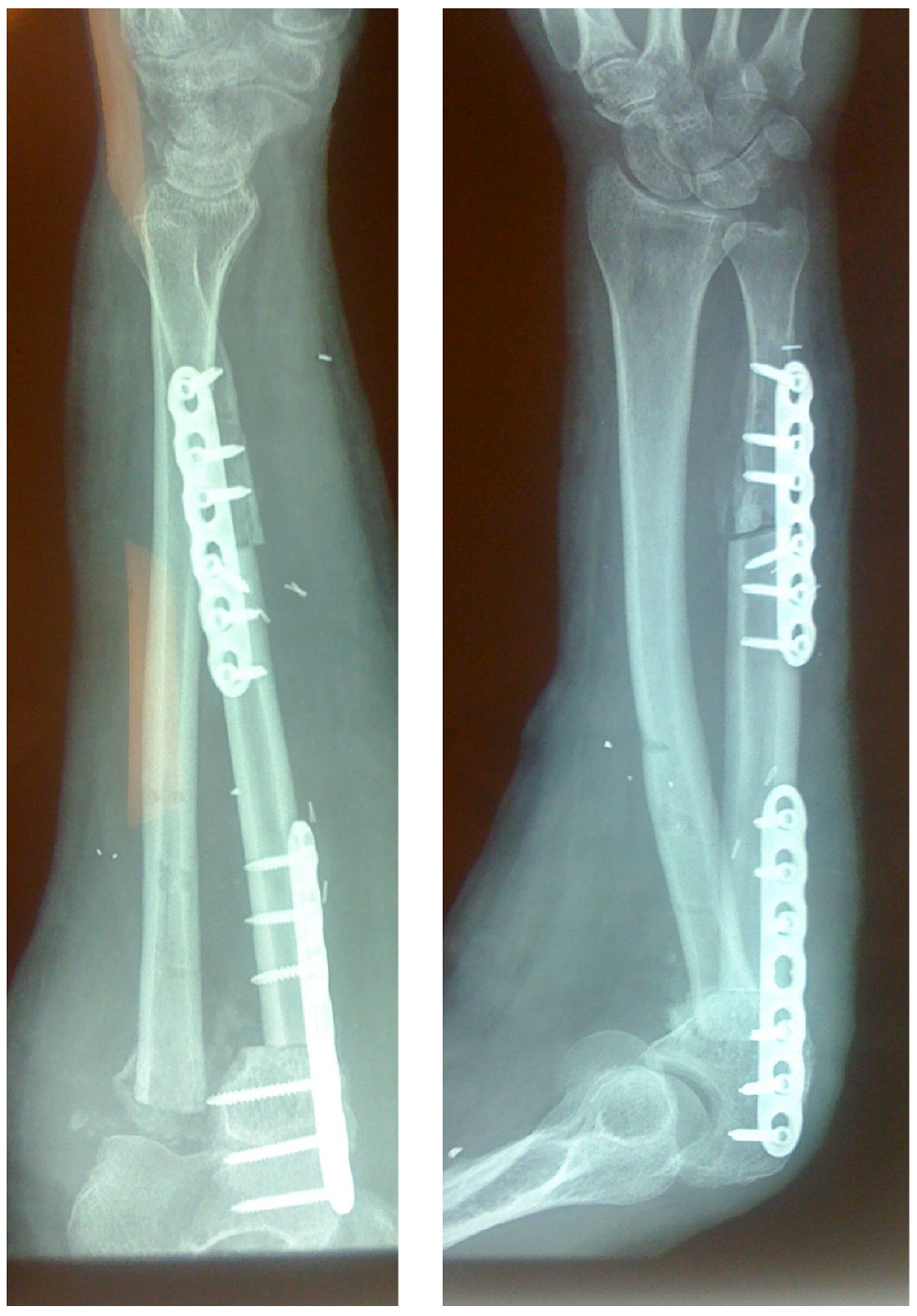
3.2. Time to Union—Complications
3.3. Functional Outcomes (ROM—DASH Score)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiland, A.J.; Weiss, A.P.C.; Moore, J.R.; Tolo, V.T. Vascularized Fibular Grafts in the Treatment of Congenital Pseudarthrosis of the Tibia. J. Bone Jt. Surg. Am. 1990, 72, 654–662. [Google Scholar] [CrossRef]
- Tamai, S.; Sakamoto, H.; Hori, Y. Vascularized Fibula Transplantation-a Report of 8 Cases in the Treatment of Traumatic Bony Defect or Pseudarthrosis of Long Bones. Int’l. J. Mirosurg. 1980, 2, 205–212. [Google Scholar]
- Tetsworth, K.D.; Burnand, H.G.; Hohmann, E.; Glatt, V. Classification of Bone Defects: An Extension of the Orthopaedic Trauma Association Open Fracture Classification. J. Orthop. Trauma 2021, 35, 71–76. [Google Scholar] [CrossRef]
- Perry, C.R. Bone Repair Techniques, Bone Graft, and Bone Graft Substitutes. Clin. Orthop. Relat. Res. 1999, 360, 71–86. [Google Scholar] [CrossRef]
- Walter, M. Resection de l’extrémité Inférieure Du Radius Pour Ostéosarcome: Greffe de l’extremité Supérieure Du Péroné. Bull. Mem. Soc. Chir. Par. 1911, 37, 739–747. [Google Scholar]
- Banic, A.; Hertel, R. Double Vascularized Fibulas for Reconstruction of Large Tibial Defects. J. Reconstr. Microsurg. 1993, 9, 421–428. [Google Scholar] [CrossRef]
- Ian Taylor, G.; Miller, G.D.H.; Ham, F.J. The Free Vascularized Bone Graft. A Clinical Extension of Microvascular Techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef]
- Allsopp, B.J.; Hunter-Smith, D.J.; Rozen, W.M. Vascularized versus Nonvascularized Bone Grafts: What Is the Evidence? Clin. Orthop. Relat. Res. 2016, 474, 1319–1327. [Google Scholar] [CrossRef]
- Heitmann, C.; Erdmann, D.; Levin, L.S. Treatment of Segmental Defects of the Humerus with an Osteoseptocutaneous Fibular Transplant. J. Bone Joint. Surg. Am. 2002, 84, 2216–2223. [Google Scholar] [CrossRef]
- Yajima, H.; Tamai, S.; Mizumoto, S.; Inada, Y. Vascularized Fibular Grafts in the Treatment of Osteomyelitis and Infected Nonunion. Clin. Orthop. Relat. Res. 1993, 293, 256–264. [Google Scholar] [CrossRef]
- Repo, J.P.; Sommarhem, A.; Roine, R.P.; Sintonen, H.; Halonen, T.; Tukiainen, E. Free Vascularized Fibular Graft Is Reliable in Upper Extremity Long-Bone Reconstruction with Good Long-Term Outcomes. J. Reconstr. Microsurg. 2016, 32, 513–519. [Google Scholar] [CrossRef]
- Landau, M.J.; Badash, I.; Yin, C.; Alluri, R.K.; Patel, K.M. Free Vascularized Fibula Grafting in the Operative Treatment of Malignant Bone Tumors of the Upper Extremity: A Systematic Review of Outcomes and Complications. J. Surg. Oncol. 2018, 117, 1432–1439. [Google Scholar] [CrossRef]
- Gummesson, C.; Atroshi, I.; Ekdahl, C. The Disabilities of the Arm, Shoulder and Hand (DASH) Outcome Questionnaire: Longitudinal Construct Validity and Measuring Self-Rated Health Change after Surgery. BMC Musculoskelet. Disord. 2003, 4, 1–6. [Google Scholar] [CrossRef]
- Themistocleous, G.S.; Goudelis, G.; Kyrou, I.; Chloros, G.D.; Krokos, A.; Galanos, A.; Gerostathopoulos, N.E.; Soucacos, P.N. Translation into Greek, Cross-Cultural Adaptation and Validation of the Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH). J. Hand Ther. 2006, 19, 350–357. [Google Scholar] [CrossRef]
- Davey, P.A.; Simonis, R.B. Modification of the Nicoll Bone-Grafting Technique for Nonunion of the Radius and/or Ulna. J. Bone Jt. Surg. Br. 2002, 84, 30–33. [Google Scholar] [CrossRef]
- Flamans, B.; Pauchot, J.; Petite, H.; Blanchet, N.; Rochet, S.; Garbuio, P.; Tropet, Y.; Obert, L. Pertes de Substance Osseuse à La Main et Au Poignet Traitées En Urgence Par Technique de La Membrane Induite (Technique de Masquelet). Chir. Main 2010, 29, 307–314. [Google Scholar] [CrossRef]
- Walker, M.; Sharareh, B.; Mitchell, S.A. Masquelet Reconstruction for Posttraumatic Segmental Bone Defects in the Forearm. J. Hand Surg. Am. 2019, 44, 342.e1–342.e8. [Google Scholar] [CrossRef]
- Micev, A.J.; Kalainov, D.M.; Soneru, A.P. Masquelet Technique for Treatment of Segmental Bone Loss in the Upper Extremity. J. Hand Surg. Am. 2015, 40, 593–598. [Google Scholar] [CrossRef]
- Reid, R.L.; Baker, G.I. The Single-Bone Forearm-A Reconstructive Technique. Hand 1973, 5, 214–219. [Google Scholar] [CrossRef]
- Ditsios, K.; Iosifidou, E.; Kostretzis, L.; Konstantinou, P.; Pinto, I.; Theodoroudis, I.; Hatzokos, I. Combined Bone Transportation and Lengthening Techniques for the Treatment of Septic Nonunion of the Forearm Followed by Tendon Transfer. Case Rep. Orthop. 2017, 2017, 9672126. [Google Scholar] [CrossRef]
- van Isacker, T.; Barbier, O.; Traore, A.; Cornu, O.; Mazzeo, F.; Delloye, C. Forearm Reconstruction with Bone Allograft Following Tumor Excision: A Series of 10 Patients with a Mean Follow-up of 10 Years. Orthop. Traumatol. Surg. Res. 2011, 97, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Choo, A.; O’Connor, D.P.; Brinker, M.R. Treatment of Infected Forearm Nonunions with Large Complete Segmental Defects Using Bulk Allograft and Intramedullary Fixation. J. Hand Surg. Am. 2016, 41, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; He, H.; Han, L.; Zhang, S.; Yang, L.; Han, W.; Wang, X.; Gao, J.; Zhao, J.; et al. Clinical Guidelines for Indications, Techniques, and Complications of Autogenous Bone Grafting. Chin. Med. J. 2024, 137, 5–7. [Google Scholar] [CrossRef]
- Gan, A.W.T.; Puhaindran, M.E.; Pho, R.W.H. The Reconstruction of Large Bone Defects in the Upper Limb. Injury 2013, 44, 313–317. [Google Scholar] [CrossRef]
- Cano-Luís, P.; Andrés-Cano, P.; Ricón-Recarey, F.J.; Giráldez-Sánchez, M.A. Treatment of Posttraumatic Bone Defects of the Forearm with Vascularized Fibular Grafts. Follow up after Fourteen Years. Injury 2018, 49 (Suppl. 2), S27–S35. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Wen, G.; Chai, Y. Reconstruction of Complex Tissue Defect of Forearm with a Chimeric Flap Composed of a Sural Neurocutaneous Flap and a Vascularized Fibular Graft: A Case Report. Microsurgery 2018, 38, 790–794. [Google Scholar] [CrossRef]
- Jupiter, J.B.; Gerhard, H.J.; Guerrero, J.; Nunley, J.A.; Levin, L.S. Treatment of Segmental Defects of the Radius with Use of the Vascularized Osteoseptocutaneous Fibular Autogenous Graft. J. Bone Jt. Surg. Am. 1997, 79, 542–550. [Google Scholar] [CrossRef]
- Adani, R.; Delcroix, L.; Innocenti, M.; Marcoccio, I.; Tarallo, L.; Celli, A.; Ceruso, M. Reconstruction of Large Posttraumatic Skeletal Defects of the Forearm by Vascularized Free Fibular Graft. Microsurgery 2004, 24, 423–429. [Google Scholar] [CrossRef]
- Shahzad, F.; Fazzalari, A.; Zoghbi, Y.; Coriddi, M.R.; Chapman, T.R.; Mehrara, B.J.; Disa, J.J.; Cordeiro, P.G.; Healey, J.; Athanasian, E. Reconstruction of Oncologic Upper Extremity Defects with Fibula Free Flaps Has High Union Rates and Excellent Functional Outcomes. J. Surg. Oncol. 2023, 128, 1416–1427. [Google Scholar] [CrossRef]
- Claxton, M.R.; Shirley, M.B.; Bakri, K.; Rose, P.S.; Moran, S.L.; Houdek, M.T. Utility of the Free Vascularized Fibula Flap to Reconstruct Oncologic Defects in the Upper Extremity. Anticancer Res. 2020, 40, 2751–2755. [Google Scholar] [CrossRef]
- Dell, P.C.; Sheppard, J.E. Vascularized Bone Grafts in the Treatment of Infected Forearm Nonunions. J. Hand Surg. Am. 1984, 9, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.N.; Dailiana, Z.H.; Innocenti, M.; Mathoulin, C.L.; Mattar, R.; Sauerbier, M. Vascularized Bone Grafts for Upper Limb Reconstruction: Defects at the Distal Radius, Wrist, and Hand. J. Hand Surg. Am. 2010, 35, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Boretto, J.G.; Holc, F.; Gallucci, G.L.; Donndorff, A.; Rellán, I.; De Carli, P. Fibula Flap in Upper Extremity Segmental/Critical Size Bone Defects Fixed with Locking Plates. Single-Inst. Obs. Cohort. Inj. 2023, 54 (Suppl. 6), 110737. [Google Scholar] [CrossRef]
- Mattar, R.; Azze, R.J.; Ferreira, M.C.; Starck, R.; Canedo, A.C. Vascularized Fibular Graft for Management of Severe Osteomyelitis of the Upper Extremity. Microsurgery 1994, 15, 22–27. [Google Scholar] [CrossRef]
- Yücetürk, A.; Tuncay, C.; Işiklar, U.; Tandoğan, R. Vascularised Bone Graft Applications in Upper Extremity Problems. Microsurgery 1998, 18, 160–162. [Google Scholar] [CrossRef]
- Noaman, H.H. Management of Upper Limb Bone Defects Using Free Vascularized Osteoseptocutaneous Fibular Bone Graft. Ann. Plast. Surg. 2013, 71, 503–509. [Google Scholar] [CrossRef]
- Atilgan, N. The Use of Free Fibula Flap in Different Extremities and Our Clinical Results. Cureus 2023, 15, e47450. [Google Scholar] [CrossRef]
- Balakrishnan, T.M.; Madhurbootheswaran, S.; Janardhanam, J. Single-Stage Reconstruction with Innate Chimeric-Free Fibula Flap in Limb-Preserving Excision of Upper Limb Sarcomas. J. Hand Microsurg. 2024, 16, 100007. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 512193. [Google Scholar] [CrossRef]

| Case | Sex | Age (Years) | Type of Injury (Diagnosis) | Site of Bone Defect | Initial Treatment (Previous Operation) | Time from Injury to FVFG (Months) | Graft Length (cm) | Bone Union (Months) | Forearm ROM (Degrees) | Wrist ROM (Degrees) | DASH Score | Follow-Up (Months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronation | Supination | Flexion | Extension | |||||||||||
| 1 | F | 14 | Giant cell tumor | R | - | - | 8 | 3 | 85 | 80 | 60 | 30 | 7.5 | 98 |
| 2 | M | 27 | Closed fracture | R | ORIF + bone graft | 12 | 10 | 4 | 70 | 60 | 40 | 20 | 12.5 | 74 |
| 3 | F | 32 | Closed fracture | U | ORIF | 17 | 9 | 4.5 | 50 | 60 | 30 | 20 | 18.3 | 82 |
| 4 | M | 29 | Closed fracture | R | ORIF + bone graft | 8 | 12 | 4 | 50 | 60 | 25 | 30 | 17.5 | 78 |
| 5 | M | 38 | Open fracture | U | External fixation | 1 | 9.5 | 5 | 70 | 40 | 45 | 40 | 11.7 | 64 |
| 6 | F | 24 | Closed fracture | R | ORIF | 10 | 8.5 | 3 | 30 | 20 | 30 | 25 | 20.8 | 78 |
| 7 | M | 31 | Giant cell tumor | R | - | - | 11 | 4.5 | 30 | 50 | 0 | 0 | 45 | 110 |
| 8 | M | 26 | Giant cell tumor | R | - | - | 10.5 | 4 | 70 | 50 | 35 | 20 | 8.3 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, P.; Kostretzis, L.; Ditsiou, A.Z.; Samaras, I.; Papadopoulos, P.; Ditsios, K. Outcomes of Free Vascularized Fibular Grafts in Treating Massive Forearm Skeletal Defects. J. Pers. Med. 2024, 14, 973. https://doi.org/10.3390/jpm14090973
Konstantinou P, Kostretzis L, Ditsiou AZ, Samaras I, Papadopoulos P, Ditsios K. Outcomes of Free Vascularized Fibular Grafts in Treating Massive Forearm Skeletal Defects. Journal of Personalized Medicine. 2024; 14(9):973. https://doi.org/10.3390/jpm14090973
Chicago/Turabian StyleKonstantinou, Panagiotis, Lazaros Kostretzis, Athina Zacharoula Ditsiou, Ioannis Samaras, Pericles Papadopoulos, and Konstantinos Ditsios. 2024. "Outcomes of Free Vascularized Fibular Grafts in Treating Massive Forearm Skeletal Defects" Journal of Personalized Medicine 14, no. 9: 973. https://doi.org/10.3390/jpm14090973
APA StyleKonstantinou, P., Kostretzis, L., Ditsiou, A. Z., Samaras, I., Papadopoulos, P., & Ditsios, K. (2024). Outcomes of Free Vascularized Fibular Grafts in Treating Massive Forearm Skeletal Defects. Journal of Personalized Medicine, 14(9), 973. https://doi.org/10.3390/jpm14090973






