Effects of Haptic Feedback Interventions in Post-Stroke Gait and Balance Disorders: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Elegibility Criteria
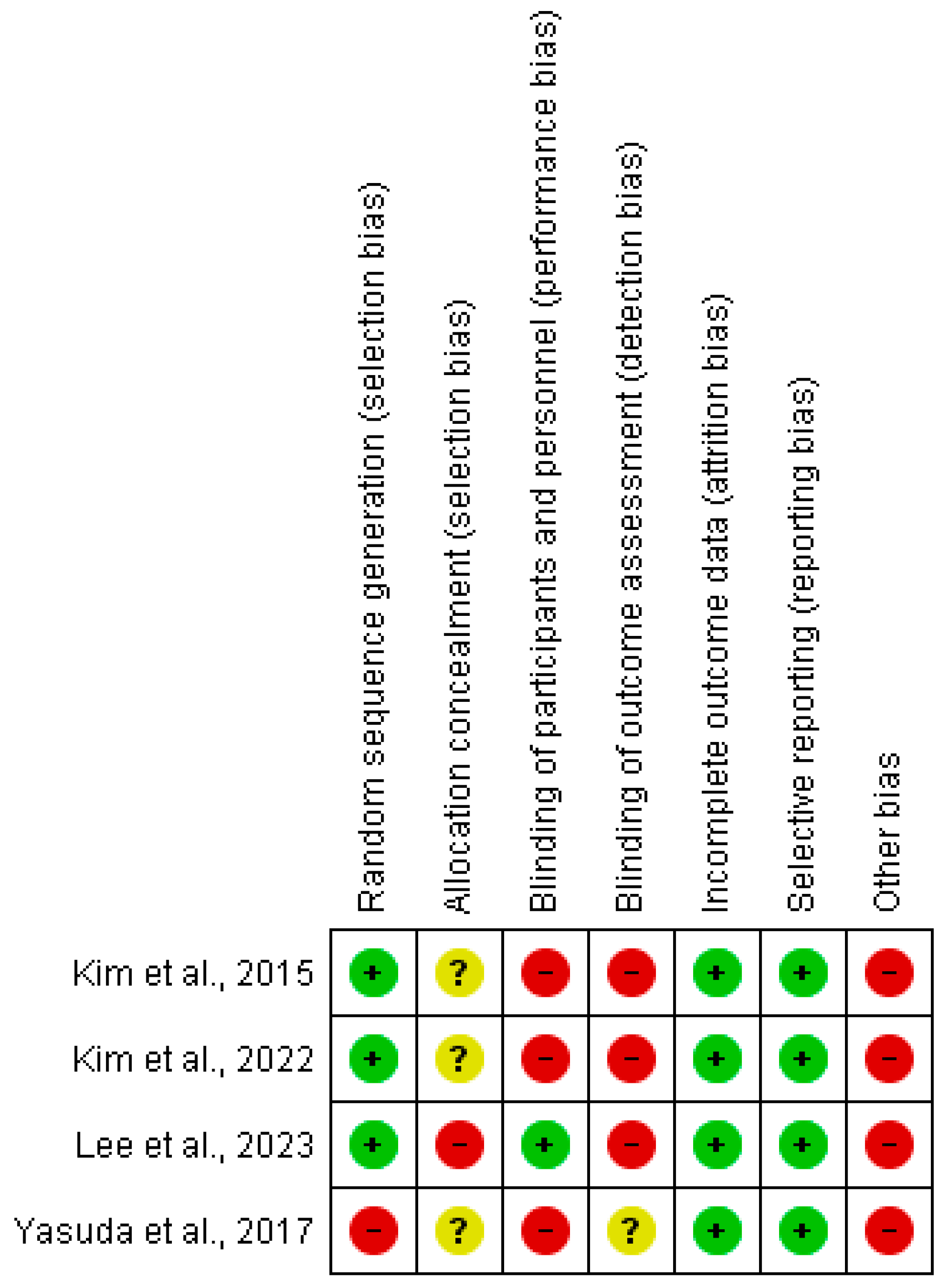
2.3. Assessment of the Methodological Quality and Risk of Bias
2.4. Selection Process and Data Extraction
2.5. Data Synthesis and Statistical Analysis
3. Results
3.1. Synthesis of Results
3.2. Participant Characteristics
3.3. Intervention Characteristics
| Authors (Year) | Haptic Feedback |
|---|---|
| Schonhaut et al. (2024) [52] | Vibration: Tractors attached to hip and trunk. Hip abductor vibration adjusted in real time according to pelvis movement. Trunk vibration as the other condition for comparison. |
| Lee et al. (2023) [47] | Electrical Stimulation: Low-frequency electrical output in LL triggered when weight shifting is detected by an insole pressure-measuring device. |
| Kim et al. (2022) [48] | Vibration: Pressure sensor-based vibrotactile biofeedback system that gives vibration inputs in calves related to torso tilt. |
| Lee et al. (2022) [56] | Kinesthetic: Tactile inputs to the neck, similar to a light touch in relation to the ML and AP directions. |
| Lee et al. (2021) [53] | Vibration: Haptic bracelet that gives feedback by vibration cues related to arm swing movement in gait. |
| Afzal et al. (2019) [55] | Vibration: Insoles with a Force-Sensitive Resistor in the foot to determine swing and stance phase and give vibrotactile stimuli accordingly in the swing phase of the paretic leg. |
| Yasuda et al. (2018) [54] | Vibration: Vibrotactile biofeedback bilaterally attached to the ASIS and PSIS enables perception of the center of pressure during balance tasks. The system gives the information to both the therapist and patient. |
| Afzal et al. (2018) [58] | Kinesthetic and vibration: Haptic cane device that provides kinesthetic information and vibrators that provide tactile feedback on the leg during the swing phase. Insoles to provide contact ground information are also part of the system. |
| Yasuda et al. (2017) [49] | Vibration: Vibrotactile biofeedback bilaterally attached to the ASIS and PSIS gave information about direction of body sway (CoP). |
| Ma et al. (2017) [51] | Vibration: Plantar force acquisition unit and a vibration feedback unit on the affected side of the patient. Vibrational cues given when excessive foot inversion occurred. |
| Kim. et al. (2015) [50] | Electrical Stimulation: FES therapeutic unit set to the minimum sensory stimulation level. Activated in LL when weight shift is achieved. |
| Afzal et al. (2015) [57] | Kinesthetic: Kinesthetic feedback given by Phantom Omni® (patients’ hand grasping a handle). Feedback information in the form of light directional force indicating body movement to maintain balance. |
| Badke et al. (2011) [59] | Electrical Stimulation: Electrotactile feedback disposed in tongue (intraoral device that gives stimulus related to postural control). |
| Authors (Year) | Study Type (n) | Intervention and Dose | Variables: Outcome Measurement | Results |
|---|---|---|---|---|
| Schonhaut et al. (2024) [52] | CS (n = 40) | IED: Walking trials under different feedback conditions: no vibration, hip vibration and trunk vibration. Only one session (16 min/session). | Foot placement modulation: treadmill (other specifications not provided). Sacrum displacement and velocity (standing): method not specified. | Greater foot modulation in hip and trunk vibration modes (p < 0.01) and in constant mode of vibration (p = 0.01). Better standing and significant sacrum displacement (p < 0.01) with non-paretic side vibration. Paretic side vibration only affected to the sacrum displacement (p > 0.05). |
| Lee, K. (2023) [47] | RCT (n = 60) EG (n = 30) CG (n = 30) | EG: Balance training (BT) with WS as main exercise and electrical stimulation (ES) as feedback in LL. CG: Balance training without electrical stimulation. 30 sessions (50 min/session). 5 sessions/week. 6 weeks (total of 25 h). No follow up. | Static Balance Ability (sway speed and velocity moment): balance platform. Dynamic balance ability: TUG, FRT and BBS. Lower-extremity motor function: FM-LL. Activities of Daily Living: MBI. | Both groups showed improvement in all variables, but the experimental group showed greater improvement than the control (p < 0.05). |
| Kim et al. (2022) [48] | RCT (cross-over) (n = 24) | IED: Different feedback conditions while standing. All participants measured under three conditions in a randomized order: tactile BF (vibration); visual BF (mirror), and none feedback. 1 session for each condition (7.5 min/session; 3 sessions; 22.5 min). 24 h of washout between sessions. | Static Balance Ability (sway length and sway velocity): Wii Balance Board. Weight-Distribution Symmetry Index: Wii Balance Board. | Significant differences (p < 0.01) in sway length for tactile biofeedback. Tactile feedback also showed a significantly slower sway velocity and constant weight-distribution symmetry index compared with other conditions (p < 0.01). |
| Lee et al. (2022) [56] | CR (n = 1) | IED: Tasks of stance and gait balance protocol. Different conditions were carried out as Romberg and Straight-line tests with and without feedback. Only 1 session (min/session not specified). | Balance (trunk tilt): IMU sensor. Gait speed: IMU sensor. | Feedback device did not have effects on gait speed. No feedback condition and feedback conditions both showed improvement in balance. |
| Lee et al. (2021) [53] | CR (n = 1) | IED: Walking trials under different conditions: normal walk and different feedback in both paretic and non-paretic arms and backward and forwards movements. Only 1 session (min/session not specified). | Angle of arm swing: device on bracelets. Gait Parameters (velocity, stride length and SR): IMUs on lower limbs. ML and AP tilts: IMUs. | Arm swing modifications reached except in two feedback conditions (more complex feedback). Velocity and stride length increased in all feedback conditions. SR also improved under feedback conditions, as well as ML and AP tilts. |
| Afzal et al. (2019) [55] | CS (n = 8) | IED: Walking trials under different conditions: no feedback and feedback under different proportional or inversely proportional time and intensity changes. Only 1 session (min/session not specified). | Gait speed: handheld stopwatch. SR (calculated with ratio of stance-times): designed program connected to sensors and feedback device. | Statistically significant differences for SR in feedback trials. Significant differences between proportional time and intensity change feedback, and between inversely proportional time and intensity change feedback. No significant differences in gait speed. |
| Yasuda, K. et al. (2018) [54] | CS (n = 9) | Balance training (standing and WS) with vibrotactile BF. 8 sessions (45 min/session). 2 sessions/week. 4 weeks (total of 6 h). No follow up. | Patient’s postural stability (CoP pressure data in spatial variability, distance of sway and standard derivation of CoP time series): Wii Balance Board. Functional balance performance: BBS, FRT and TUG. | Significant improvement in CoP spatial variability, BBS, FRT and TUG between pre and post-tests (p > 0.05). |
| Afzal, MR. et al. (2018) [58] | CS (n = 10) | IED: Walking trials under different conditions: normal walk, tactile feedback, kinesthetic feedback at different walking speeds and both tactile and kinesthetic feedback at different walking speeds. Only 1 session (min/session not specified). | Stance Symmetry Ratio (SSR): insoles with sensors. Muscle activity: EMG. Balance (ML trunk tilt): smartphone. | In tactile, kinesthetic (normal speed) and tactile and kinesthetic (20% increase speed), SRR showed improvement. ML tilt was better in kinesthetic, and tactile and kinesthetic feedback conditions, but without statistical difference. Better muscle activity in kinesthetic feedback and tactile and kinesthetic feedback (normal speed). |
| Yasuda et al. (2017) [49] | CT (n = 17) EG (n = 9) CG (n = 8) | EG: balance task (bipedal stance) with BF information. 5 rep. of balance task (15 s each) with 1 min interval between rep. with BF. CG: balance tasks. 5 rep. of balance task (15 s each) with 1 min interval between rep. Only one session (5.25 min/session). No follow up. | Postural Stability (CoP spatial variability, CoP velocity of displacement and Mean CoP distance in the AP and ML directions): Wii Balance Board. | Only the CoP spatial variability and the mean distance in the ML direction were significantly lower in the experimental group (p > 0.05). |
| Ma et al. (2017) [51] | CS (n = 8) | IED: Walking trials under different conditions: biofeedback turned off (BFOff) and biofeedback turned on (BFOn). Only 1 session (min/session not specified). | Kinematic variables (foot, ankle, knee, hip and pelvic movements): Vicon Nexus 1.8.1 3D motion capture system. Plantar pressure distribution: in-shoe plantar pressure measurement system. | Stance (p < 0.05) and stride (p < 0.01) times significantly increased for both limbs. Foot inversion in swing phase of the affected limb significantly decreased in BFOn condition (p < 0.05). Peak knee flexion in swing phase and peak hip abduction in stance phase of the unaffected limb decrease (p < 0.05). In BFOn condition, plantar pressure distribution of affected limb increased significantly (p < 0.01) as well as average plantar pressure of both limbs (p < 0.05). |
| Kim. et al. (2015) [50] | RCT (n = 30) EG (n = 13) CG (n = 12) | EG: Weight shift (WS) training with electrical sensory stimulation feedback in LL (15 min/day) + CRehab (30 min/day). CG: General weight shift (WS) training (15 min/day) + CRehab (30 min/day). 20 sessions (45 min/session). 5 sessions/week. 4 weeks (total of 15 h). No follow up. | Balance in standing posture (CoP path lengths, CoP velocities and foot forces (FF)): Zebris Platform. | Improvements in CoP path length in experimental group with significant difference between groups (p < 0.05). Both groups showed improvement in FF, but there were better results in EG. Even though, no significant difference between groups for FF or CoP velocities. |
| Afzal et al. (2015) [57] | CS (n = 8) | IED: Balance trials while maintaining stance position under feedback and no feedback conditions. Only 1 session (min/session not reported). | Trunk tilt values: smartphone Body sway (mean velocity displacement, planar deviation, ML and AP trajectories): smartphone | Mean velocity displacement and planar deviation exhibited significant values when comparing no feedback and feedback conditions (p < 0.05). |
| Badke et al. (2011) [59] | CS (n = 29) | Segmental movement exercises and balance training (maintaining challenging postures) with TEF. 35 sessions (60 min/session). 2 sessions/day. 5 days/week. 8 weeks (total of 80 h). No follow up. | Balance: BBS. Gait ability: DGI. Balance and mobility: TUG, ABC. Quality of life: SIS. | Statistically significant improvement in BBS, DGI, TUG, ABC and almost all spheres of SIS. |
| Authors (Year) | Age (Mean) | Sex (M/F) | Phase and Time Post-Stroke (Mean) | Type of Stroke |
|---|---|---|---|---|
| Schonhaut et al. (2024) [52] | 63.5 | 27/13 | Chronic 69.5 | Not reported |
| Lee, K. (2023) [47] | 67.6 years | 33/26 | Chronic 15.25 ± 5.85 months | Thirty-seven ischemic Twenty-two hemorrhagic |
| Kim et al. (2022) [48] | 63 years | 18/6 | Chronic 15.54 ± 9.00 months | Not reported |
| Lee et al. (2022) [56] | 60 years | 1/0 | Early Subacute 37 days | Hemorrhagic |
| Lee et al. (2021) [53] | 64 years | 1/0 | Early Subacute 26 days | Ischemic |
| Afzal et al. (2019) [55] | 54.5 years | 6/2 | Early Subacute 23.9 ± 9.3 days | Five ischemic Three hemorrhagic |
| Yasuda et al. (2018) [54] | 65.8 years | 7/2 | Chronic 81.56 months | Four ischemic Five hemorrhagic |
| Afzal et al. (2018) [58] | 57.7 years | 6/4 | Early Subacute 62.5 ± 26.6 days | Five ischemic Five hemorrhagic |
| Yasuda et al. (2017) [49] | 65.1 years | 13/4 | Chronic 38.16 months | Ten ischemic Seven hemorrhagic |
| Ma et al. (2017) [51] | 53.5 years | 8/1 | Chronic 45 months | Six ischemic Two hemorrhagic |
| Kim. et al. (2015) [50] | 59.6 years | 17/8 | Late Subacute 12.8 ± 7.6 weeks | Not reported |
| Afzal et al. (2015) [57] | 52 years | 6/2 | Late Subacute 70.0 ± 41.4 days | One ischemic Seven hemorrhagic |
| Badke et al. (2011) [59] | 59 years | 20/9 | Chronic 52.2 ± 34.8 months | Not reported |
3.4. Methodological Quality and Risk of Bias
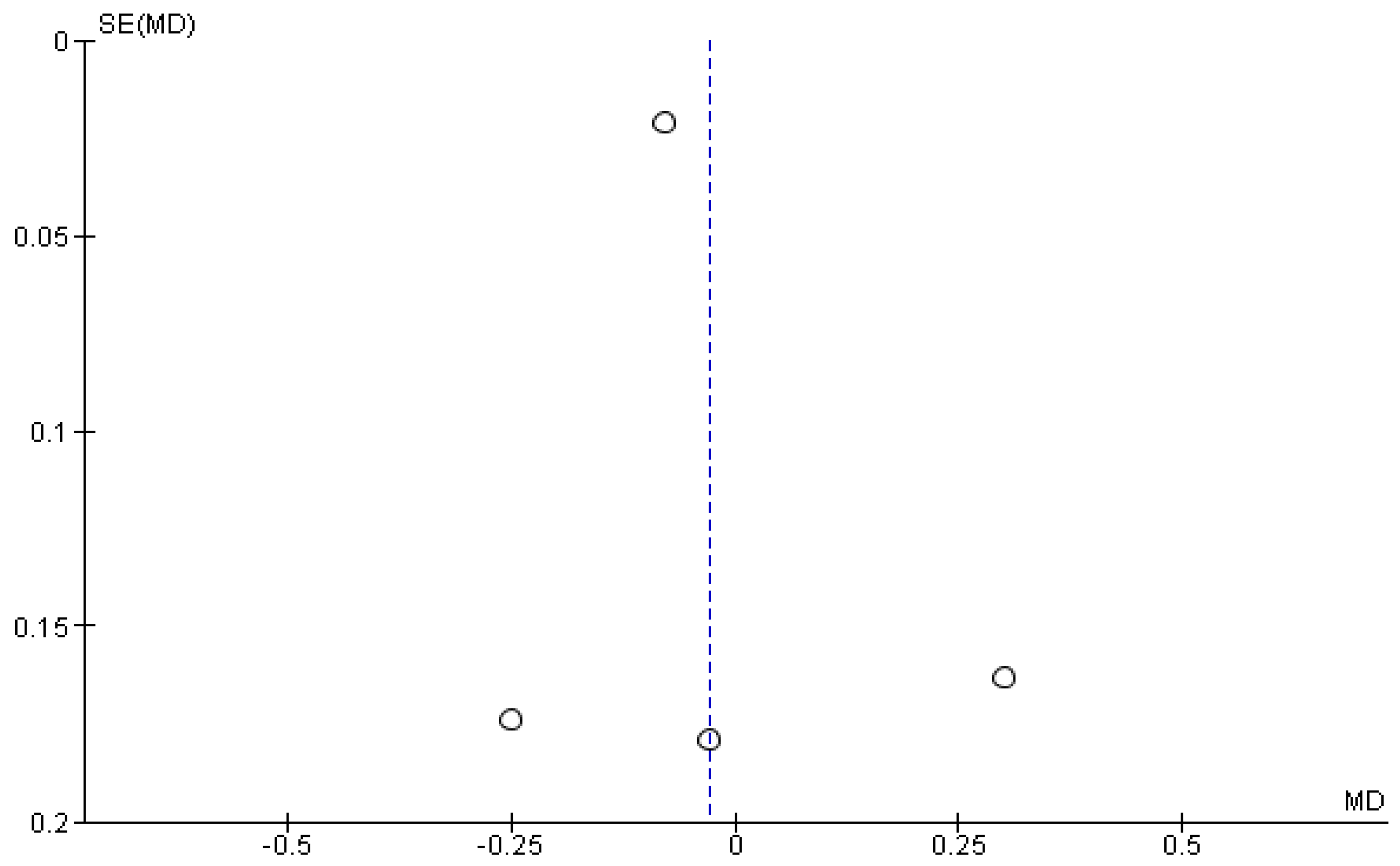
3.5. Results of the Meta-Analysis
3.6. Immediate Effects on Balance and Gait
3.7. Post-Training Effects on Balance
3.7.1. Static Balance
3.7.2. Dynamic Balance
3.8. Post-Training Effects on Gait
3.9. Other Functional Outcomes and Post-Training Effects
4. Discussion
4.1. Immediate Effects on Balance
4.2. Immediate Effects on Gait
4.3. Post-Training Effects on Balance
4.3.1. Static Balance
4.3.2. Dynamic Balance
4.4. Post-Training Effects on Gait
4.5. Other Outcomes
4.6. Clinical Implications
4.7. Study Limitations and Future Research Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soto, A.; Guillén-Grima, F.; Morales, G.; Muñoz, S.; Aguinaga-Ontoso, I.; Fuentes-Aspe, R. Prevalence and Incidence of Ictus in Europe: Systematic Review and Meta-Analysis. An. Sist. Sanit. Navar. 2022, 45, e0979. [Google Scholar] [CrossRef]
- SEMI; SEMERGEN; Freno al ictus; FEI; Feasan. El Atlas Del Ictus En España. 2019. Available online: https://www.sen.es/actividades/91-articulos/2617-el-atlas-del-ictus (accessed on 23 May 2024).
- Moore, S.A.; Boyne, P.; Fulk, G.; Verheyden, G.; Fini, N.A. Walk the Talk: Current Evidence for Walking Recovery after Stroke, Future Pathways and a Mission for Research and Clinical Practice. Stroke 2022, 53, 3494–3505. [Google Scholar] [CrossRef]
- Hollands, K.L.; Pelton, T.A.; Tyson, S.F.; Hollands, M.A.; van Vliet, P.M. Interventions for Coordination of Walking Following Stroke: Systematic Review. Gait Posture 2012, 35, 349–359. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Gray, V.L.; Creath, R.A.; Binder-Macleod, S.A.; Rogers, M.W. Control of Lateral Weight Transfer Is Associated with Walking Speed in Individuals Post-Stroke. J. Biomech. 2017, 60, 72–78. [Google Scholar] [CrossRef]
- Khan, F.; Chevidikunnan, M.F. Prevalence of Balance Impairment and Factors Associated with Balance among Patients with Stroke. A Cross Sectional Retrospective Case Control Study. Healthcare 2021, 9, 320. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Rocha, R.J.; Boening, A.; Ferreira, G.P.; Perovano, M.C. Home-Based Exercises Are as Effective as Equivalent Doses of Centre-Based Exercises for Improving Walking Speed and Balance after Stroke: A Systematic Review. J. Physiother. 2022, 68, 174–181. [Google Scholar] [CrossRef]
- Mille, M.L.; Johnson-Hilliard, M.; Martinez, K.M.; Zhang, Y.; Edwards, B.J.; Rogers, M.W. One Step, Two Steps, Three Steps More... Directional Vulnerability to Falls in Community-Dwelling Older People. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1540–1548. [Google Scholar] [CrossRef]
- Morone, G.; Spitoni, G.F.; De Bartolo, D.; Ghanbari Ghooshchy, S.; Di Iulio, F.; Paolucci, S.; Zoccolotti, P.; Iosa, M. Rehabilitative Devices for a Top-down Approach. Expert. Rev. Med. Devices 2019, 16, 187–195. [Google Scholar] [CrossRef]
- De Bartolo, D.; Paolucci, S. From Movement to Thought and Back: A Review on the Role of Cognitive Factors Influencing Technological Neurorehabilitation. Funct. Neurol. 2019, 34, 131–144. [Google Scholar]
- De Angelis, S.; Princi, A.A.; Dal Farra, F.; Morone, G.; Caltagirone, C.; Tramontano, M. Vibrotactile-Based Rehabilitation on Balance and Gait in Patients with Neurological Diseases: A Systematic Review and Metanalysis. Brain Sci. 2021, 11, 518. [Google Scholar] [CrossRef]
- Hatala, R.; Cook, D.A.; Zendejas, B.; Hamstra, S.J.; Brydges, R. Feedback for Simulation-Based Procedural Skills Training: A Meta-Analysis and Critical Narrative Synthesis. Adv. Health Sci. Educ. Theory Pract. 2014, 19, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.G.S.; Fan, E.S.L.; Lam, P.K.; Kwok, W.T.; Ma, C.Z.H.; Lam, F.M.H. The Effect of Adding Real-Time Postural Feedback in Balance and Mobility Training in Older Adults: A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2024, 123, 105439. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Moriana, G.; Moreno, A.J.; Sevillano, J.L. Technology-Based Feedback and Its Efficacy in Improving Gait Parameters in Patients with Abnormal Gait: A Systematic Review. Sensors 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Carrobles, J.A. Bio/Neurofeedback. Clin. Salud 2016, 27, 125–131. [Google Scholar] [CrossRef]
- Huang, H.; Wolf, S.L.; He, J. Recent Developments in Biofeedback for Neuromotor Rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 11. [Google Scholar] [CrossRef]
- Xu, J.; Bao, T.; Lee, U.H.; Kinnaird, C.; Carender, W.; Huang, Y.; Sienko, K.H.; Shull, P.B. Configurable, Wearable Sensing and Vibrotactile Feedback System for Real-Time Postural Balance and Gait Training: Proof-of-Concept. J. Neuroeng. Rehabil. 2017, 14, 102. [Google Scholar] [CrossRef]
- See, A.R.; Choco, J.A.G.; Chandramohan, K. Touch, Texture and Haptic Feedback: A Review on How We Feel the World around Us. App. Sci. 2022, 12, 4686. [Google Scholar] [CrossRef]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef]
- Arpaia, P.; Coyle, D.; Donnarumma, F.; Esposito, A.; Natalizio, A.; Parvis, M. Visual and Haptic Feedback in Detecting Motor Imagery within a Wearable Brain–Computer Interface. Measurement 2023, 206, 112304. [Google Scholar] [CrossRef]
- Faure, C.; Fortin-Cote, A.; Robitaille, N.; Cardou, P.; Gosselin, C.; Laurendeau, D.; Mercier, C.; Bouyer, L.; McFadyen, B.J. Adding Haptic Feedback to Virtual Environments with a Cable-Driven Robot Improves Upper Limb Spatio-Temporal Parameters during a Manual Handling Task. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2246–2254. [Google Scholar] [CrossRef]
- Afzal, M.R.; Pyo, S.; Oh, M.-K.; Park, Y.S.; Yoon, J. Identifying the Effects of Using Integrated Haptic Feedback for Gait Rehabilitation of Stroke Patients. In Proceedings of the International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017. [Google Scholar]
- Lee, B.C.; Chen, S.; Sienko, K.H. A Wearable Device for Real-Time Motion Error Detection and Vibrotactile Instructional Cuing. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Tsetserukou, D.; Hosokawa, S.; Terashima, K. LinkTouch: A Wearable Haptic Device with Five-Bar Linkage Mechanism for Presentation of Two-DOF Force Feedback at the Fingerpad. In Proceedings of the 2014 IEEE Haptics Symposium (HAPTICS), Houston, TX, USA, 23–26 February 2014; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2014; pp. 307–312. [Google Scholar]
- Yem, V.; Vu, K.; Kon, Y.; Kajimoto, H. Effect of Electrical Stimulation Haptic Feedback on Perceptions of Softness-Hardness and Stickiness While Touching a Virtual Object. In Proceedings of the 2018 IEEE Conference on Virtual Reality and 3D User Interfaces (VR), Tuebingen/Reutlingen, Germany, 18–22 March 2018; pp. 89–96. [Google Scholar]
- Verite, F.; Bachta, W.; Morel, G. Closed Loop Kinesthetic Feedback for Postural Control Rehabilitation. IEEE Trans. Haptics. 2014, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Paoloni, M.; Mangone, M.; Scettri, P.; Procaccianti, R.; Cometa, A.; Santilli, V. Segmental Muscle Vibration Improves Walking in Chronic Stroke Patients with Foot Drop: A Randomized Controlled Trial. Neurorehabil Neural Repair. 2010, 24, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Hebert, D.; Boger, J.; Lacheray, H.; Gardner, D.; Apkarian, J.; Mihailidis, A. A Haptic-Robotic Platform for Upper-Limb Reaching Stroke Therapy: Preliminary Design and Evaluation Results. J. NeuroEngineering Rehabil. 2008, 5, 15. [Google Scholar] [CrossRef]
- Piggott, L.; Wagner, S.; Ziat, M. Haptic Neurorehabilitation and Virtual Reality for Upper Limb Paralysis: A Review. Crit. Rev. Biomed. Eng. 2016, 44, 1–32. [Google Scholar] [CrossRef]
- Valdés, B.A.; Schneider, A.N.; Van der Loos, H.F.M. Reducing Trunk Compensation in Stroke Survivors: A Randomized Crossover Trial Comparing Visual and Force Feedback Modalities. Arch. Phys. Med. Rehabil. 2017, 98, 1932–1940. [Google Scholar] [CrossRef]
- Sahu, A.; Naqvi, W.M. Upper Limb Functional Independence in Subacute Stroke Patients: A Study Protocol Investigating the Impact of Haptic Enhanced Virtual Reality System. J. Crit. Rev. 2020, 7, 446–451. [Google Scholar] [CrossRef]
- Moral-Munoz, J.A.; Luque-Moreno, C.; Lucena-Anton, D. Virtual Reality for Motor Recovery in Stroke Rehabilitation. In Ischemic Stroke Therapeutics; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 331–344. [Google Scholar]
- Spencer, J.; Wolf, S.L.; Kesar, T.M. Biofeedback for Post-Stroke Gait Retraining: A Review of Current Evidence and Future Research Directions in the Context of Emerging Technologies. Front. Neurol. 2021, 12, 637199. [Google Scholar] [CrossRef]
- Afzal, M.R.; Pyo, S.; Oh, M.-K.; Park, Y.S.; Lee, B.-C.; Yoon, J. Haptic Based Gait Rehabilitation System for Stroke Patients. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Republic of Korea, 9–14 October 2016; pp. 3198–3203. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document); University of Oxford: Oxford, UK, 2011. [Google Scholar]
- Moseley, A.M.; Elkins, M.R.; Van der Wees, P.J.; Pinheiro, M.B. Using Research to Guide Practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review-Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Cochrane Training: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 8 July 2024).
- GRADEpro. Guideline Development Tool [Software]; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2022; Available online: https://gradepro.org (accessed on 8 July 2024).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Lee, K. Balance Training with Weight Shift-Triggered Electrical Stimulation for Stroke Patients: A Randomized Controlled Trial. Brain Sci. 2023, 13, 225. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Shin, W.S. Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke. Brain Sci. 2022, 12, 358. [Google Scholar] [CrossRef]
- Yasuda, K.; Kaibuki, N.; Harashima, H.; Iwata, H. The Effect of a Haptic Biofeedback System on Postural Control in Patients with Stroke: An Experimental Pilot Study. Somatosens. Mot. Res. 2017, 34, 65–71. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Cha, Y.-J. Effect of Weight Shift Training with Electrical Sensory Stimulation Feedback on Standing Balance in Stroke Patients. J. Korean Soc. Phys. Med. 2015, 10, 257–263. [Google Scholar] [CrossRef][Green Version]
- Ma, C.Z.H.; Zheng, Y.P.; Lee, W.C.C. Changes in Gait and Plantar Foot Loading upon Using Vibrotactile Wearable Biofeedback System in Patients with Stroke. Top. Stroke Rehabil. 2017, 25, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Schonhaut, E.B.; Howard, K.E.; Jacobs, C.J.; Knight, H.L.; Chesnutt, A.N.; Dean, J.C. Altered Foot Placement Modulation with Somatosensory Stimulation in People with Chronic Stroke. J. Biomech. 2024, 166, 112043. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Eizad, A.; Kim, Y.; Park, Y.; Oh, M.K.; Yoon, J. Use of Vibrotactile Bracelets to Study Effects of Arm Swing Variation on Overground Gait. IEEE Access 2021, 9, 90896–90907. [Google Scholar] [CrossRef]
- Yasuda, K.; Saichi, K.; Kaibuki, N.; Harashima, H.; Iwata, H. Haptic-Based Perception-Empathy Biofeedback System for Balance Rehabilitation in Patients with Chronic Stroke: Concepts and Initial Feasibility Study. Gait Posture 2018, 62, 484–489. [Google Scholar] [CrossRef]
- Afzal, M.R.; Lee, H.; Eizad, A.; Lee, C.H.; Oh, M.K.; Yoon, J. Effects of Vibrotactile Biofeedback Coding Schemes on Gait Symmetry Training of Individuals with Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1617–1625. [Google Scholar] [CrossRef]
- Lee, H.; Eizad, A.; Park, J.; Kim, Y.; Hwang, S.; Oh, M.K.; Yoon, J. Development of a Novel 2-Dimensional Neck Haptic Device for Gait Balance Training. IEEE Robot. Autom. Lett. 2022, 7, 2511–2518. [Google Scholar] [CrossRef]
- Afzal, M.R.; Byun, H.Y.; Oh, M.K.; Yoon, J. Effects of Kinesthetic Haptic Feedback on Standing Stability of Young Healthy Subjects and Stroke Patients. J. NeuroEngineering Rehabil. 2015, 12, 27. [Google Scholar] [CrossRef]
- Afzal, M.R.; Pyo, S.; Oh, M.K.; Park, Y.S.; Yoon, J. Evaluating the Effects of Delivering Integrated Kinesthetic and Tactile Cues to Individuals with Unilateral Hemiparetic Stroke during Overground Walking. J. Neuroeng. Rehabil. 2018, 15, 33. [Google Scholar] [CrossRef]
- Badke, M.B.; Sherman, J.; Boyne, P.; Page, S.; Dunning, K. Tongue-Based Biofeedback for Balance in Stroke: Results of an 8-Week Pilot Study. Arch. Phys. Med. Rehabil. 2011, 92, 1364–1370. [Google Scholar] [CrossRef]
- Grefkes, C.; Grefkes, C.; Fink, G.R.; Fink, G.R. Recovery from Stroke: Current Concepts and Future Perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Monrobel, M.; Escobio-Prieto, I.; Magni, E.; Galan-Mercant, A.; Lucena-Anton, D.; Pinero-Pinto, E.; Luque-Moreno, C. Descriptive Analysis of Post-Stroke Patients in a Neurological Physical Therapy Unit. Front. Neurol. 2023, 14, 1056415. [Google Scholar] [CrossRef] [PubMed]
- Łapot, T.; Rokosz, K.; Kumięga, K. Therapeutic and Treatment Procedures in the Acute Phase of Stroke. Health Promot. Phys. Act. 2022, 18, 33–40. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the Minimum Clinically Important Difference: A Review of Concepts and Methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Poole, C.; Kuss, O. The Ongoing Tyranny of Statistical Significance Testing in Biomedical Research. Eur. J. Epidemiol. 2010, 25, 225–230. [Google Scholar] [CrossRef]
- Nardone, A.; Godi, M.; Grasso, M.; Guglielmetti, S.; Schieppati, M. Stabilometry Is a Predictor of Gait Performance in Chronic Hemiparetic Stroke Patients. Gait Posture 2009, 30, 5–10. [Google Scholar] [CrossRef]
- Van Bladel, A.; De Ridder, R.; Palmans, T.; Van der Looven, R.; Verheyden, G.; Meyns, P.; Cambier, D. Defining Characteristics of Independent Walking Persons after Stroke Presenting with Different Arm Swing Coordination Patterns. Hum. Mov. Sci. 2024, 93, 103174. [Google Scholar] [CrossRef]
- Bower, K.J.; McGinley, J.L.; Miller, K.J.; Clark, R.A. Instrumented Static and Dynamic Balance Assessment after Stroke Using Wii Balance Boards: Reliability and Association with Clinical Tests. PLoS ONE 2014, 9, e115282. [Google Scholar] [CrossRef]
- Bruyneel, A.V.; Dubé, F. Best Quantitative Tools for Assessing Static and Dynamic Standing Balance after Stroke: A Systematic Review. Physiother. Can. 2021, 73, 329–340. [Google Scholar] [CrossRef]
- Jonsdottir, J.; Maestanza Mattos, F.G.; Torchio, A.; Corrini, C.; Cattaneo, D. Fallers after Stroke: A Retrospective Study to Investigate the Combination of Postural Sway Measures and Clinical Information in Faller’s Identification. Front. Neurol. 2023, 14, 1157453. [Google Scholar] [CrossRef]
- Johansson, J.; Nordström, A.; Gustafson, Y.; Westling, G.; Nordström, P. Increased Postural Sway during Quiet Stance as a Risk Factor for Prospective Falls in Community-Dwelling Elderly Individuals. Age Ageing 2017, 46, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Savcun Demirci, C.; Aydogan Arslan, S.; Yakut, H.; Sertel, M.; Kutluhan, S. Comparison of Psychometric Properties of the Postural Assessment Scale for Stroke Patients with Berg Balance Scale and Brunel Balance Assessment for Chronic Stroke. Adnan Menderes Üniversitesi Sağlık Bilim. Fakültesi Derg. 2021, 5, 479–487. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of Gait Performance Tests in Men and Women with Hemiparesis after Stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, J.; Cattaneo, D. Reliability and Validity of the Dynamic Gait Index in Persons With Chronic Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and Future of Gait Assessment in Clinical Practice: Towards the Application of Novel Trends and Technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N.; Kumar, D. Minimal Clinically Important Difference of the Lower-Extremity Fugl–Meyer Assessment in Chronic-Stroke. Top. Stroke Rehabil. 2016, 23, 233–239. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wang, C.H.; Wu, S.C.; Chen, P.C.; Sheu, C.F.; Hsieh, C.L. Establishing the Minimal Clinically Important Difference of the Barthel Index in Stroke Patients. Neurorehabilit. Neural Repair. 2007, 21, 233–238. [Google Scholar] [CrossRef]
- Duncan, P.W.; Wallace, D.; Min Lai, S.; Johnson, D.; Embretson, S.; Jacobs Laster, L. The Stroke Impact Scale Version 2.0 Evaluation of Reliability, Validity, and Sensitivity to Change. Stroke 1999, 30, 2131–2140. [Google Scholar] [CrossRef]
- Ostrowska, P.M.; Studnicki, R.; Rykaczewski, M.; Spychała, D.; Hansdorfer-Korzon, R. Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial. Int. J. Environ. Res. Public Health. 2022, 19, 16214. [Google Scholar] [CrossRef]
- Park, S.H.; Hsu, C.J.; Lin, J.T.; Dee, W.; Roth, E.J.; Rymer, W.Z.; Wu, M. Increased Motor Variability Facilitates Motor Learning in Weight Shift toward the Paretic Side during Walking in Individuals Post-Stroke. Eur. J. Neurosci. 2021, 53, 3490–3506. [Google Scholar] [CrossRef]
- Aruin, A.S.; Rao, N.; Sharma, A.; Chaudhuri, G. Compelled Body Weight Shift Approach in Rehabilitation of Individuals with Chronic Stroke. Top. Stroke Rehabil. 2012, 19, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hyun Nam, S.; Min Son, S.; Kim, K. Changes of Gait Parameters Following Constrained-Weight Shift Training in Patients with Stroke. J. Phys. Ther. Sci. 2017, 29, 673–676. [Google Scholar]
- Yun, N.; Joo, M.C.; Kim, S.C.; Kim, M.S. Robot-Assisted Gait Training Effectively Improved Lateropulsion in Subacute Stroke Patients: A Single-Blinded Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 827–836. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001; ISBN 9241545429. [Google Scholar]


| Databases | Search Strategy |
|---|---|
| Embase | “feedback system” AND (“tactile stimulation” OR “vibration sense” OR electrostimulation) AND “cerebrovascular accident” AND (training OR program OR exercise OR intervention OR rehabilitation OR physiotherapy OR therapy) AND (gait OR balance OR “lower limb” OR walking OR mobilization). |
| Medline/PubMed Web of Science Scopus | Feedback AND (haptic* OR vibr* OR electric* OR tactile) AND stroke AND (training OR program* OR exercise OR intervention OR rehab* OR physiotherap* OR therapy) AND (gait OR balance OR “lower limb” OR walk* OR ambul*). |
| Authors (Year) | Total | Items | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Lee et al. (2023) [47] | 8/10 | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Kim et al. (2022) [48] | 6/10 | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Kim et al. (2015) [50] | 4/10 | yes | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Certainty Assessment | № of Patients | Effect | Certainty | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirect Evidence | Imprecision | Other Considerations | EG | CG | Relative (95% CI) | Absolute (95% CI) | ||
| 4 | CTs | Very serious | Not serious | Serious | Not serious | Publication bias is strongly suspected Low association | 75 | 74 | - | MD −0.03 (−0.21 to 0.15) | ⨁◯◯◯ Very Low | CoP velocity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Risquet, M.; Cáceres-Matos, R.; Magni, E.; Luque-Moreno, C. Effects of Haptic Feedback Interventions in Post-Stroke Gait and Balance Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 974. https://doi.org/10.3390/jpm14090974
Gomez-Risquet M, Cáceres-Matos R, Magni E, Luque-Moreno C. Effects of Haptic Feedback Interventions in Post-Stroke Gait and Balance Disorders: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2024; 14(9):974. https://doi.org/10.3390/jpm14090974
Chicago/Turabian StyleGomez-Risquet, Maria, Rocío Cáceres-Matos, Eleonora Magni, and Carlos Luque-Moreno. 2024. "Effects of Haptic Feedback Interventions in Post-Stroke Gait and Balance Disorders: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 14, no. 9: 974. https://doi.org/10.3390/jpm14090974
APA StyleGomez-Risquet, M., Cáceres-Matos, R., Magni, E., & Luque-Moreno, C. (2024). Effects of Haptic Feedback Interventions in Post-Stroke Gait and Balance Disorders: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 14(9), 974. https://doi.org/10.3390/jpm14090974







