Effect of the Applied Voltages on the Corrosion–Wear Behavior of Thermal Spray Al Coating
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of Substrates
2.2. Thermal Spraying of Al Coatings
2.3. Mechanical Properties of the Coatings
2.4. Characterization of the Coatings
2.5. Corrosion and Wear Evaluations
2.6. Quantitative Analysis of the Mutual Corrosion and Wear
3. Results
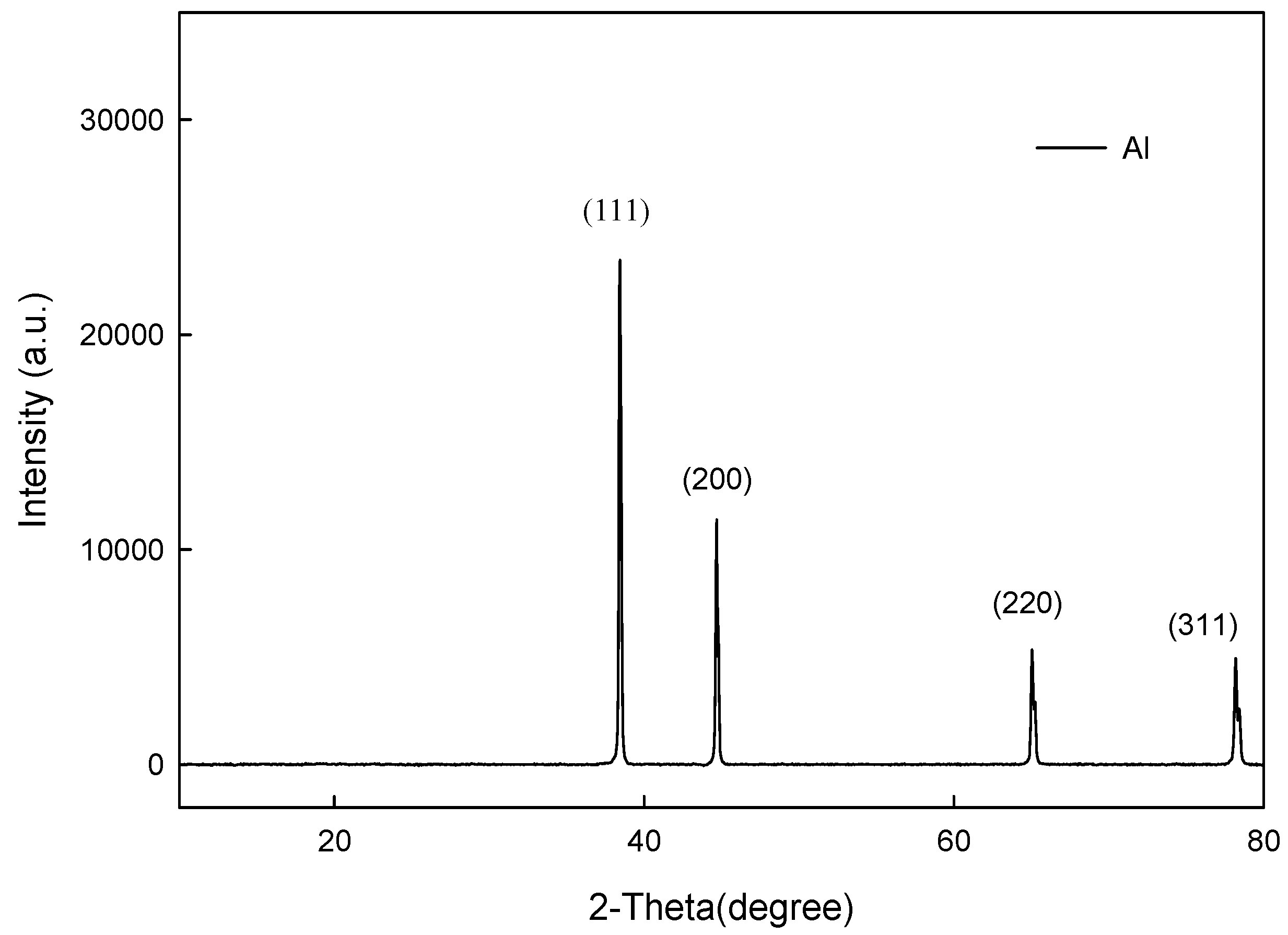
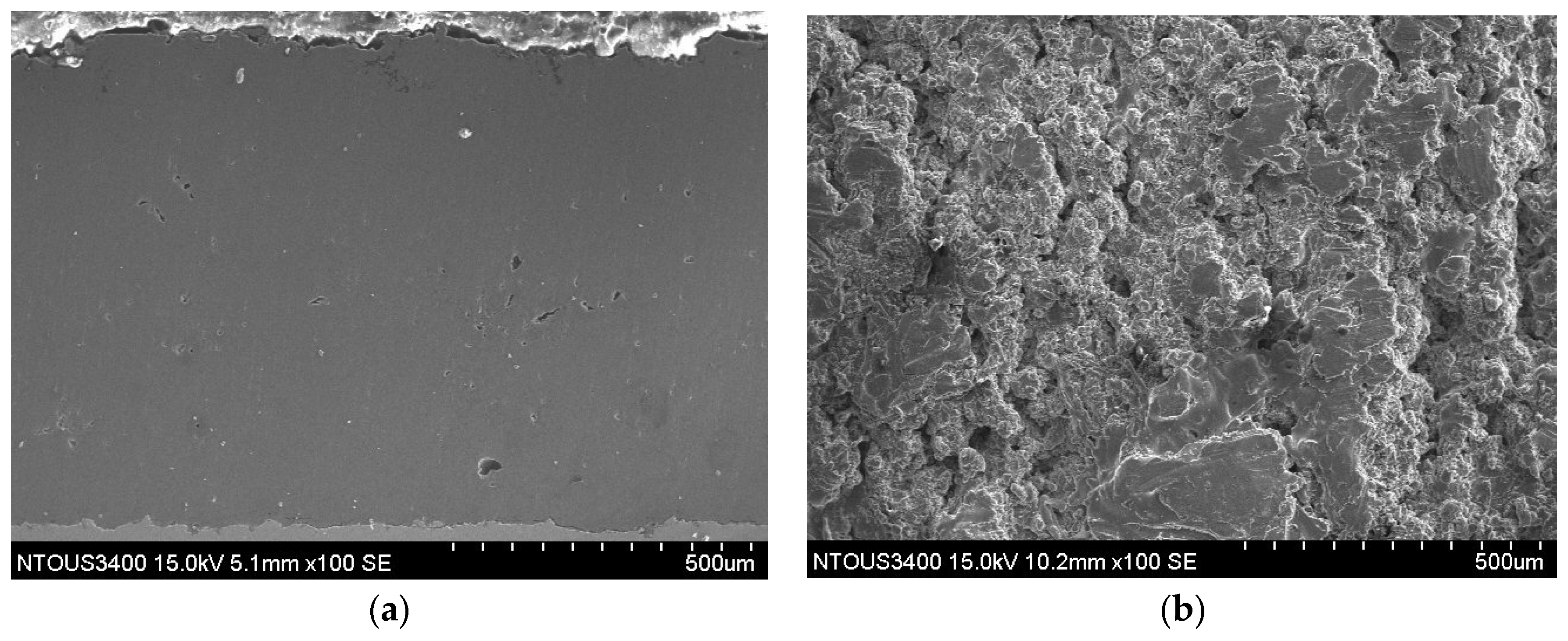
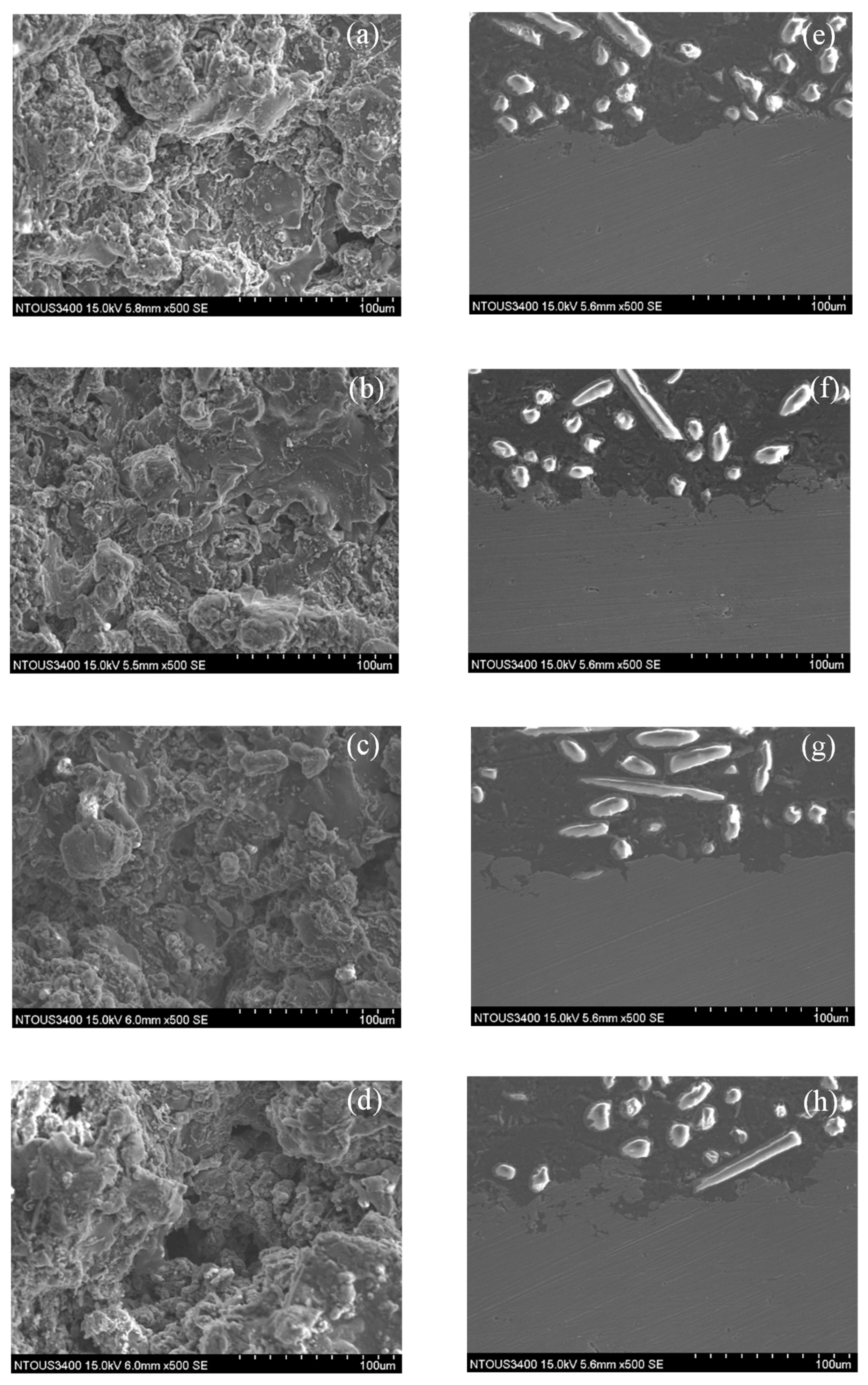
3.1. Microstructures of the Spray Al Coating
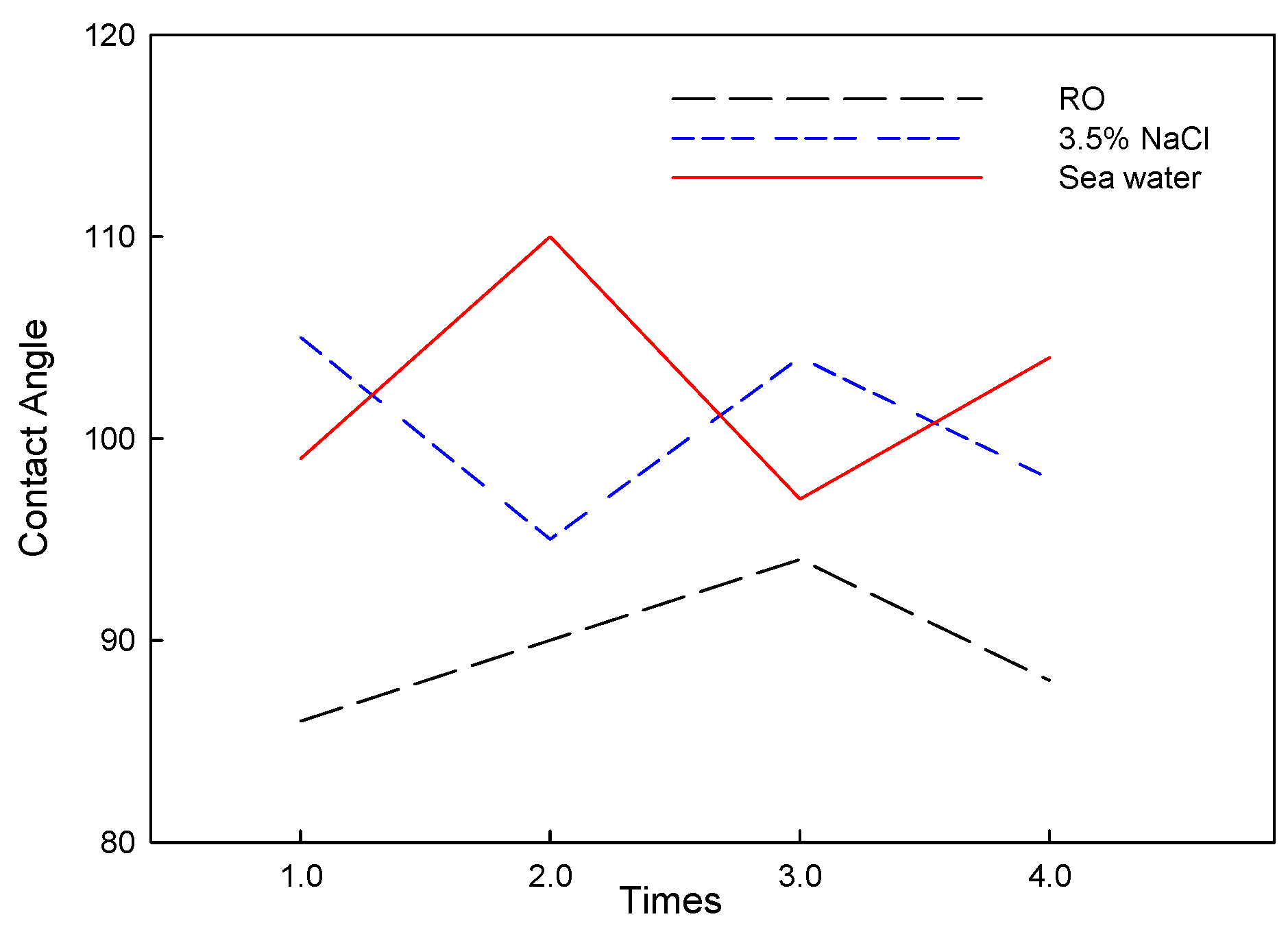
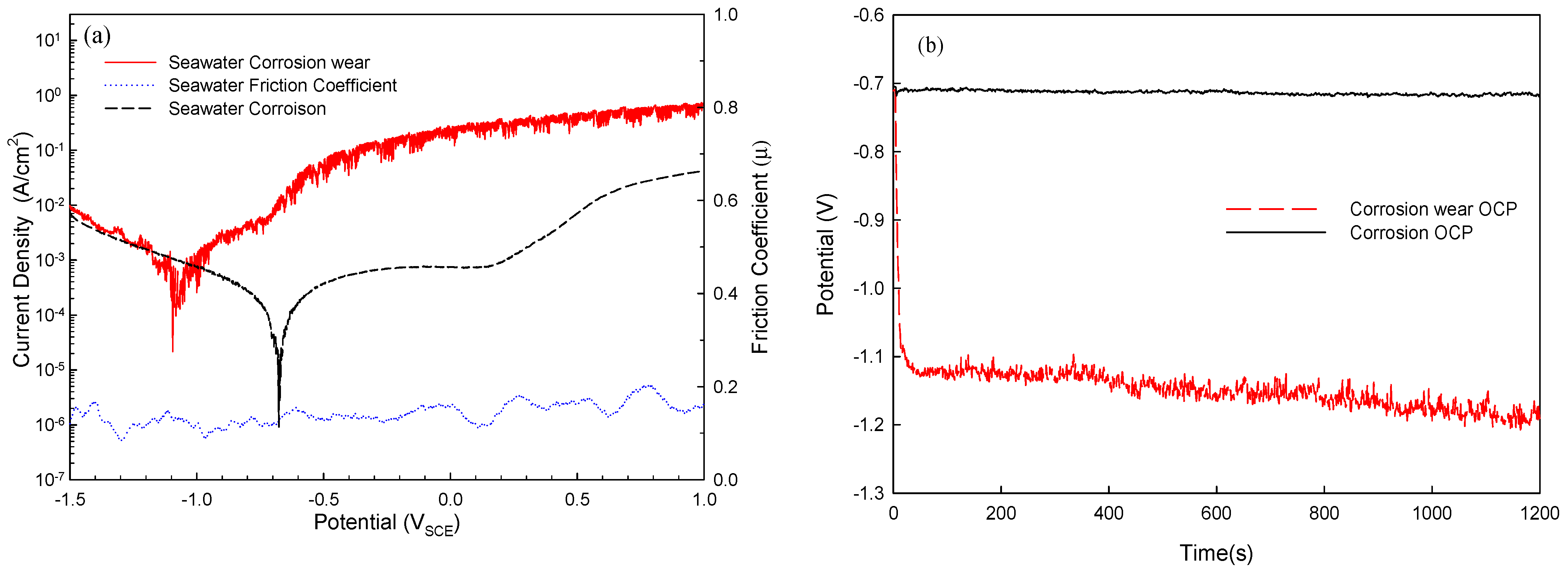
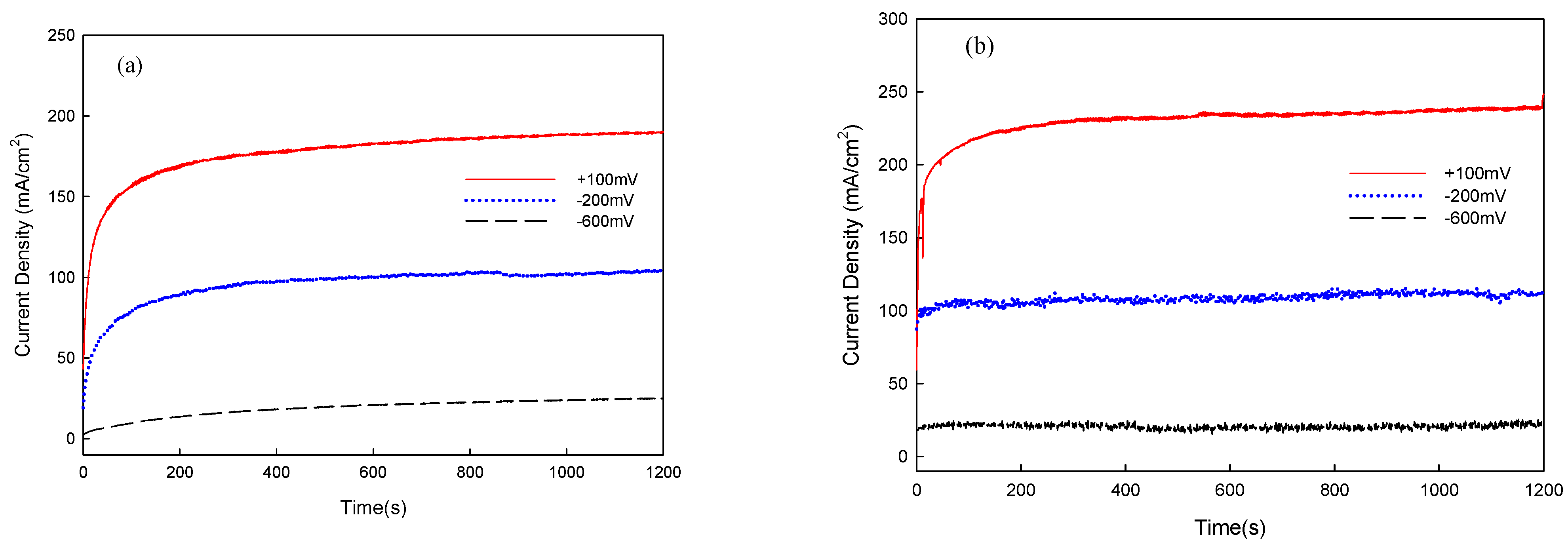
3.2. Electrochemical Analysis of Corrosion and Corrosion–Wear Behavior of Al Coating
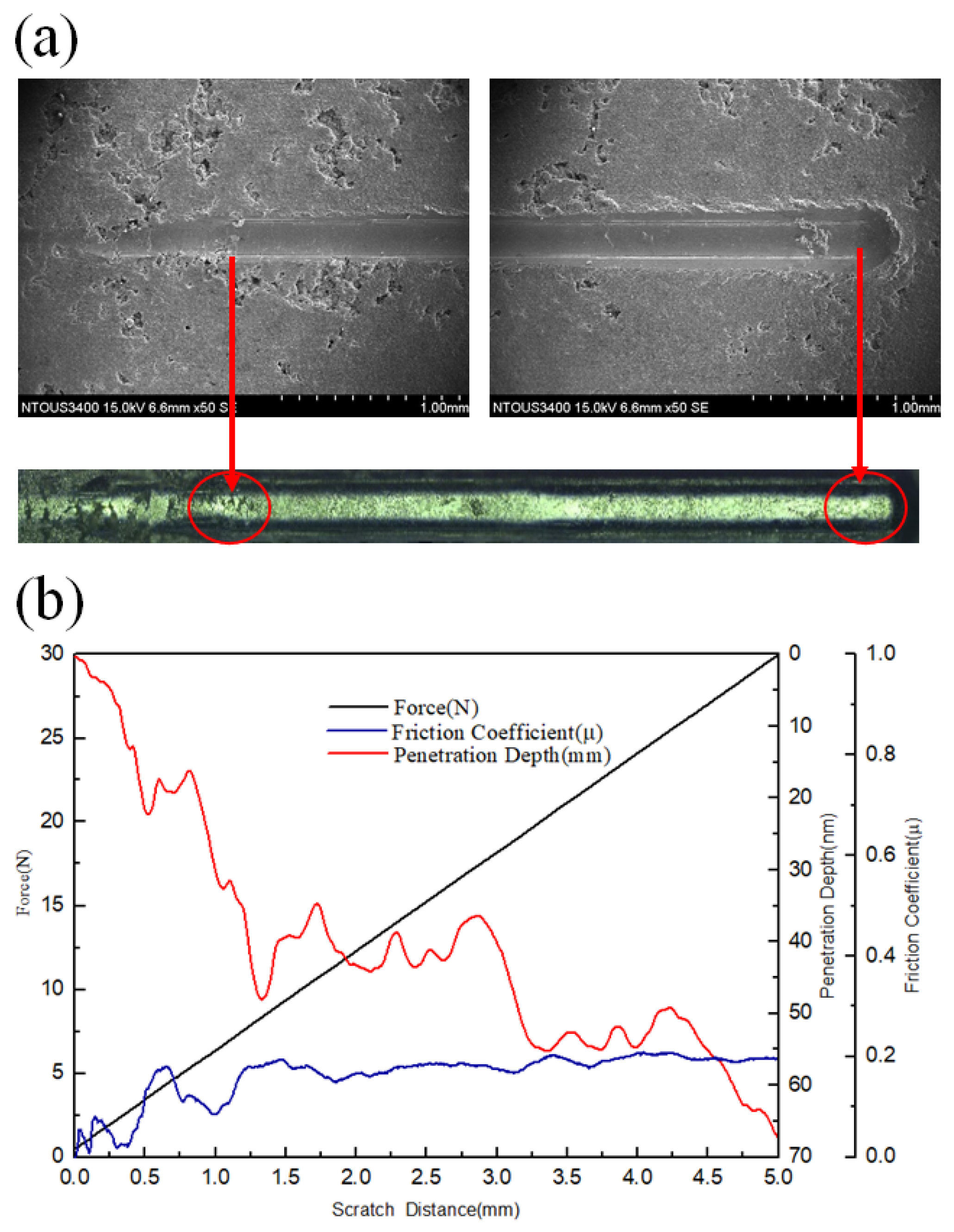
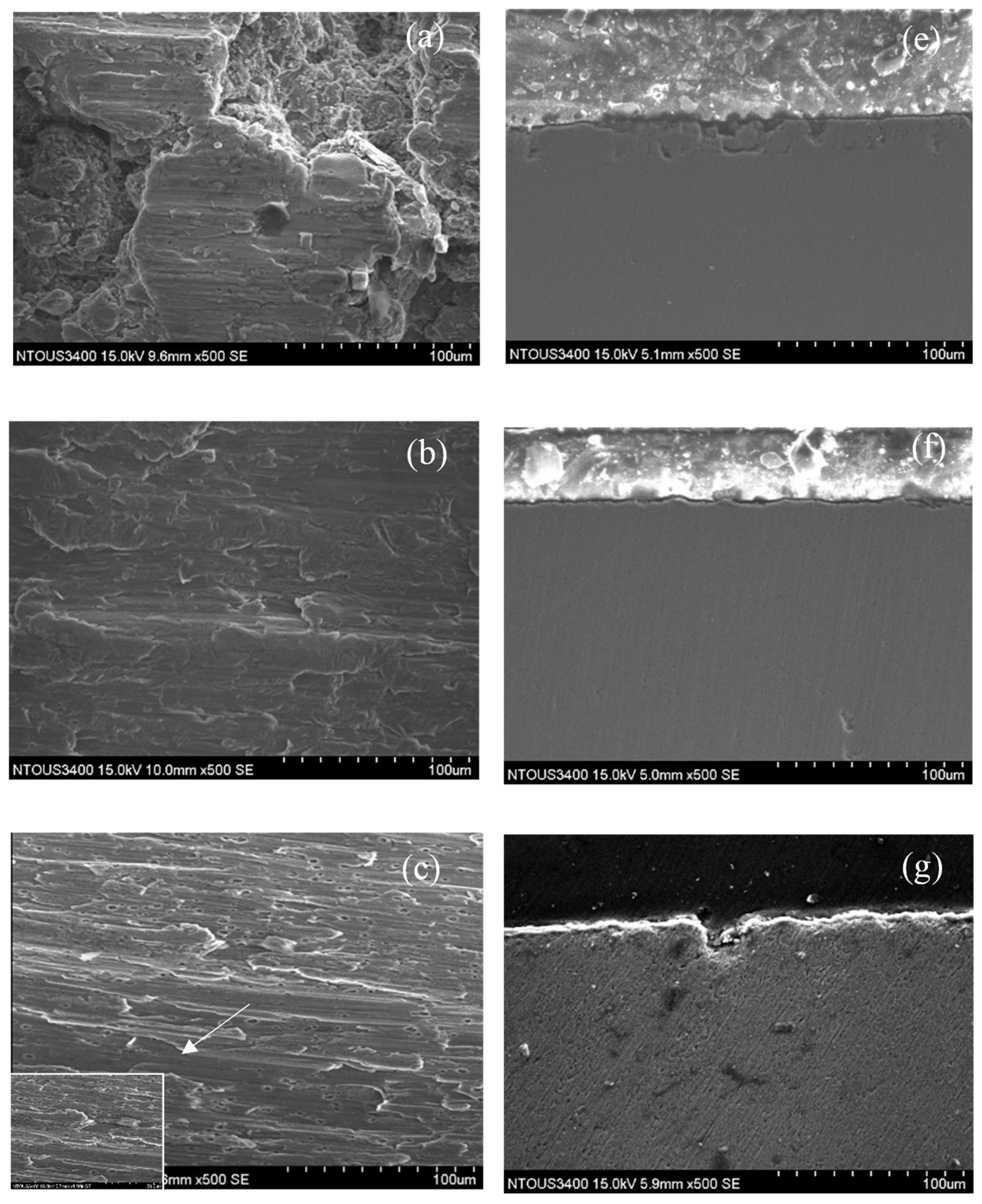
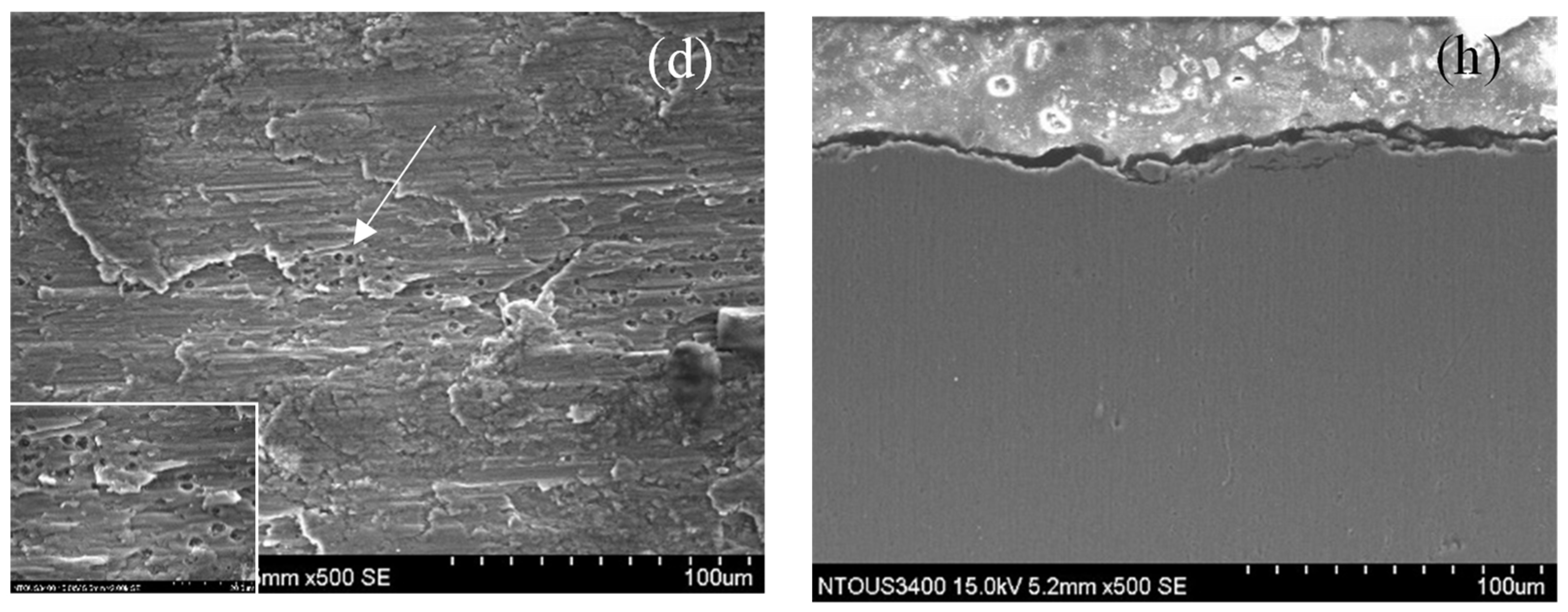
3.3. Corrosion and Corrosion–Wear Characteristics in the Spray Al Coating
4. Discussion
4.1. XPS Analysis of Thermal Spray Al Coating after the Corrosion–Wear Test
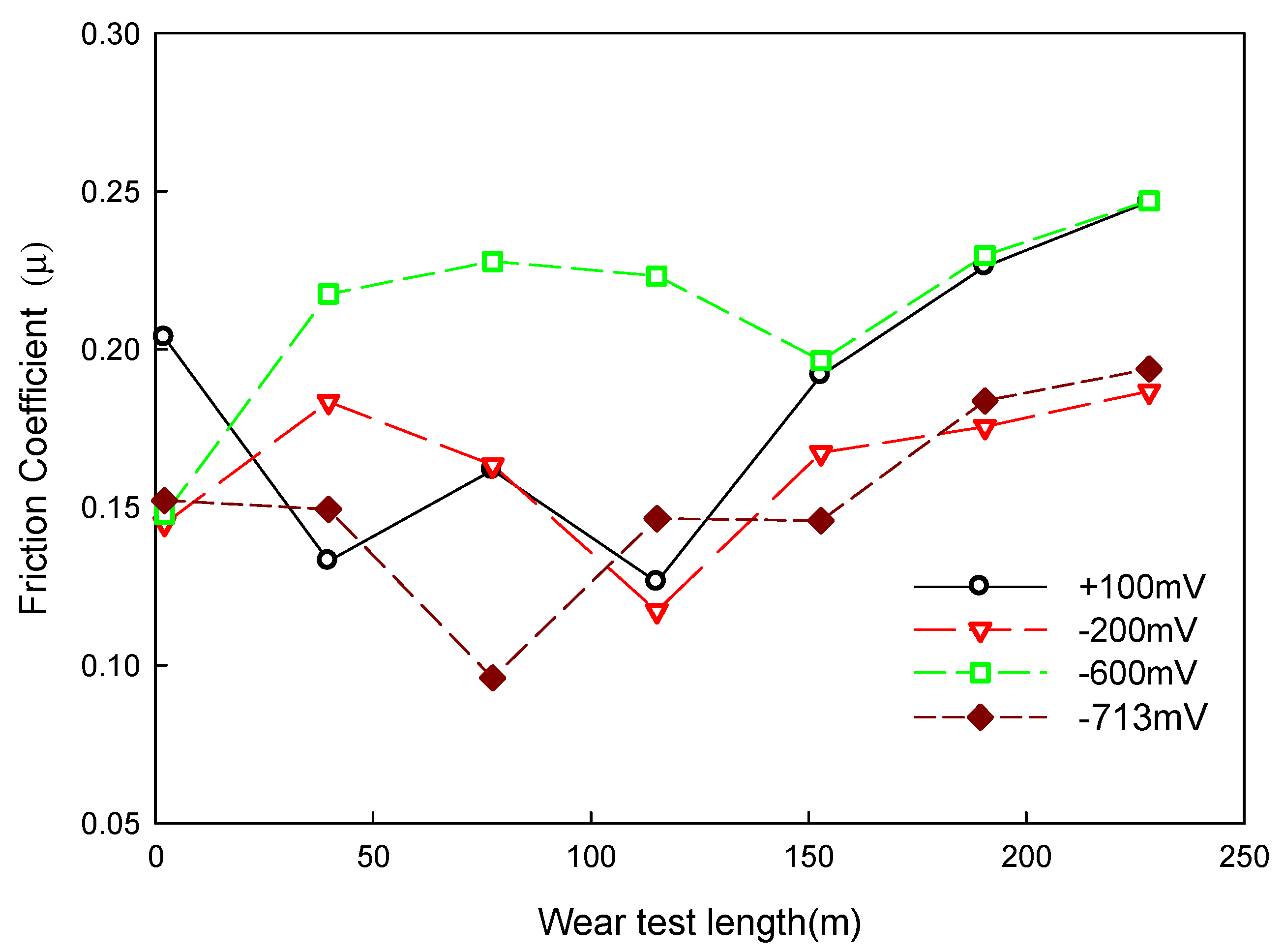
4.2. Relation between Corrosion–Wear and Friction Coefficient
4.3. Corrosion of Al Coating and Corrosion–Wear of Al Coating Interaction
5. Conclusions
- In the corrosion test, the weight loss increased as the polarization potentials rose, and the weight loss was only 15 mg with an applied potential of +100 mVSCE, indicating that the coating had excellent corrosion resistance.
- In the results from the corrosion–wear of the Al coating test, the weight loss was from 56 to 178 mg, with the applied polarization potentials from −713 mVSCE to +100 VSCE. The surface morphologies changed from wear scratch to inner voids in the scratches, which explained that the coating suffered not only from wear but also from serious corrosion.
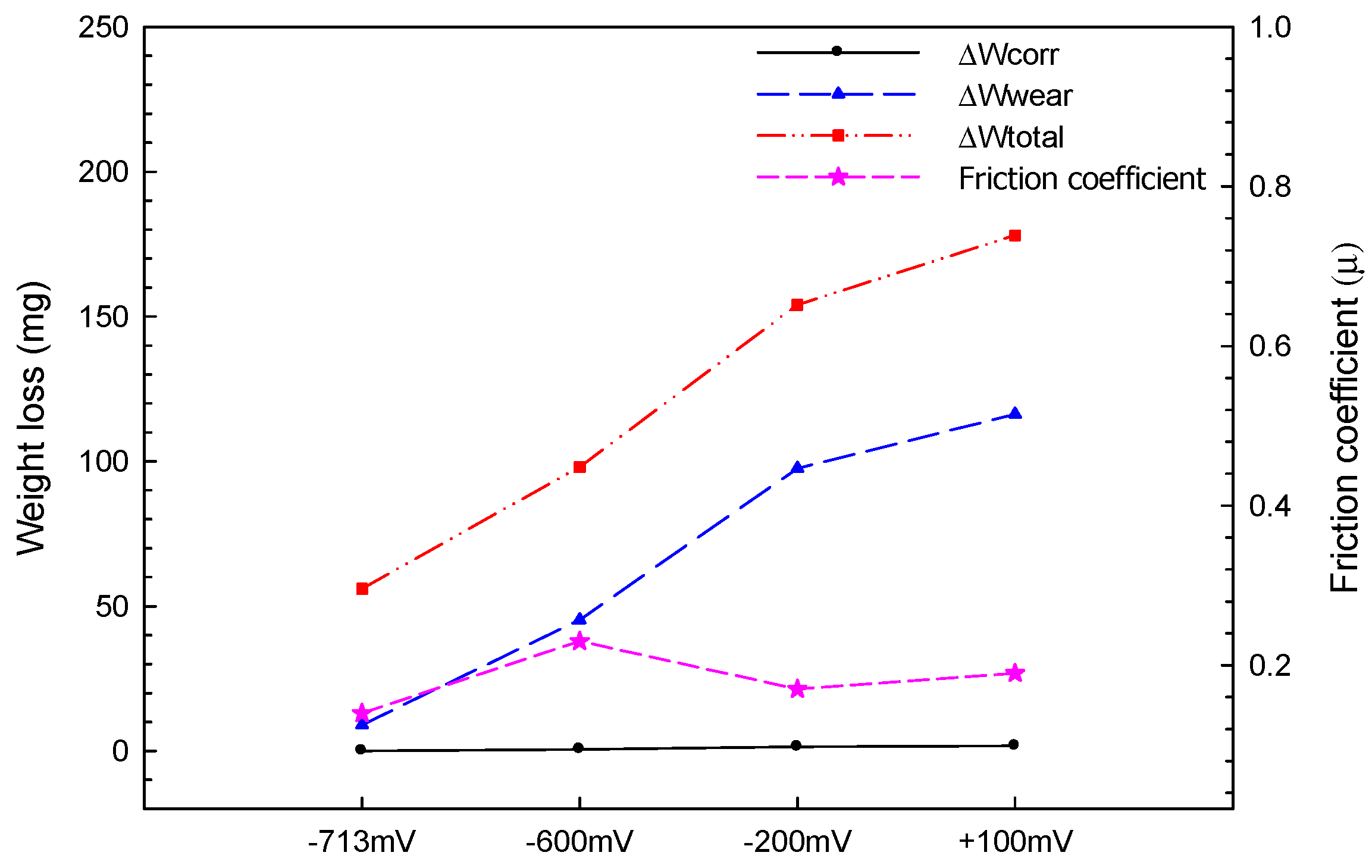
- Mutual corrosion of Al coating and corrosion–wear of Al coating were the primary causes of deterioration of the pure Al coating in seawater in the corrosion–wear test. Via the quantitative analysis, ∆Wcorr changed somewhat as the applied polarization potentials increased; however, ∆Wwear continued rising due to the mutual corrosion and wear and primarily caused the weight loss of the coating.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Ortegaa, A.; Areitioaurtenaa, O.; Alvesa, S.A.; Goitandiaa, A.M.; Elexpea, I.; Aranab, J.L.; Bayóna, R. Development of a superhydrophobic and bactericide organic topcoat to be applied on thermally sprayed aluminum coatings in offshore submerged components. Prog. Org. Coat. 2019, 137, 105376. [Google Scholar] [CrossRef]
- Chen, T.C.; Chou, C.C.; Yung, T.Y.; Tsai, K.C.; Huang, J.Y. Wear behavior of thermally sprayed Zn/15Al, Al and Inconel 625 coatings on carbon steel. Surf. Coat. Technol. 2016, 303, 78–85. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Wang, D.; Xue, L.; Zeng, B.; Qin, B.; Tang, Z. Effect of liquid oxy-nitriding at various temperatures on wear and molten aluminum corrosion behaviors of AISI H13 steel. Corros. Sci. 2021, 178, 109088. [Google Scholar] [CrossRef]
- Lin, M.Y.; Hsiao, P.S.; Sheu, H.H.; Chang, C.C.; Tsai, M.S.; Wuu, D.S.; Lee, H.B. Improving the Corrosion Resistance of 6061 Aluminum Alloy Using Anodization and Nickel-Cobalt based Sealing Treatmen. Int. J. Electrochem. Sci. 2021, 16, 211053. [Google Scholar] [CrossRef]
- Park, I.C.P.; Kim, S.J. Corrosion behavior in seawater of arc thermal sprayed Inconel 625 coatings with sealing treatment. Surf. Coat. Technol. 2017, 325, 729–737. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Mohedano, M.; Casajús, P.; Merino, S. Al/SiC thermal spray coatings for corrosion protection of Mg-Al alloys in humid and saline environments. Surf. Coat. Technol. 2010, 268, 826. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Mohedano, M.; Casajús, P. Corrosion behaviour of a magnesium matrix composite with a silicate plasma electrolytic oxidation coating. Surf. Coat. Technol. 2010, 204, 2767. [Google Scholar] [CrossRef]
- Jiang, Q.; Miao, Q.; Liang, W.P.; Ying, F.; Tong, F.; Xu, Y.; Ren, B.L.; Yao, Z.J.; Zhang, P.Z. Corrosion behavior of arc sprayed Al-Zn-Si-RE coatings on mild steel in 3.5 wt% NaCl solution. Electrochim. Acta 2014, 115, 644–656. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, X.; Geng, G. Dry sliding wear behavior and corrosion resistance of NiCrBSi coating deposited by activated combustion-high velocity air fuel spray process. Mater. Des. 2010, 31, 913–917. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Liang, B. Wear characterization of thermal spray welded Ni-Cr-B-Si-RE alloy coatings. J. Mater. Process. Technol. 2009, 209, 1368–1374. [Google Scholar] [CrossRef]
- Uozato, S.; Nakata, K.; Ushio, M. Corrosion and wear behaviors of ferrous powder thermal spray coatings on aluminum alloy. Surf. Coat. Technol. 2003, 169–170, 691–694. [Google Scholar] [CrossRef]
- Edrisy, A.; Perry, T.; Cheng, Y.T.; Alpas, A.T. Wear of thermal spray deposited low carbon steel coatings on aluminum alloys. Wear 2001, 251, 1023–1033. [Google Scholar] [CrossRef]
- Frenk, A.; Kurz, W. Microstructural effects on the sliding wear resistance of a cobalt-based alloy. Wear 1994, 174, 81–91. [Google Scholar] [CrossRef]
- Qi, X.; Eigen, N.; Aust, E.; Gärtner, F.; Klassen, T.; Bormann, R. Two-body abrasive wear of nano- and microcrystalline TiC–Ni-based thermal spray coatings. Surf. Coat. Technol. 2006, 200, 5037. [Google Scholar] [CrossRef]
- Aruna, S.T.; Balaji, N.; Shedthi, J.; Grips, V.K.W. Effect of critical plasma spray parameters on the microstructure, microhardness and wear and corrosion resistance of plasma sprayed alumina coatings. Surf. Coat. Technol. 2012, 208, 92. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn–Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- Kuroda, S.; Kawakita, J.; Takemoto, M. An 18-year exposure test of thermal-sprayed Zn, Al, and Zn-Al coatings in marine environment. Corrosion 2006, 62, 635–647. [Google Scholar] [CrossRef]
- Han, M.S.; Woo, Y.B.; Ko, S.C.; Jeong, Y.J.; Jang, S.K.; Kim, S.J. Effects of thickness of Al thermal spray coating for STS 304. Trans. Nonferrous Met. Soc. China 2009, 19, 925–929. [Google Scholar] [CrossRef]
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Pragathi, P.; Elansezhian, R. Wear and corrosion behaviour on pure aluminium matrix reinforced with SiC and spent catalyst by using the stir casting method. Mater. Today Proc. 2021, 38, 3246–3252. [Google Scholar]
- Dinga, H.; Zhoua, G.; Dai, Z.; Bua, Y.; Jiang, T. Corrosion wear behaviors of 2024Al in artificial rainwater and seawater at fretting contact. Wear 2009, 267, 292–298. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, W.; Xie, Z.; Dai, H.; Wang, E.; Chen, Y.; Liu, L. Enhancing anti-wear and anti-corrosion performance of cold spraying aluminum coating by high current pulsed electron beam irradiation. Vacuum 2020, 182, 109772. [Google Scholar] [CrossRef]
- López-Ortega, A.; Arana, J.L.; Rodríguez, E.; Bayón, R. Corrosion, wear and tribocorrosion performance of a thermally sprayed aluminum coating modified by plasma electrolytic oxidation technique for offshore submerged components protection. Corros. Sci. 2018, 143, 258–280. [Google Scholar] [CrossRef]
- Lee, H.B. Synergy between corrosion and wear of electrodeposited Ni-W coating. Tribol. Trans. 2013, 50, 407–419. [Google Scholar] [CrossRef]
- Lee, H.B.; Wuu, D.S.; Lee, C.Y.; Lin, C.S. Wear and immersion corrosion of Ni-P electrodeposit in NaCl solution. Tribol. Int. 2010, 43, 235–244. [Google Scholar] [CrossRef]
- Lee, H.B.; Wu, M.Y. A Study on the Corrosion and Wear Behavior of Electrodeposited Ni–W–P Coating. Metall. Mater. Trans. A 2017, 48, 4667–4680. [Google Scholar] [CrossRef]
- Greczynski, G.; Petrov, I.; Greene, J.E.; Hultman, L. Al capping layers for nondestructive x-ray photoelectron spectroscopy analyses of transition-metal nitride thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2015, 33, 05E101. [Google Scholar] [CrossRef] [Green Version]


| Ecorr (V) | icorr (μA/cm2) | Friction Coefficient | |
|---|---|---|---|
| Corrosion–wear of Al coating | −1.051 | 450 | 0.13 |
| Corrosion of Al coating | −0.672 | 57 | - |
| Potential (V) | Weight Loss (mg) | Friction Coefficient | |
|---|---|---|---|
| Corrosion–wear of Al coating | −1.151 | 56 | 0.14 |
| Corrosion of Al coating | −0.713 | 2 | - |
| Applied Voltage | I | Weight Loss (mg) | Ra (μm) |
|---|---|---|---|
| −713 mVSCE | - | 2 | 9.35 |
| −600 mVSCE | 19.0 | 7 | 10.57 |
| −200 mVSCE | 95.4 | 10 | 11.18 |
| +100 mVSCE | 214.2 | 15 | 13.46 |
| Applied Voltage | I | Weight Loss (mg) | Ra (μm) | Friction Coefficient |
|---|---|---|---|---|
| −713 mVSCE | - | 56 | 2.64 | 0.14 |
| −600 mVSCE | 24.1 | 98 | 3.63 | 0.23 |
| −200 mVSCE | 108.4 | 154 | 4.96 | 0.17 |
| +100 mVSCE | 229.9 | 178 | 5.54 | 0.19 |
| Element | Spectral Line | Formula | Binding Energy (eV) | ||
|---|---|---|---|---|---|
| NIST | Reference | Experimental on the Surface | |||
| Al | 2p | Al | 72.9 | 72.9 | 72.9 |
| Al | 2p | Al2O3 | 74.6 | 74.2 | 74.6 |
| Applied Voltage | Wtotal (mg) | Wwear (mg) | Wcorr (mg) | ΔW (mg) | |
|---|---|---|---|---|---|
| ΔWcorr | ΔWwear | ||||
| −713 mVSCE | 56 | 45 | 2 | 0 | 9 |
| −600 mVSCE | 98 | 45 | 7 | 0.57 | 45.3 |
| −200 mVSCE | 154 | 45 | 10 | 1.45 | 97.55 |
| +100 mVSCE | 178 | 45 | 15 | 1.76 | 116.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.-Y.; Sheu, H.-H.; Chang, J.-K.; Chen, C.-H.; Hong, Y.-H.; Lin, M.-H.; Lee, H.-B. Effect of the Applied Voltages on the Corrosion–Wear Behavior of Thermal Spray Al Coating. Lubricants 2022, 10, 197. https://doi.org/10.3390/lubricants10080197
Jian S-Y, Sheu H-H, Chang J-K, Chen C-H, Hong Y-H, Lin M-H, Lee H-B. Effect of the Applied Voltages on the Corrosion–Wear Behavior of Thermal Spray Al Coating. Lubricants. 2022; 10(8):197. https://doi.org/10.3390/lubricants10080197
Chicago/Turabian StyleJian, Shun-Yi, Hung-Hua Sheu, Jun-Kai Chang, Chih-Hung Chen, Yuan-Huan Hong, Ming-Hsun Lin, and Hung-Bin Lee. 2022. "Effect of the Applied Voltages on the Corrosion–Wear Behavior of Thermal Spray Al Coating" Lubricants 10, no. 8: 197. https://doi.org/10.3390/lubricants10080197
APA StyleJian, S.-Y., Sheu, H.-H., Chang, J.-K., Chen, C.-H., Hong, Y.-H., Lin, M.-H., & Lee, H.-B. (2022). Effect of the Applied Voltages on the Corrosion–Wear Behavior of Thermal Spray Al Coating. Lubricants, 10(8), 197. https://doi.org/10.3390/lubricants10080197




